Effect of N-acetyl-cysteine treatment on sensorineural hearing loss: a meta-analysis
Abstract
N-acetyl-cysteine (NAC) is an efficacious treatment for sensorineural hearing loss in animal models, such as noise-induced hearing loss (NIHL), however previous research into the effect of NAC on patients with hearing loss produced contradictory results. In this study, we investigated the effect of NAC treatment on sensorineural hearing loss. PubMed, Web of Science and Embase databases were searched in their entirety using the key words: hearing loss, NAC, N-acetylcysteine, and sensorineural hearing loss. Studies which included assessment of hearing loss with pure-tone threshold (PTA) data were selected. Eligible studies regarding the effects of NAC treatment on patients with hearing loss were collected by two independent reviewers. A total of 1197 individuals were included from seven published studies. Two studies reported data for a sudden idiopathic sensorineural hearing loss (SISNHL) group. Three studies reported data for a NIHL group. Other studies reported data for drug-induced hearing loss. The meta-analysis demonstrated that the overall effect of NAC treatment on sensorineural hearing loss was invalid. However, NAC treatment was linked with improved patient outcomes of hearing tests in cases of sudden hearing loss, but did not prevent hearing loss induced by noise or ototoxicity. However, there is a need for better-designed studies with larger samples to further prove the correlation between the effect of NAC and hearing loss.
INTRODUCTION
Hearing loss has become a common concern among diseases that cause disability, affecting approximately 5.3% of the worldwide population.1 Deafness has a profound influence not only on daily communication, but also on physical and psychological health, social relationships, and quality of life.2 According to the location of the lesion, hearing loss is divided into four types, of which sensorineural deafness is one, and the leading factors causing disabling sensorineural hearing loss can be congenital or acquired. Common causes of acquired hearing loss in adults are aging (presbycusis), noise, ototoxic drugs, inflammation and other unknown aetiologies. The most likely mechanism explaining hearing loss which has been demonstrated by studies, is concerned with reactive oxygen species (ROS), which damage auditory hair cells by activating apoptotic cell death programs.3 The accumulation of ROS in cells causes an imbalance between free radicals and antioxidants in the body, and this imbalanced mechanism and subsequent apoptotic program are important contributors to deafness.4, 5 It has been hypothesised that the use of antioxidants may inactivate or reduce the generation of free radicals causing damage to cells.6 Recent studies have indicated that antioxidants such as glutathione (GSH), which is a critical antioxidant in the human body, can reduce the production of ROS, and thus protect hair cells from damage.7 NAC, as a precursor of GSH which therefore increases its production, has been clinically adopted to prevent deafness.8
To date, many clinical trials have been performed to investigate the association between NAC and deafness caused by a single factor. However, the outcomes of these studies are controversial. Thus, this meta-analysis was conducted to analyse the effect of NAC on sensorineural hearing loss. Patients were divided into subgroups according to the different aetiologies of hearing loss, with the aim of better elucidating the connections between NAC and sensorineural hearing loss.
METHODS
Search strategy
We searched for all relevant publications using PubMed, Web of Science and Embase databases. However, the grey literature databases were not searched. Search terms included the following key words: hearing loss, NAC, N-acetylcysteine, sensorineural hearing loss. A compound search strategy was used to search all relevant studies regardless of language or publication status. Relevant studies were identified in the computerized databases up to September 2019, without limitations of language. However, a paper needed to have at least the title and/or abstract in English to be included in the present selection. All references were loaded into EndNote, and titles and abstracts were examined after duplicates were removed independently by two reviewers. The computerized search was supplemented by a manual search of the bibliographies of all retrieved articles. Potentially related papers were evaluated for inclusion against pre-specified eligibility and exclusion criteria. Reference lists of all studies identified by the above methods and bibliographies of systematic reviews or meta-analyses were examined. Final agreement on study inclusion was determined by consensus between two reviewers.
Inclusion and exclusion criteria of publications
Original studies were carefully checked. Selected studies were included if they satisfied the following criteria.
Inclusion criteria: (1) Only clinical trials concerning NAC and sensorineural hearing loss were selected. (2) All participants were diagnosed with sensorineural deafness (whether induced by ototoxic drugs, noise-induced, or sudden hearing loss). (3) All individuals were allocated to either a group given supplementation with NAC through the oral route or a control group without NAC therapy. (4) All observational data from the studies were intended to identify differences between the experimental and a control group.
The exclusion criteria were: (1) Case reports, abstracts, comments, review articles, duplicate publications, and editorials. (2) Other therapies than NAC and hearing loss. (3) The available information was not adequate for data extraction.
Data extraction
Two authors selected articles and glanced over the reference lists of retrieved papers to further identify possibly relevant articles. The process of data extraction was performed by two authors independently, in duplicate, adopting a standardized data extraction form. Disagreements were solved by consensus between two authors. The relevant information was extracted from each publication relating to the information on the intervention (the number of individuals in the study groups (treated with NAC) and control groups, the doses and duration of use of NAC), basic information (the name of the first author, publication year, study title), and other information (age of subjects, types of hearing loss, assessment tools).
Statistical analysis
This meta-analysis was completed using Review Manager 5.3. Subgroup analysis was performed to analyse heterogeneity between different types of hearing loss and NAC. The random effects model was applied, relying upon the P-value of the Chi-squared statistic when P was < 0.05, and the Higgins I2 test was used to assess heterogeneity (I2 < 25%, which was assumed homogeneous; I2 = 25%–50%, which was assumed as low heterogeneity; I2 = 50%–75%, which was assumed as moderate heterogeneity; I2 > 75%, which was assumed as high heterogeneity). If the I2-value was > 50%, the selected articles were considered to have moderate or high heterogeneity, so the random effects model was used to combine effect size, while if the I2-value was < 50%, the fixed effects model was used to combine effect size. Standardized mean difference (SMD) and 95% confidence interval (95% CI) were calculated to estimate the relationship between sensorineural hearing loss and NAC in each subgroup. The statistical significance was set at a P < 0.05.
Assessment of the risk of bias
Two independent researchers evaluated the quality of the articles in terms of five aspects: generation of the appropriate sequence; allocation concealment; blinding of the participants, the researcher and the evaluators; handling of missing data for subsequent final judgment. This information was adopted to inform the assessment of the risk of bias and the quality, and the Cochrane risk of bias tool was used for quality assessment of these studies.
RESULTS
Included studies
Approximately sixty-three relevant articles were initially selected from electronic databases. From these, 35 articles were cursorily eliminated based on their titles and abstracts which were not in accord with the inclusion/exclusion criteria or were duplicates, leaving 28 studies remaining. Of these, 21 articles were excluded because their data were incomplete. The completed process to identify relevant searches is shown in Figure 1.
Characteristics of included studies
Seven articles covering data from a total of 1197 participants were included in this meta-analysis. Two studies reported data for a sudden hearing loss group. Three studies reported data for a NIHL group. Other studies reported data for drug-induced hearing loss. The name of the first author, publication year, study title, study design, age of subjects, the number of individuals in each group, the type of hearing loss, the doses and duration of NAC treatment, assessment tools, and amounts of drugs administered are shown in Table 1.
| References | Age(years) | Type of hearing loss | Patient number(n) | Duration of NAC | Dose of NAC(mg/day) | Study and control group |
|---|---|---|---|---|---|---|
| Kopke R, et al.9 2015 | mean=19.63; range:18-35 | noise induced hearing loss | all: 566 NAC: 277 control(placebo): 289 |
13 days | 2700 | study group: NAC control group: placebo |
| Doosti A, et al.10 2014 | mean=39.12, SD= ± 5.00; range:28-50 | noise induced hearing loss | all: 48 NAC: 16 ginseng: 16 control: 16 |
14 days | 1200 | study group 1: NAC study group 2: ginseng control group: placebo |
| Zhenmin G, et al.11 2011 | mean=27.3, SD= ± 6.5; range:19-35 | noise induced hearing loss | all: 363 NAC: 223 control: 140 |
14 days | 1200 | study group: NAC control group: placebo |
| Chen CH, et al.12 2016 | group NAC: mean=44, SD= ±13 group control: mean=48, SD= ±12 |
sudden hearing loss | all: 70 NAC: 35 control: 35 |
3 months | 1200 | study group: NAC+corticosteroid control group: corticosteroid alone |
| Angeli SI, et al.13 2012 | mean=48.6, SD= ±14.0 | sudden hearing loss | all: 56 steroids+NAC: 27 steroids alone: 29 |
14 days | 1200 | study group: steroids+NAC control group: steroids |
| Tokgoz B, et al.14 2011 | group NAC: mean=45.0, SD= ±13.2 group control: mean=49.9, SD= ±15.2 |
drug-induced ototoxicity | all: 60 NAC: 30 control: 30 |
28 days | 1200 | study group: NAC+drug treatment control group: drug treatment |
| Feldman L, et al.15 2007 | group NAC: mean=65.8, SD= ±12.5 group control: mean=59.8, SD= ±11.5 |
drug-induced ototoxicity | all: 40 NAC: 20 control: 20 |
(7 ± 3) days | 1200 | study group: NAC+drug treatment control group: drug treatment |
Assessment of the risk of bias
The risk of bias in papers at the main outcome level is shown in Table 2. Of the seven included studies, two did not report the method used for sequence generation. As for allocation concealment, none of the papers offered sufficient information on this process to allow judgment. All studies explained the blinding of the evaluators, as well as the justification for missing data, when errors occurred.
| References | Sequence generation | Allocation concealment | Participant and researcher blinding | Blinding of evaluators | Handling of missing data |
|---|---|---|---|---|---|
| Chen CH, et al.12 | High | Unkown | Low | Low | Low |
| Angeli SI, et al.13 | High | Unkown | Low | Low | Low |
| Doosti A, et al.10 | Low | Unkown | Low | Low | Low |
| Kopke R, et al.9 | Low | Unkown | Low | Low | Low |
| Zhenmin G, et al.11 | Low | Unkown | Low | Low | Low |
| Tokgoz B, et al.14 | Low | Unkown | Low | Low | Low |
| Feldman L, et al.15 | Low | Unkown | Low | Low | Low |
Meta-analysis and subgroup analysis results
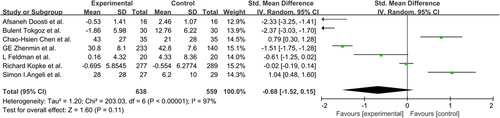
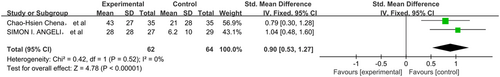
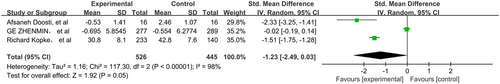
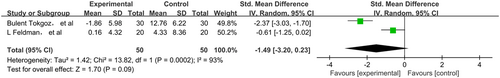
The pooled data were calculated using the random-effects model as there was highly significant heterogeneity among the studies. The total SMD in the studies with sensorineural hearing loss gave a corresponding value of −2.11 (95%CI, −6.74–2.25, P > 0.05) (Figure 2). However, in subgroup analysis, the total SMD in the studies with sudden hearing loss had a corresponding value of 0.90 (95%CI, 0.53–1.27, P < 0.05) (Figure 3). As such, the analysis revealed that the effect of NAC therapy was significant in ameliorating sudden hearing loss. The total SMD in the studies with NIHL had a corresponding value of −1.23 (95%CI, −2.49–0.03, P = 0.05) (Figure 4). The total SMD in the studies of ototoxicity had a corresponding value of −1.94 (95%CI, −3.20–0.23, P > 0.05) (Figure 5). The meta-analysis demonstrated that the overall effect of NAC treatment on sensorineural hearing loss was invalid. However, NAC treatment was linked with improved patient outcomes of hearing tests in sudden hearing loss, but did not prevent noise-induced hearing loss or ototoxicity-induced hearing loss.
DISCUSSION
In our meta-analysis, a total of 1197 individuals, divided into three categories, were included from seven published studies. Subjects were randomly assigned either to a group that was administered NAC or another that was not as the control group.9-15 The seven included trials evaluated the effect of NAC therapy on different types of sensorineural deafness. Although aging is one common cause of sensorineural hearing loss, and most studies have shown that the mechanism of presbycusis is related to oxidative stress,16, 17 the effect of NAC therapy on age-related hearing loss in animal models is still controversial.18, 19 At present, there are no published results of clinical trials for presbycusis, so we did not cover the current popular presbycusis-related research in our study. One drawback of this analysis is that the doses and duration of use of NAC may have been different in different studies. Additionally in these studies, the tools used to evaluate hearing loss were varied, thus we eventually chose the results of PTA as the relevant data. We analysed all the data from these articles and performed subgroup analysis, and finally found that treatment with NAC had a therapeutic effect on sudden deafness.
The subgroup analysis of treatment for sudden hearing loss showed significant differences in outcomes with the use of NAC (SMD = 0.90, 95%CI = 0.53–1.27). Analysis of these studies showed that treatment with NAC, alone or in combination with steroids, has an effect on sudden hearing loss.12, 13 Since clinical evidence suggests there is a state of altered oxidative stress in sudden hearing loss, a plausible explanation for the beneficial effects of NAC involves the limitation of ROS-induced apoptosis.20 On the other hand, corticosteroids can affect the signalling pathway of apoptosis which eventually reduces the damage to auditory hair cells, so we speculated that NAC may potentiate the anti-apoptotic effect of simultaneously administering corticosteroids.21 In contrast, the NIHL group showed no significant improvement with NAC treatment (SMD = −1.23, 95%CI = 2.49–0.03). The reason for this result may be explained by the fact that some studies have shown that exposure to intense noise over time not only affects the metabolic activity of mitochondria in cells, which may increase the accumulation of ROS, but also damages blood flow of the organ of Corti and the mechanical structure of the cell membrane, as well as changing the normal physiological structure and function of the cochlea, ultimately resulting in damage to the cochlea and sensorineural deafness.22 Ototoxic drugs give rise to high-frequency hearing loss in the first place, so in this meta-analysis we eliminated hearing threshold at high frequencies from publications for correlation. The group of patients with hearing loss induced by drugs also showed no significant improvement with the administration of NAC (SMD = −1.23, 95%CI = 2.49–0.03). In the subgroup of patients with noise-induced and drug-induced hearing loss, there were few relevant studies, clinical heterogeneity may be a possible source of heterogeneity. In the noise-induced hearing loss subgroup, the main source of heterogeneity may be the different conditions of noise exposure. Although the patients enrolled in three studies were exposed to noise, the duration and intensity of the noise exposure were different. The mechanism of long-term chronic noise exposure induced hearing loss is different from blast trauma induced hearing loss, which may be the possible main source of heterogeneity in the noise-induced hearing loss. In the drug-induced hearing loss subgroup, the features of participants were different, for example in research by Feldman et al,15 patients with end-stage renal disease (ESRD) were recruited, with the aim of preventing gentamicin-induced hearing loss. However, in the study by Tokgoz et al,14 participants with continuous ambulatory peritoneal dialysis (CAPD) peritonitis were enrolled and treated with NAC to prevent amikacin-induced hearing loss, which may be the possible main source of heterogeneity in the drug-induced hearing loss group.
ROS constitute one of the pathogenic mechanisms by which drugs cause ototoxicity, but other studies have demonstrated that reactive nitrogen species (RNS) and apoptosis also mediate the death of auditory hair cells.23, 24 Animal experiments have shown that the use of NOS (nitric oxide synthase) inhibitors can alleviate the ototoxicity of aminoglycosides to the cochlea.25, 26 Consequently, NAC is ineffective in preventing damage to the cochlea through other mechanisms in drug-induced hearing loss. Besides the above reasons, in the cochlea, the susceptibility of hair cells to oxidative stress varies with their position. Compared with the apical outer hair cells, the basal coil outer hair cells, which are responsible for high frequency hearing, are more vulnerable, and as a result, their intrinsic defence systems against oxidative stress caused by varied aetiologies are different,27 which may explain the different effects of NAC treatment on different types of hearing loss.
According to Clinical Practice Guidelines, steroid is the first-line treatment for sudden idiopathic sensorineural hearing loss (SISNHL); however, approximately 50% of patients experience no or limited hearing improvement after steroid treatment.28 NAC therapy is effective for SISNHL, especially when combined with steroids; nevertheless, the mechanism is still unclear. Some researchers have found that in animal models, NAC can be involved in the inflammatory response, which is one of the aetiologies of SISNHL.29, 30 In addition, previous research suggested that NAC may be a steroid sensitizer and may thus help to treat steroid-resistant asthma in mice,31 which could be a new potential mechanisms of NAC action in the treatment of SISNHL, and may be of potential value in clinical application. It is expected that more high-quality studies and clinical trials will be carried out in the future to improve and refine these hypotheses and provide conclusions.
In spite of these important findings, our study did have some limitations. Firstly, in the SISNHL subgroup, recovery rates of patients with SISNHL without treatment ranged from 30% to 60%, with many patients experiencing symptomatic improvement within 2 weeks of onset. Therefore, it is possible that patients treated within 2 weeks of the onset of symptoms experience spontaneous hearing recovery unrelated to the treatment; conversely, it is also possible that after 2 weeks there is irreversible damage that would not respond to any treatment. The delay in presentation and treatment in our series may explain the noted difference in hearing improvement. These results warrant further investigation with more rigorous randomized trials. Secondly, these studies really represent three entirely different aetiologies for sensorineural hearing loss, with not only different causes, but different prevention and treatment plans. For example, sudden hearing loss is only diagnosed after the fact/onset and then treatment started. In contrast noise-induced loss is treated before exposure. These differences are likely to affect the results of analysis. In addition, our meta-analysis included seven clinical trials, each of which might have experimental bias. Hence, more accurate outcomes without these variables require further study to prove the relationship between them.
CONCLUSION
This meta-analysis demonstrated that the overall effect of NAC treatment on sensorineural hearing loss was invalid. However, in subgroup analysis, NAC treatment was linked with improved patient outcomes of hearing tests in cases of sudden hearing loss, but did not prevent hearing loss caused by noise or ototoxic drugs. However, there is a need for better-designed studies with larger samples to further prove the correlation between the effect of NAC and hearing loss.
COMPETING INTERESTS
No competing interests declared.
AUTHOR CONTRIBUTIONS
All authors contributed significantly to this work. Xue Bai and Miao Wang performed the research study, collected the data; Xun Niu and Hong Yu analyzed the data; Jianxin Yue and Yu Sun designed the study and revised the manuscript.




