Impact of baseline renal function on all-cause mortality in patients who underwent cardiac resynchronization therapy: A systematic review and meta-analysis
Abstract
Background
Cardiac resynchronization therapy (CRT) improves both morbidity and mortality in selected patients with heart failure and increased QRS duration. However, chronic kidney disease (CKD) may have an adverse effect on patient outcome. The aim of this systematic review was to analyze the existing data regarding the impact of baseline renal function on all-cause mortality in patients who underwent CRT.
Methods
Medline database was searched systematically, and studies evaluating the effect of baseline renal function on all-cause mortality in patients who underwent CRT were retrieved. We performed three separate analyses according to the comparison groups included in each study. Data were analyzed using Review Manager software (RevMan version 5.3; Oxford, UK).
Results
We included 16 relevant studies in our analysis. Specifically, 13 studies showed a statistically significant higher risk of all-cause mortality in patients with impaired baseline renal function who underwent CRT. The remaining three studies did not show a statistically significant result. The quantitative synthesis of five studies showed a 19% decrease in all-cause mortality per 10-unit increment in estimated glomerular filtration rate (eGFR) [HR: 0.81, 95% CI (0.73–0.90), p<0.01, 86% I2]. Additionally, we demonstrated that patients with an eGFR<60 mL/min/1.73 m2 had an all-cause mortality rate of 66% [HR: 1.66, 95% CI (1.37–2.02), p<0.01, 0% I2], which was higher than in those with an eGFR≥60 mL/min/1.73 m2.
Conclusion
Baseline renal dysfunction has an adverse effect on-all cause mortality in patients who underwent CRT.
1 Introduction
Chronic kidney disease (CKD) represents a prevalent comorbidity in patients with heart failure (HF). Accumulating evidence suggests that nearly one third of patients with HF have concomitant stage III or greater CKD [1]-[5]. In addition, the majority of individuals with advanced kidney disease who initiate renal replacement therapy will develop clinical HF or left ventricular dysfunction [6]. The combination of these two conditions results in a nearly three times greater mortality risk compared with patients without significant kidney disease [7]. Of note, landmark randomized trials including the COMPANION [8], CARE-HF [9], MADIT-CRT [5], and RAFT studies [10] clearly demonstrated that cardiac resynchronization therapy (CRT) improved morbidity and mortality in patients with HF who had a reduced left ventricular ejection fraction (LVEF≤35%), left bundle branch block morphology, and QRS duration ≥150 msec on electrocardiogram (ECG). However, patients with an indication for CRT often have significant comorbidities such as atrial fibrillation, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, anemia, and/or kidney dysfunction, which may have a negative impact on patient outcomes. CKD is a common comorbidity in this setting [11]. The aim of this systematic review and meta-analysis was to investigate whether reduced renal function at baseline has an adverse effect on all-cause mortality in patients treated with biventricular pacing devices.
2 Materials and methods
2.1 Search strategy
The MEDLINE database was manually searched using PubMed web-based search engine without year or language restriction or any other limits until July 9, 2016. The following algorithm was used: “(cardiac resynchronization therapy OR CRT OR biventricular pacing) AND (renal failure OR kidney disease OR dialysis)”. Further, the reference lists of all included studies as well as relevant review articles were searched.
2.2 Study selection
2.2.1 Inclusion/exclusion criteria
Randomized control trials and observational studies reporting original adjusted data about the impact of baseline renal function on all-cause mortality in patients who underwent CRT were included in our analysis. The exclusion criteria were: studies not reporting data on the study outcome, studies reporting only unadjusted data, review articles, letters to the editor, editorial comments, studies reporting data on mixed ICD/CRT populations, and studies reporting data on a combined endpoint only. Additionally, studies with potentially overlapping cohorts were excluded from the quantitative synthesis. In such cases, we included the cohort with the largest sample size.
2.3 Data extraction
The information extracted for each study was: i) publication details (first author׳s last name, journal, year of publication), ii) general characteristics of the study (country of origin, study design, single or multi-center, enrollment period, follow-up duration, number of patients included), iii) characteristics of the study population [age, gender, type of cardiomyopathy, LVEF, New York Heart Association (NYHA) HF classification, history of atrial fibrillation, QRS duration, type of CRT device, mean glomerular filtration rate (GFR), mean creatinine level], and iv) the results reported in the study [adjusted hazard ratio (HR), relative risk (RR), odds ratio (OR) with 95% confidence intervals (CI)] regarding the impact of baseline renal function on all-cause mortality.
2.4 Statistical analysis
Data were analyzed using Review Manager software (RevMan, version 5.3; Oxford, UK). Adjusted HR for the impact of baseline renal function on all-cause mortality, was used in the analysis. Moreover, we performed three separate analyses according to the comparison groups provided in the included studies.
The statistical heterogeneity of the study was assessed using the I2 index. We considered low, medium, and high heterogeneity to have approximate values: 25% (I2=25), 50% (I2=50), and 75% (I2=75), respectively [12]. Funnel plots were constructed using RevMan software to assess publication bias. Fixed effect models were utilized in the analysis because of the low heterogeneity of the included studies. Funnel plots showed no significant publication bias.
3 Results
3.1 Studies and patients
A total of 225 studies were initially identified through the database search (Fig. 1). Subsequently, 178 studies were excluded at the title/abstract level, and 31 studies were excluded at the full text level, according to the criteria mentioned above. As a result, 16 studies [13]-[28] satisfied our inclusion criteria and were included in our analysis.
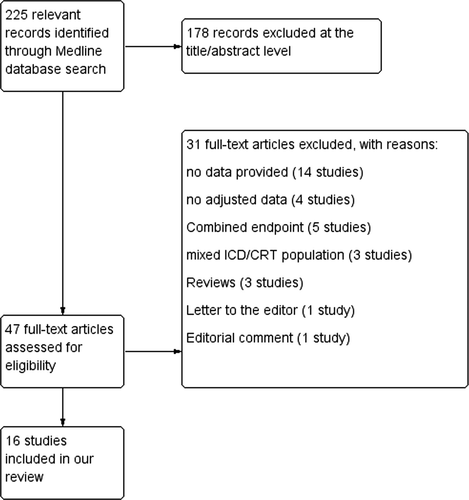
Medline database search strategy.
Of the 16 studies included in this analysis, 13 demonstrated that baseline renal dysfunction had a significant adverse effect on all-cause mortality in patients who underwent CRT, and the remaining three studies did not. Table 1 summarizes the main characteristics of the aforementioned studies along with their individual effect on the investigated outcome.
| First Author [ref] | Journal | Year | N | Enrol-lment period | Ischemic CMP (%) | Mean follow-up (months) | Age (mean) | Males (%) | LVEF (%) | Type of CRT (pts) | Total deaths | NYHA (% of pts) | mean GFR (ml/kg/1.73 m2) | Parameter (GFR in ml/kg/ 1.73 m2, Cre in mg/ml) | Point esti-mate type | Point estimate (all cause mortality) | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Daly DD [13] | PACE | 2016 | 415 | 2002–2011 | 60.7 | 51.6 | 67.3 | 72.2 | 25.4 | CRT-D (415) | 163 | III/IV: 86.1 | n/a | GFR increment 10-units | HR | 0.94 | 0.87–1.02 |
| Gronda E [14] | Cardiology Journal | 2015 | 375 | 1999–2009 | 48.8 | med: 43 | 66.6 | 80.8 | 28 | CRT-D (277) | 93 | III (mean) | 59.9 | GFR increment 10-units | HR | 0.88 | 0.78–0.98 |
| Eisen A [25] | J Cardiovasc Electrophysiol | 2014 | 453 | 2010–2012 | 69.8 | 12 | 66.2 | 86.5 | <30%: 78.8 | CRT-D (453) | 24 | II-IV: 96 | n/a | GFR< 30 | HR | 0.9 | 0.1–7.5 |
| Bogdan S [15] | J Cardiovasc Electrophysiol, | 2014 | 179 | 2007–2010 | 69 | 48 | 68 | 85 | 24.2 | CRT-P (26)/ CRT-D (153) | 73 | III-IV: 90 | 57.17 | GFR< 60/ increment 1-unit | HR | 2.03/0.97 | 1.14–3.61/ 0.96–0.99 |
| Hoke U [20] | Circ Cardiovasc Qual Outcomes | 2014 | 208 | 2000–2010 | 69 | med: 38.6 | 78 | 79 | 27 | CRT-D/ CRT-P | 84 | III (mean) | 51 | GFR increment 1-unit | HR | 0.975 | 0.959–0.995 |
| Khatib M [23] | European Journal of heart failure | 2014 | 608 | 2000–2011 | 42 | 36 | 66.9 | 77 | 24.8 | CRT-D (404) | 174 | II: 23, III: 67, IV: 10 | 63.5 | GFR< 60 | HR | 1.61 | 1.14–2.30 |
| Hoke U [16] | Diabetes Care | 2013 | 710 | n/a | 57 | 38 | 66 | 75.5 | 25 | CRT | 255 | III (mean) | 69 | GFR increment 1-unit | HR | 0.977 | 0.969–0.985 |
| Morani G [24] | Europace | 2013 | 374 | 2004–2007 | 56 | med: 55 | 69 | 80 | 27 | CRT-P (108)/ CRT-D (266) | 117 | II: 24, III: 62, IV: 14 | 58 | GFR< 60 | HR | 1.45 | 0.91–2.32 |
| Kreuz J [26] | Europace | 2012 | 239 | 2001–2010 | 58.2 | med: 43 | 66.7 | 80.3 | 26 | CRT-D (239) | 59 | II: 27.6, III: 72.4 | n/a | Cre increment 0.2-units | HR | 1.98 | 1.7–3 |
| Verbrugge FH [19] | J Cardiac Fail | 2012 | 172 | 2008–2011 | 48 | 18 | 71 | 68 | 29 | CRT-P (98)/ CRT-D (74) | 21 | II: 31, III: 59, IV: 9 | n/a | GFR< 60 | HR | 3.11 | 1.10–8.81 |
| Van Bommel RJ [17] | JACC | 2011 | 490 | 1999–2007 | 59.8 | 26 | 65.5 | 80 | 24 | n/a | 106 | III (mean) | 69.4 | GFR increment 1-unit | HR | 0.97 | 0.96–0.98 |
| Lin G [18] | European Heart Journal | 2011 | 482 | 1999–2005 | 61.6 | 36.45 | 68.4 | 79.7 | 22.3 | CRT-D (385) | 215 | n/a | 51 median | GFR< 60 | HR | 1.61 | 1.16–2.28 |
| Adelstein EC [22] | Pace | 2010 | 787 | 1999–2007 | 56 | 34 | 67 | 73 | 22 | CRT-D (787) | 230 | IV: 6 | 60 | GFR increment 10-units | Corrected HR for survival improvement | 1.21 | 1.13–1.30 |
| Van Bommel RJ [21] | European Heart Journal | 2010 | 716 | n/a | 59 | 25 | 67 | 79.1 | 25 | CRT-D (660)/ CRT-P (56) | 141 | II: 20. III: 72, IV: 8 | 65 | GFR decrement 10-units | HR | 1.18 | 1.09–1.27 |
| Bai R [28] | J Cardiovasc Electrophysiol | 2008 | 542 | 1999–2005 | 66.6 | 27.1 | 66.4 | 77.1 | 19.9 | CRT-D (395)/ CRT-P (147) | 130 | III: 80.6, IV: 19.4 | n/a | Cre> 1.4 | OR | 4.885 | 1.607–14.850 |
| Shalaby A [27] | Pace | 2008 | 330 | 2003–2005 | 63.6 | 19.7 | 67.3 | 81.8 | 22.4 | CRT-D (330) | 66 | III (mean) | n/a | Cre 1.4–3/Cre increment 0.1-unit | HR | 1.89/ 1.11 | 1.06–3.39/ 1.04–1.17 |
- List of abbreviations: N: number of patients, CMP: Cardiomyopathy, CRT: Cardiac Resynchronization Therapy, CRT-D: Cardiac Resynchronization Therapy-Defibrillator, CRT-P: Cardiac Resynchronization Therapy-Pacemaker, NYHA: New York Heart Association, GFR: Glomerular Filtration Rate, Cre: Serum Creatinine levels, HR: Hazard Ratio, OR: Odds Ratio
3.2 Quantitative synthesis
Our search revealed two studies investigating the impact of 10-unit increments in GFR on all-cause mortality. We noted an 8% decrease in all-cause mortality per 10-unit increment in GFR [HR: 0.92, 95% CI (0.86–0.98), p=0.01, 0% I2; Fig. 2]. Likewise, quantitative synthesis of three studies demonstrated that a 1-unit increment in GFR was associated with a 3% decrease in all-cause mortality [HR: 0.97, 95% CI (0.97–0.98), p<0.01, 23% I2; Fig. 3]. Furthermore, quantitative synthesis of five studies demonstrated that patients with an estimated GFR (eGFR) <60 mL/min/1.73 m2 had a 66% increase in all-cause mortality [HR: 1.66, 95% CI (1.37–2.02), p<0.01, 0% I2] than those with an eGFR≥60 mL/min/1.73 m2 (Fig. 4). Finally, we performed an analysis in which studies reporting an outcome change of 1-unit increment in GFR were considered after imputing the effect of 10-unit increments in GFR. In this analysis, the quantitative synthesis of five studies showed that a 10-unit increment in GFR was associated with a 19% decrease in all-cause mortality [HR: 0.81, 95% CI (0.73–0.90), p<0.01, 86% I2; Fig. 5].
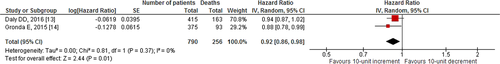
Forest plot showing the impact of 10-unit increment in GFR, on all-cause mortality in patients who underwent CRT.
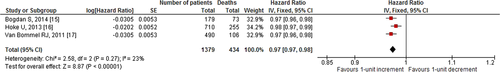
Forest plot showing the impact of 1-unit increment in GFR, on all-cause mortality in patients who underwent CRT.
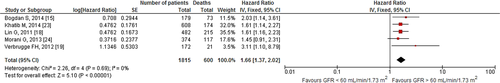
Forest plot showing the impact of GFR<60 mL/min/1.73 m2 on all-cause mortality in patients who underwent CRT.
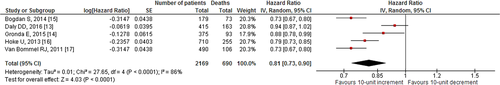
Forest plot showing the impact of 10-unit increment in GFR on all-cause mortality in patients who underwent CRT (after imputing the effect for 10-unit GFR change in the three studies which reported outcome for 1-unit GFR increase).
4 Discussion
Τo the best of our knowledge, this is the first meta-analysis demonstrating the impact of baseline renal function on all-cause mortality in HF patients who underwent CRT. Specifically, we demonstrated that i) there was a 19% decrease in all-cause mortality per 10-unit increment in GFR, ii) 1-unit increment in GFR was associated with a 3% decrease in all-cause mortality, and iii) eGFR <60 mL/min/1.73 m2 was associated with a 66% increase in all-cause mortality.
Several major randomized controlled trials evaluated the value of CRT in patients with HF, reduced ejection fraction (LVEF≤35%), and a wide QRS in the standard 12-lead ECG [9], [29], [30]. However, there are limited data on patients with CKD and advanced renal failure. Indeed, CKD is a common problem among patients with cardiovascular disease. Several mechanisms can explain the presence of CKD in patients with HF including decreased cardiac output, elevated venous pressure leading to renal congestion, chronic anemia, elevated vasoconstrictive neurohormonal factors, and medications [25], [31]. The CARE-HF study enrolled 813 patients with HF (NYHA III or IV; QRS ≥120 msec) and compared the optimal medical therapy with CRT [32]. The primary outcome of all-cause mortality or unplanned hospitalization for a major cardiovascular event was not different for patients with eGFR ≥ 60 mL/min/1.73 m2 and those with eGFR ≤ 60 mL/min/1.73 m2 [32]. Interestingly, a post-hoc analysis of the COMPANION trial revealed that CRT candidates with baseline renal dysfunction had significantly higher risk of sudden cardiac death (HR: 1.69; 95% CI: 1.06–2.69, p=0.03) [8]. The MADIT-CRT study in 1820 patients with HF (NYHA I or II, ischemic or non-ischemic cardiomyopathy, LVEF≤30%, QRS ≥130 msec) did not include a subgroup analysis of patients with chronic renal dysfunction [5]. Furthermore, the MIRACLE study conducted in 453 patients with HF (NYHA III or IV, LVEF>35%, QRS≥130 ms) showed an improvement in GFR in patients who underwent CRT (baseline GFR 30–60 mL/min/1.73 m2) in comparison with the control group [30]. Additionally, the RAFT study examined 1,798 patients (NYHA II or III, LVEF≤30%, QRS≥120 ms or paced QRS≥200 ms) with an implantable cardioverter defibrillator (ICD) or those who underwent CRT [10]. Remarkably, the authors failed to show any significant difference in the primary outcome of patients with GFR ≥ 60 mL/min/1.73 m2 and those with GFR≤60 mL/min/1.73 m2 [10]. Finally, the REVERSE trial enrolled 610 patients (NYHA I or II, LVEF≥40%, QRS≥120 ms) who underwent CRT with or without defibrillator. No significant difference was evident between patients with GFR≥82.7 mL/min/1.73 m2 and those with GFR≤82.7 mL/min/1.73 m2 [33].
The pathophysiology of the adverse effect of CKD in patients who have undergone CRT remains unclear. Several underlying mechanisms have been proposed including volume expansion secondary to sodium retention, increased oxidative stress, hypertension, atherosclerosis, insulin resistance with impaired glucose tolerance, medial vascular calcification secondary to elevated phosphate levels, and left ventricular hypertrophy secondary to anemia [34]. In fact, renal dysfunction is not uncommon in this setting and has been shown to affect prognosis in patients with HF. A retrospective analysis of renal function in 6630 patients with LVEF≤35% from the SOLVD registry, showed that one-third patients had an eGFR<60 mL/min/1.73 m2, and every 10 mL/min/1.73 m2 reduction in eGFR was associated with a 6.4% increase in mortality [HR: 1.064, 95% CI (1.033–1.096), p<0.001] [3]. Similarly, Hillege et al. reported that an impaired renal function was independently associated with increased risk of death, cardiovascular death, and hospitalization for patients with congestive HF who had either preserved or reduced LVEF [4]. This study which included 2680 patients with HF, showed that each 10 mL/kg/1.73 m2 decrease in GFR, from the baseline level of 75 mL/min/1.73 m2, was associated with a significant increase in mortality [HR 1.09, 95% CI (1.06–1.14)] [4]. Finally, a meta-analysis of 80,098 patients with HF showed that any renal impairment (HR 1.56, p<0.001) or moderate to severe renal impairment (HR 2.31, p<0.001) was associated with a higher risk of all-cause mortality [35].
There are very few studies investigating the role of CRT in the improvement of renal function in patients with HF and chronic renal dysfunction [14], [36]. An observational study revealed that patients with baseline CKD showed an improvement in eGFR (mean change: 4.24±14.2 mL/min/1.73 m2), and patients without baseline CKD showed a decrease in GFR (mean change eGFR: 3.01±20.9 mL/min/1.73 m2) [14]. Recently, a study including 260 patients demonstrated that the severity of baseline CKD was significantly associated with an increased risk of death, transplantation, or left ventricular assist device [37]. Additionally, renal response (increase in eGFR) was common after CRT and was associated with a significant decrease in the risk of the aforementioned primary outcomes [37]. The causes of renal dysfunction in HF are complex, reflecting pre-existing renal damage, pharmacological treatment, and net renal perfusion pressure [38]. It should be acknowledged that CRT cannot affect the pre-existing renal damage. However, increase in arterial pressure and fall in venous pressure might improve renal perfusion and delay or reverse renal dysfunction [36], [39]. Treatment of HF with CRT has been associated with improved renal function potentially through several mechanisms [17], [36], [40]. Indeed, CRT improves LV systolic function, systemic hemodynamic status, and prerenal circulation [41]. In support of these speculations Fung et al. showed reverse remodeling of the left ventricle, defined as a reduction in LV end-systolic volume by 10%, after CRT [42]. Specifically, Fung et al. were among the first to identify a potential link between impaired renal function and poor clinical outcomes in patients who underwent CRT [42]. They retrospectively investigated echocardiographic assessment and renal function tests before and 3 months after CRT in 85 consecutive patients. Of note, change in eGFR after CRT was significantly correlated with changes in LV end-systolic volumes, end-diastolic volumes, ejection fraction, and mortality. Successful left ventricular reverse remodeling was the only independent predictor of preservation of renal function after CRT [42]. This study indicated that changes in eGFR at three months after CRT might predict long-term prognosis in these patients [42]. In addition, lack of significant left ventricular reverse remodeling after CRT may indicate a high-risk group with potential for rapid decline in renal function [42]. In addition, CRT decreases the central venous pressure that plays a crucial role in the progression of renal failure [43]. Furthermore, reduced activity of sympathetic nerve and renin-angiotensin-aldosterone system and lower levels of N-terminal pro-brain natriuretic peptide (NT-proBNP) have been reported after CRT [36], [44], [45]. This favorable neurohormonal modulation may contribute to the CRT associated improvement in renal function.
The negative impact of CKD on mortality, especially arrhythmic deaths has been demonstrated in patients with ICD [46]. Additionally, a retrospective analysis demonstrated that CKD was associated with adverse prognosis after ICD implantation, but not so after CRT defibrillator (CRT-D) implantation [25]. There is no evidence regarding the efficacy of CRT alone (without defibrillator therapy) in patients with end stage renal disease. Similarly, in the ICD group, cost-effectiveness and safety represent significant issues in the CRT group, including an increased risk of infection and procedural complications in patients with CKD. In a study that evaluated patients with HF, CRT implantation was associated with a median incremental cost of $107,800 per quality-adjusted life-year gained [47]. However, as expected, this benefit varied according to the presence of comorbidities, which indicates that patients with a short life expectancy, such as those with CKD, should be considered carefully. It should be emphasized that adequate recommendations regarding the use of CRT in patients with renal dysfunction cannot be made based on the current literature alone.
5 Limitations
A few potential limitations of this research should be acknowledged. This study focused primarily on observational studies, which were mainly retrospective analyses. Additionally, the quantification of eGFR in the included studies was performed using either the Cockcroft–Gault equation or Modification of Diet in Renal Disease equation. Another limitation of our study was the variation in interpretation of the studied outcomes between the different studies. As a result, out of the 16 studies included in the systematic review, only 9 were included in quantitative synthesis which were further categorized into three types [impact of GFR < 60 mL/min/1.73 m2 on all-cause mortality, impact of 1-unit increment in GFR on all-cause mortality, and impact of 10-unit increment in GFR on all-cause mortality]. Moreover, in the final analysis, studies reporting the outcome change of 1-unit in GFR increase were considered after imputing the effect for 10-unit, and hence, extrapolation bias cannot be excluded. However, when our analysis was limited to two studies that directly reported the effect of 10-unit increment, the overall estimate still remained significant.
6 Conclusions
In conclusion, this meta-analysis revealed an adverse effect of baseline renal dysfunction on all-cause mortality in patients who underwent CRT. Additional studies are needed in this field in order to identify the CRT patients with CKD that will have a favorable outcome regarding morbidity and mortality.
Disclosures
None.
Conflict of interest
All authors declare no conflict of interest related to this study.
Acknowledgements
None.




