Heart rate variability monitored by the implanted device predicts response to CRT and long-term clinical outcome in patients with advanced heart failure
Abstract
Background:
Few data exist on the long-term changes and the prognostic value of heart rate variability (HRV) assessed by implanted devices in heart failure (HF) patients treated with resynchronization therapy (CRT).
Aims:
To analyze the long-term changes in the standard deviation of 5-minute median atrial–atrial sensed intervals (SDANN), and assess its role in predicting CRT efficacy and major cardiovascular events.
Methods and results:
We included 509 consecutive patients implanted with CRT devices. At 12-month follow-up, 44 patients had died and 86 patients had at least one HF hospitalisation. A significant increase in SDANN occurred after 4 weeks of CRT (from 69±22 ms to 82±27 ms, p<0.001). A further increase in SDANN was observed 6 months after implantation. Multivariable analysis identified SDANN as the sole predictor of major cardiovascular events (p=0.03) among several baseline parameters. SDANN≤65 ms at the first week and SDANN≤76 ms after 4 weeks of CRT yielded the best prediction of all-cause mortality and urgent heart transplantation on Kaplan–Meier analysis (log-rank test p=0.015 and p=0.011, respectively for week 1 and 4 values). Moreover, relative reduction in LVESV after CRT significantly correlated with SDANN at week 1 (r=−0.596, p=0.012), and week 4 (r=−0.703, p=0.001).
Conclusions:
Device-monitored HRV is a useful tool to identify, early after implantation, patients with a low likelihood of long-term benefits from CRT and at high risk for cardiovascular events.
1. Introduction
In heart failure (HF) patients, prognosis and disease progression may be related to the autonomic imbalance of chronic sympathetic activation and vagal withdrawal as a consequence of low cardiac output 1. Depressed heart rate variability (HRV), a sign of autonomic dysfunction, has been found to be an independent predictor of mortality in HF patients 2 3 4. It has been reported that cardiac resynchronization therapy (CRT) improves HRV reflecting a shift of cardiac autonomic balance toward a more favourable profile, less dependent on sympathetic activation 5 6 7. These changes in autonomic cardiac control may contribute to the long-term benefits of CRT, including a reduction in mortality. However, the pathophysiological mechanisms by which CRT may improve HRV are not yet fully understood. Today, few data exist on the long-term changes and the prognostic value of HRV assessed by implanted devices in HF patients treated with CRT 5,7, and the time-course of HRV and left ventricular functional changes have not been defined.
This study was undertaken with a twofold aim: a) to analyze the short and long-term changes of a time domain measure of HRV, the standard deviation of 5-minute median atrial-atrial sensed intervals (SDANN), continuously measured by implanted CRT devices; and b) to assess its role in predicting CRT efficacy and major cardiovascular events and to relate HRV with changes in left ventricular function.
2. Methods
2.1. Patient selection, device implantation and follow-up
From 1999 to 2005, patients successfully implanted with biventricular pacing devices for CRT (Medtronic Inc., Minneapolis, MN, USA), were prospectively included in the InSync/InSync ICD Italian Registries. The Registries enrolled patients with advanced symptomatic chronic HF, an ejection fraction (LVEF)≤35% and a wide QRS complex (≥130 ms). All patients provided written informed consent approved by each Hospital's Ethics Committee.
Patients enrolled in the Registries and implanted with CRT devices capable of continuous recording of HRV (CRT Model 8042, CRT-D Models 7277 and 7279) were included in the present analysis. Patients with permanent atrial fibrillation, sinus node dysfunction or requiring continuous atrial pacing were excluded. The devices and the pacing leads were implanted using standard techniques, with the target site for the left ventricular (LV) lead being the lateral or postero-lateral coronary sinus vein. When a conventional indication for an implantable cardioverter defibrillator (ICD) existed, a combined device was implanted. Before device implantation, patients underwent baseline evaluation, including demographics and medical history data collection, clinical examination, 12-lead electrocardiogram, estimation of NYHA functional class and echocardiographic assessment.
Echocardiographic evaluation included LV end-diastolic volume (LVEDV), end-systolic volume (LVESV) and LVEF assessed by Simpson's equation using the apical four-chamber view 10. The severity of mitral regurgitation was assessed from colour-flow Doppler images in the apical 4-chamber view. Mitral regurgitation was classified as mild or grade 1 (jet area/left atrium area ≤20%), moderate or grade 2 (jet area/left atrium area 20% to 40%) and severe or grade 3 (jet area/left atrium area >40%) 11. Echo-directed adjustment of the atrio-ventricular pacing interval was performed before hospital discharge and at follow-up to optimize haemodynamic function.
Pharmacological treatments were based on clinical evaluation by the attending physicians.
After implant, patients returned for a regular clinic visit at 1, 3, and 6 months, and then every 6 months thereafter. At each follow-up visit, the clinical examination, 12-lead electrocardiogram and NYHA classification assessment were repeated. A complete echocardiographic evaluation was required every 6 months. Stored data, including arrhythmic episodes, were retrieved and saved to disks for further off-line analysis. Spontaneous arrhythmic episodes detected by the device were validated by 2 independent expert electrophysiologists. Ventricular fibrillation was diagnosed if the recorded R-R interval was ≤240 ms and accompanied by changes in the morphology of stored electrograms.
The effects of CRT on echocardiographic parameters were evaluated, comparing the baseline values with those at 6-month of follow-up for surviving patients, otherwise the last observation was carried forward. Major cardiac events were considered as: death for any cause, urgent heart transplantation, and hospitalisation for HF worsening.
2.2. HRV evaluation
Continuous HRV was measured by the CRT device as SDANN and the values were stored for each complete 24 hour period. The technical feasibility of the SDANN measurement achieved by automatic algorithms of implanted devices has been previously demonstrated 12. Paced atrial beats, supraventricular and ventricular premature beats, and arrhythmic episodes were automatically excluded from the analysis by the device.
Stored SDANN data were retrieved and saved to disks. Then, weekly averages were calculated excluding the days during which the amount of valid sensed atrial beats fell below 80% at the off-line evaluation. The average of the first week after CRT implantation was used as the baseline value of SDANN 5.
2.3. Statistical analysis
Continuous data were expressed as means±standard deviation. Categorical data were expressed by percentages. Differences between mean data were compared by a t-test for Gaussian variables, and by Mann-Whitney or Wilcoxon non-parametric test for non-Gaussian variables, respectively for independent or paired samples. Differences in proportions were compared by a Chi-square analysis.
Univariable binary logistic regression analysis was utilized to evaluate the relationship between the occurrence of cardiovascular events and baseline clinical and echocardiographic characteristics. All variables associated with a statistical significance such as p-value<0.2 were considered for multivariable binary logistic regression analysis.
Receiver-operating characteristic (ROC) curve analysis was applied to assess the best cut-off value of the variables to predict major cardiovascular events. Mortality or urgent heart transplantation rate was summarized by construction of Kaplan-Meier curves and the distributions of the groups were compared by a log-rank test. SDANN values were compared with changes in echocardiographic variables by using linear regression analysis. A p-value<0.05 was considered significant for all tests. All statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL, USA).
3. Results
The study analyzed 509 patients that met the aforementioned inclusion criteria. The majority of patients (330, 65%) received a CRT-D device for primary (67%) or secondary (33%) prevention of sudden death, while the remaining 179 (35%) patients received a CRT pacemaker. All patients underwent de novo implantation. Table 1 summarizes the demographics, clinical and echocardiographic parameters and pharmacological therapy for the whole population. Medications were stable throughout the follow-up without significant changes.
| Parameter | Total n=509 |
|---|---|
| Male, n(%) | 418(82) |
| Age, years | 66±10 |
| Ischaemic aetiology, n(%) | 274(54) |
| NYHA class | 2.9±0.6 |
| QRS duration, ms | 162±28 |
| LV ejection fraction, % | 26±8 |
| LVEDV, ml | 229±89 |
| LVESV, ml | 165±76 |
| Mitral regurgitation, grade | 2.0±0.9 |
| Diabetes, n(%) | 76(15) |
| CRT-D use, n(%) | 330(65) |
| Class III antiarrhythmic use, n(%) | 160(31) |
| Diuretic use, n(%) | 489(96) |
| ACE-inhibitor or angiotensin-receptor blocker use, n(%) | 412(81) |
| β-blocker use, n(%) | 308(61) |
- a All data are presented as mean±standard deviation, unless otherwise noted. NYHA = New York Heart Association; LVEDV = Left ventricular end-diastolic volume; LVESV = Left ventricular end-systolic volume; LV = Left ventricular; ACE = Angiotensin-converting-enzyme.
At a median follow-up of 12 months (25th-75th percentile, 7 to 18 months), 44 of the 509 patients (rate 13.5 per 100 patient-years of follow-up) died or underwent urgent heart transplantation. Eighty six (17%) patients had at least one hospitalisation for worsening HF; a major cardiovascular event occurred in 97/509 (19%) patients. Among the 330 patients implanted with a CRT-D system, 43 (13%) patients had a spontaneous episode of ventricular fibrillation appropriately detected and terminated by the device.
Compared to week 1, a significant increase of SDANN occurred after 4 weeks of CRT (from 69±22 ms to 82±27 ms, p<0.001). The SDANN value further increased 6 months after device implantation (87±27 ms, p=0.001 vs. the 4th week) and the improvement persisted at 12 months (87±28 ms). Diabetic and non-diabetic patients had similar SDANN values at week 1 (70±22 vs. 70±22 ms) and 4 weeks after starting CRT (79±23 vs. 83±27 ms).
3.1. Prediction of cardiovascular events, survival analysis and left ventricular remodelling
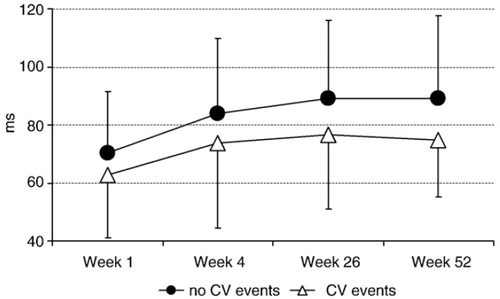
The values of SDANN were significantly lower at week 1 and after 4 weeks in patients with major cardiovascular events and stabilized later at a plateau value (Fig. 1). The patients with and without cardiovascular events during follow-up did not differ significantly in terms of baseline clinical and echocardiographic parameters, aetiology of the cardiomyopathy and pharmacological treatment, except for NYHA class and the SDANN values (Table 2).
| No CV events (n=412) | CV events (n=97) | p-value | |
|---|---|---|---|
| Male, n(%) | 334(81) | 84(87) | 0.201 |
| Age, years | 66±10 | 68±9 | 0.172 |
| Ischaemic aetiology, n(%) | 215(52) | 59(61) | 0.125 |
| NYHA class | 2.8±0.6 | 3.1±0.5 | 0.001 |
| QRS duration, ms | 162±28 | 163±28 | 0.573 |
| LV ejection fraction, % | 26±7 | 26±9 | 0.824 |
| LVEDV, ml | 229±83 | 231±115 | 0.919 |
| LVESV, ml | 164±70 | 173±100 | 0.658 |
| Mitral regurgitation, grade | 2.0±0.9 | 2.2±1.1 | 0.143 |
| Class III antiarrhythmic use, n(%) | 122(30) | 38(39) | 0.068 |
| Diuretic use, n(%) | 395(96) | 94(97) | 0.779 |
| ACE-inhibitor or angiotensin-receptor blocker use, n(%) | 333(81) | 79(81) | 0.889 |
| β-blocker use, n(%) | 255(62) | 53(55) | 0.189 |
| SDANN Week 1, ms | 72±22 | 64±22 | 0.019 |
| SDANN Week 4, ms | 84±26 | 74±29 | 0.005 |
- a CV = Cardiovascular events; NYHA = New York Heart Association; LVEDV = Left ventricular end-diastolic volume; LVESV = Left ventricular end-systolic volume; LV = Left ventricular; ACE = Angiotensin-converting-enzyme; SDANN = standard deviation of 5-minute median atrial-atrial sensed intervals.
Baseline clinical/echocardiographic parameters and the SDANN value at week 1 were evaluated for their predictive value for cardiovascular events by univariable analysis; both SDANN value and NYHA class were significantly related to the occurrence of cardiovascular events. Multivariable analysis of predictors of major cardiovascular events was performed entering into the model the SDANN value at week 1, age, ischaemic aetiology and NYHA class (all with p-value<0.2). This analysis identified low SDANN value as the sole statistically significant predictor (p=0.03; Table 3).
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Male gender | 1.50 | 0.80-2.84 | 0.209 | - | - | - |
| Age | 1.02 | 0.99-1.04 | 0.172 | 0.99 | 0.96-1.02 | 0.542 |
| Ischaemic aetiology | 1.42 | 0.90-2.24 | 0.127 | 1.69 | 0.93-3.09 | 0.088 |
| NYHA class | 1.85 | 1.01-3.41 | 0.048 | 1.76 | 0.84-3.68 | 0.137 |
| QRS duration | 1.00 | 0.99-1.01 | 0.572 | - | - | - |
| LV ejection fraction | 0.99 | 0.97-1.03 | 0.824 | - | - | - |
| LVEDV | 1.00 | 0.99-1.01 | 0.527 | - | - | - |
| LVESV | 1.01 | 0.99-1.02 | 0.291 | - | - | - |
| Mitral regurgitation | 1.27 | 0.92-1.74 | 0.244 | - | - | - |
| SDANN Week 1 | 0.84 | 0.72-0.97 | 0.020 | 0.85 | 0.73-0.98 | 0.030 |
- a For SDANN variable, 10 ms increments were considered. OR = Odds ratio; CI = Confidence interval; LVEDV = Left ventricular end-diastolic volume; LVESV = Left ventricular end-systolic volume; LV = Left ventricular; SDANN = standard deviation of 5-minute median atrial-atrial sensed intervals.
Among the patients with CRT-D systems, the SDANN values did not differ between those with (43 patients) and those without (287 patients) episodes of ventricular fibrillation detected by the device (72±22 vs. 72±22 ms at the first week and 85±28 vs. 83±27 ms after 4 weeks of CRT).
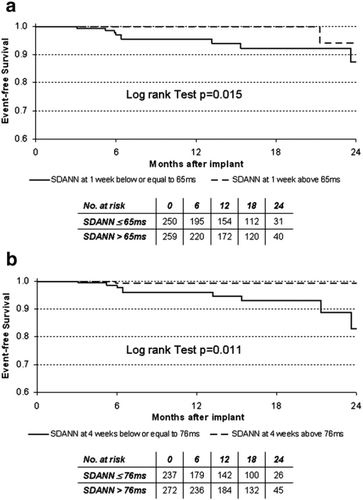
Based on the ROC curve analysis of SDANN values, the best cut-off to predict the occurrence of cardiovascular events was 65 ms at the first week (area under the curve, 0.61; confidence interval, 0.53-0.70), and 76 ms at week 4 (area under the curve, 0.64; confidence interval, 0.56-0.72). The survival curves for death or urgent heart transplantation were calculated by stratifying the population according to these cut-off values (Fig. 2). Total mortality and urgent heart transplantation were significantly greater among patients with SDANN values lower or equal to 65 ms at week 1 (log-rank test, p=0.015), and in patients that failed to increase SDANN value above 76 ms after 4 weeks of CRT (log-rank test, p=0.011).
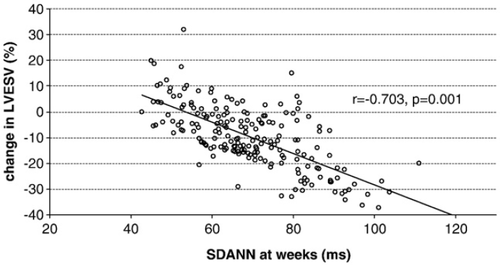
Moreover, there was a significant correlation between the relative reduction in LVESV after CRT and the SDANN values at week 1 (r=−0.596, p=0.012), and at week 4 (r=−0.703, p=0.001; Fig. 3).
4. Discussion
This is the largest study to date evaluating the relationship between CRT, HRV and clinical outcome. The main findings of the study are that: 1) the week 1 SDANN value is an independent predictor of long-term cardiovascular events in HF patients implanted with a CRT device; 2) there is a strong correlation between SDANN and favourable LV reverse remodelling induced by CRT. Thus, SDANN may be proposed as a simple and reliable parameter to identify, early in the post-implantation phase, patients at low probability of long-term benefits from CRT that may require more careful monitoring and probably additional therapeutic intervention.
Although CRT with and without defibrillation therapy significantly reduces hospitalisation and mortality in HF patients 13,14, about one third of patients do not respond 15. Much research is currently focused on the discovery of markers that could identify patients unlikely to respond to CRT.
In chronic HF, the independent prognostic value of HRV, as an index of autonomic modulation of the heart, has been demonstrated by several studies 2,16,17. Recently, device-based continuous monitoring of HRV has been proposed for diagnostic and prognostic stratification after CRT implantation. Depressed HRV 4 to 8 weeks after CRT has been shown to be associated with increased risk of cardiovascular events 5,8,18. We found SDANN value to be a predictor of further unfavourable events even at the first post-implantation week which is generally considered as the baseline value for HRV monitored by the implanted device 5. NYHA class was also found to be unequally distributed among patients with and without major cardiovascular events during the follow-up. However, neither NYHA class nor the currently used clinical predictors of long-term outcome after CRT 19 had a predictive power at multivariable analysis. The lack of independent predictive value of baseline clinical and echocardiographic parameters speaks strongly against the hypothesis that depressed SDANN values might simply identify those patients with most advanced disease. On the contrary, our findings are consistent with the hypothesis that a favourable effect of CRT may lead to early positive changes in the autonomic balance resulting in higher post-implantation SDANN values. Also, it cannot be excluded that the week 1 SDANN values may provide information on individual autonomic characteristics, which are at least in part genetically controlled 20. Thus, a more favourable autonomic trait may play a role in the likelihood of developing cardiovascular events in patients with similarly advanced HF and may also be of prognostic value for events distant in time. In favour of this latest hypothesis is the observation that, in post-myocardial infarction patients with preserved LV function, lower SDANN values and depressed baroreflex sensitivity identified those at high risk for cardiovascular events during a 5 year follow-up 21. The role of baseline SDANN as a predictor of long-term outcome is strengthened by the demonstration that a reduced HRV index before discharge after myocardial infarction was an independent risk factor for the occurrence of LV dilatation during follow-up 22. Accordingly, we have found that SDANN values after 1 week were significantly related to LV reverse remodelling as expressed by the relative reduction in LVESV 6 months after CRT, considered by Yu et al. 23 as the best volumetric parameter to predict outcome after CRT.
Moreover, SDANN after 4 weeks of CRT was markedly lower in patients with cardiovascular events compared to those without events during the follow-up and it was related to the reduction in LVESV after CRT. Of note, total mortality and urgent heart transplantation were greater among patients with SDANN values lower or equal to 65 ms at week 1 and in those who failed to increase SDANN value above 76 ms after 4 weeks of CRT.
Taken together, these findings provide insights into the two-way relationship between the autonomic nervous system modulation induced by CRT and LV reverse remodelling, which are both related to clinical outcome in HF patients treated with CRT. Chronic HF alters the autonomic nervous control of the heart by sustained sympathetic activation and parasympathetic withdrawal arising from decreased cardiac output, abnormal regional activation and distorted contraction of the heart 1,24,25. The neurohormonal activation not only increases the haemodynamic stress on the ventricle but may also exert direct toxic effects on cardiac cells and stimulate myocardial fibrosis, which can further impair the performance of the failing heart and thereby result in HF progression 26. By restoring synchrony, improving LVEF and reducing LV volumes, effective CRT may positively change the autonomic balance towards a decrease in sympathetic and an increase in vagal efferent activity, together with a reduction of renin-angiotensin system activation. In favour of this hypothesis is the observation of a reduction in sympathetic neural activity by direct recordings in patients with low LVEF during biventricular pacing compared with right ventricular pacing 27. On the other hand, the shift in cardiac autonomic balance towards a more favourable profile, less dependent on sympathetic activation, may contribute to both LV remodelling and reduction in mortality.
Although it has been suggested that HRV may identify patients at high risk for life-threatening-arrhythmias after myocardial infarction 28, we did not find any difference in SDANN values between patients with and without ventricular arrhythmias treated by the device. Our observation is consistent with both experimental 29,30 and clinical 31 32 33 studies showing that measures of autonomic tonic modulation are less powerful than measures of autonomic reflex modulation, such as baroreflex sensitivity, in predicting the likelihood and the tolerability of malignant arrhythmias.
4.1. Limitations
The method cannot be used in CRT patients with atrial fibrillation or requiring continuous atrial pacing.
Since the device-based HRV can only be measured after device implantation 5,6, it can not be excluded that the post-operative discomfort and the short stay in hospital may have influenced the trend data in the first days. However, previous studies with cross-over design 6,34 and data comparing de novo implantation with device replacement 5 have shown that HRV changes after CRT are a true treatment effect rather than a consequence of the implant procedure. The predictive value of SDANN at the first post-implantation week, suggests that a pre-implantation analysis of HRV might help in the selection of responders to CRT. However, the role of Holter-based HRV assessment to predict response to CRT remains to be addressed. On the other hand, continuous HRV measures from implanted devices provide the unique opportunity to monitor day-to-day autonomic status to detect changes heralding unfavourable events.
Pharmacological therapy at enrolment was not optimal, especially in terms of β-blockers and angiotensin-converting-enzyme inhibitors, as treatment was based on clinical evaluation by the attending physicians. Nevertheless, there were no differences in pharmacological treatment between patients with and without cardiovascular events that could affect the results.
4.2. Clinical implications
Our results strengthen the concept that the high cost devices, such as the defibrillators implanted for primary prevention, should also become monitoring systems in addition to treating potentially life-threatening arrhythmias. Thus, the diagnostic information available from implanted therapeutic devices could improve the cost-effectiveness of CRT and CRT-defibrillators by forecasting unfavourable clinical outcome.
Disclosures
Data presented in this paper are from a Medtronic sponsored registry. Tiziana De Santo and Sergio Valsecchi are employees of Medtronic, Inc. No other conflict of interest exists.
Acknowledgements
The authors would like to thank Jane Moore (Medtronic, Inc.), for her assistance in the editing of this manuscript.
Appendix A
Centers and investigators participating to the InSync/InSync ICD Italian Registry are listed below.
M.Gasparini, P.Galimberti, F.Regoli, E.Gronda, Istituto Clinico Humanitas IRCCS, Rozzano-Milano; M.Lunati, G.Cattafi, G.Magenta, M.Paolucci, R.Vecchi, Niguarda Cà Granda Hospital, Milano; M.Santini, R.Ricci, San Filippo Neri Hospital, Roma; F.Gaita, M.Bocchiardo, P.DiDonna, D.Caponi, Civile Hospital, Asti; L.Tavazzi, M.Landolina, R.Rordorf, B.Petracci, A.Vicentini, S.Savastano Policlinico S.Matteo IRCCS, Pavia; L.Padeletti, P.Pieragnoli, Careggi Hospital, Firenze; A.Vincenti, S.DeCeglia, A.Cirò, S.Gerardo Dei Tintori Hospital, Monza(MI); A.Curnis, G.Mascioli, Spedali Civili Hospital, Brescia; A.Puglisi, S.Bianchi,C.Peraldo, Fatebenefratelli Hospital, Roma; M.Sassara, A.Achilli, F.Turreni, P.Rossi, Belcolle Hospital, Viterbo; GB.Perego, S.Luca Auxologico Hospital, Milano; P.A.Ravazzi, P.Diotallevi, SS.Antonio e Biagio e Cesare Arrigo Hospital, Alessandria; M.Tritto, Mater Domini Hospital, Castellanza (VA); A.Carboni, D.Ardissino, G.Gonzi, V.Serra, Civile Hospital, Parma; G.Vergara, S.Maria Del Carmine Hospital, Rovereto (TN); G.Boriani, M.Biffi, C.Martignani, L.Frabetti, S.Orsola M.Malpighi Hospital, Bologna; G.Luzzi, Policlinico Consorziale Hospital, Bari; F.Laurenzi, S.Camillo Hospital, Roma; G.Pistis, Mauriziano Hospital, Torino; A.Cesario, G.B.Grassi Hospital, Ostia (RM); G.Zanotto, Civile Hospital, Verona; S.Orazi, S.Camillo Hospital, Rieti; R.Ometto, C.Bonanno, S.Bortolo Hospital, Vicenza; G.Molon, E.Barbieri, S.Cuore Don Calabria Hospital, Negrar (VR); A.Raviele, G.Gasparini, Umberto I Hospital, Mestre (VE); G.Botto, M.Luzi, A.Sagone, S.Anna Hospital, Como; A.Vado, S.Croce e Carle Hospital, Cuneo; A.Montenero, Multimedica Hospital, Sesto S.Giovanni (MI); G.Inama, Maggiore Hospital, Crema; B.Sassone, Civile Hospital, Bentivoglio (BO); M.Briedda, F.Zardo, S.Maria degli Angeli Hospital, Pordenone; E.Bertaglia, ULSS13 Hospital, Mirano (VE); A.Proclemer, S.Maria della Misericordia Hospital, Udine; F.Zanon, Civile Hospital Rovigo; M.Disertori, L.Gramegna, M.DelGreco, D.Dallafior, S.Chiara Hospital, Trento; C.Tomasi, A.Maresta, M.Piancastelli, S.Maria delle Croci Hospital, Ravenna; A.Bridda, S.Martino Hospital, Belluno; R.Mantovan, Cà Foncello Hospital, Treviso; A.Fusco, A.Vicentini, Polispecialistica Pederzoli Hospital, Peschiera del Garda (VR); P.Baraldi, S.Agostino Hospital, Modena; G.Lonardi, Civile Hospital, Legnago (VR); W.Rahue, S.Maurizio Hospital, Bolzano; P.Delise, Civile Hospital Conegliano (TV); C.Menozzi, S.Maria Nuova, Reggio Emilia; P.Babudri, Borgo Roma Hospital, Verona; R.Marconi, C.eG. Mazzoni Hospital, Ascoli Piceno; G.De Fabrizio F.Alfano, G.Moscati, Avellino; G.Barbato, Maggiore Hospital, Bologna; P.Gelmini, Civile Hospital Desenzano del Garda (BS); DiSabato, S.Leopoldo Mandic Hospital, Merate (LC); S.Ricci, Ramazzini Hospital, Carpi (MO); M.D.Aulerio, S.Biagio Hospital, Domodossola (VB); G.L.Morgagni, R.Latini, Civile Hospital Macerata, G.Bardelli, Fornaroli Hospital, Magenta (MI); R.Paulichl, F.Tappeiner Hospital, Merano (BZ); M.Bernasconi, M.Marzegalli, S.Carlo Borromeo, Milano; G.Neri, Montebelluna Hospital, Treviso; E.Occhetta, Hospital Maggiore della Carità, Novara; P.Bocconcelli, S.Salvatore Hospital, Pesaro; A.Capucci, Civile Hospital, Piacenza; A.Campana, S.Giovanni di Dio e Ruggi d'Aragona Hospital, Salerno; N.Dibelardino, Civile Hospital, Velletri (RM); A.Vaglio, Giovanni e Paolo Hospital, Venezia.




