Totally epicardial cardiac resynchronization therapy system implantation in patients with heart failure undergoing CABG
Abstract
Background:
Systolic dyssynchrony is present in a considerable number of patients with heart failure (HF) undergoing coronary artery bypass grafting (CABG). Surgical revascularization offers an optimal setting for totally epicardial cardiac resynchronization therapy (CRT) system implantation.
Aim:
To assess the efficacy of totally epicardial CRT implantation during CABG, in patients with HF.
Methods:
Twenty three patients with HF and dyssynchrony underwent totally epicardial CRT system implantation during CABG. This randomised, single-blind, cross-over study compared clinical and echocardiographic parameters during two periods: 3 months of active CRT (CRT+) and 3 months of inactive CRT (CRT−) pacing.
Results:
Twenty two patients underwent randomisation and completed both study periods. In the CRT+ group more patients improved by two NYHA classes (p=0.028), had a longer 6-minute walk test distance (p=0.047) and better quality of life (p=0.003) compared with the CRT− group. Echocardiography revealed an improved LV ejection fraction (p<0.001), smaller LV end-systolic volume (p=0.04), reduced mitral regurgitation (p=0.026) and improved LV synchrony in the CRT+ group compared with the CRT− group.
Conclusion:
CRT delivered by a totally epicardial system implanted during CABG is associated with additional improvement of clinical and echocardiographic parameters in patients with HF and systolic dyssynchrony.
1. Introduction
The efficacy of cardiac resynchronization therapy (CRT) in patients with advanced heart failure (HF) and systolic dyssynchrony is well-established [1,2]. However, the benefit from CRT is limited by several technical issues associated with the coronary sinus lead implantation. A recent analysis of data from 469 patients enrolled in the CARE-HF trial shows that the implantation failure rate at first attempt, ranges from 10% to 18% depending on the centres experience [3]. Successful left ventricular lead placement in the lateral position is accomplished in 88% of patients. Furthermore, the risk of potential adverse events must be taken into account in the setting of the transvenous implantation procedure: lead dislodgements requiring re-intervention occur in about 5% of cases, coronary sinus dissections or perforations in 2%, and phrenic nerve stimulation necessitating LV lead repositioning in about 1.7% of patients. Even though the risk of particular adverse events is relatively low, the overall major adverse event rate is considerable, as events occur in about 15% of implanted patients [3].
Coronary artery disease (CAD) represents the most frequent aetiology of HF [4]. A large number of patients with CAD present with lesions in coronary arteries which are suitable for coronary artery bypass grafting (CABG). Those patients with co-existing systolic ventricular dyssynchrony could benefit from totally epicardial CRT system implantation concomitant with surgical coronary revascularization.
On-pump CABG offers an optimal setting for totally epicardial CRT system implantation. With excellent exposition of the heart, there are almost no anatomical limitations for implanting the left ventricular lead at optimal site. The implantation of the epicardial leads is a relatively straightforward procedure. The potential risks associated with intravenous/intracardiac location of leads are avoided. In addition, two therapeutic procedures are performed during one intervention, thereby minimising the impact on the patient.
Based on the above mentioned assumptions, we designed a single-blind randomised, controlled cross-over study to determine whether CRT delivered using a totally epicardial implantation procedure concomitant with the CABG procedure, is associated with additional benefit in patients with HF and systolic dyssynchrony.
2. Methods
The investigation conforms with the principles outlined in the Declaration of Helsinki and the design of the study was approved by the local Ethics Committee. All patients gave their written informed consent prior to enrollment. The inclusion criteria were: CAD scheduled for elective CABG, HF of at least class III according to New York Heart Association (NYHA), left ventricular ejection fraction ≤35%, sinus rhythm, presence of inter- or intra-ventricular dyssynchrony during the pre-operative echocardiographic study, optimal medical treatment of HF for at least the previous month including beta-blockers, ACE-inhibitors and diuretics. The criteria for exclusion were: lack of patient compliance, chronic atrial fibrillation, any heart pathology requiring surgical intervention other than CABG, indications for pacing or implantation of a cardioverter-defibrillator (ICD), inability to walk, reduced life expectancy (less than 1 year).
Evaluation of suitability for elective CABG was performed by two cardiac surgeons not involved in the study and was based on the ACC/AHA guidelines for CABG surgery [5]. Patients with mild-to-moderate angina symptoms (Canadian Cardiovascular Society, CCS class I or II) underwent dobutamine stress echocardiography (DSE) to identify viable myocardium. The DSE methodology used has been described previously [6]. The operative risk was assessed by means of the EuroSCORE (European System for Cardiac Operative Risk Evaluation) scale [7]. In patients with high operative risk (EuroSCORE≥6), an intra-aortic balloon pump (IABP) was inserted prior to surgery.
2.1. Study design
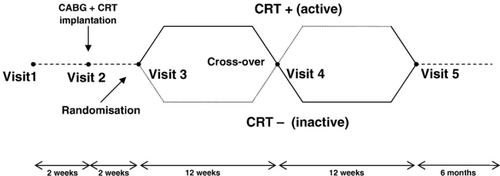
Enrollment was performed between September 2005 and October 2006. All patients underwent an elective CABG with concomitant totally epicardial CRT system implantation. After implantation, the CRT system was programmed to be inactive. After two weeks of post-operative cardiac rehabilitation, patients entered the 6-month cross-over phase of the study. During the cross-over phase two pacing modes were compared: biventricular pacing (CRT system active) mode and ventricular inhibited (VVI) mode (CRT system inactive) at a basic rate of 40 bpm (Fig. 1). Each patient was randomised to receive one pacing mode for a period of 3 months then crossed-over to the other pacing mode for the second 3 months. After this single-blind, cross-over phase the CRT system was programmed according to the patients' preference (active or inactive) and the follow-up was continued for another 6 months to assess the pacing and sensing parameters.
2.2. Echocardiography
Patients were studied using the GE Vivid 7 (Horten, Norway) ultrasound system with a 2.0–2.5 MHz transducer. Complete 2-D, M-mode and Doppler evaluation was performed. Six standard imaging planes were acquired: parasternal long- and short-axis, apical 2-, 4- and 5-chamber and apical-long axis views. Left ventricular (LV) dimensions were measured by two-dimension guided M-mode in parasternal long-axis view. LV volumes were assessed in 4-chamber apical view. LV ejection fraction (LVEF) was calculated by the modified biplane Simpson's method. The severity of mitral regurgitation (MR) was estimated in an apical 2- and 4-chamber view as a grade [1 2 3 4] and as the percentage jet area relative to the left atrial size (MR area ratio) in the apical 4-chamber view.
2.2.1. Dyssynchrony assessment
Atrio-ventricular (AV) synchrony was assessed by measuring the ratio of left ventricular diastolic filling time (DFT) from the Doppler spectrum of the mitral inflow in 4-chamber apical view to the duration of cardiac cycle. AV dyssynchrony was defined as DFT less than 40% of the cardiac cycle [8].
The left pre-ejection interval (LPEI) was measured from the onset of QRS complex from the surface ECG to the beginning of the aortic flow curve in pulsed-wave Doppler in apical 5-chamber view. The right pre-ejection interval (RPEI) was measured from the QRS onset to the beginning of the pulmonary flow curve in pulsed-wave Doppler in the parasternal short-axis view. Inter-ventricular dyssynchrony was defined as the time-difference between left and right pre-ejection intervals exceeding 40 ms [9].
Intra-ventricular dyssynchrony was assessed by tissue Doppler imaging using the pulsed-wave Doppler mode. Regional differences between the intervals from onset of QRS complex to the onset (electro-mechanical delay, EMD) and peak of systolic velocity (electro-systolic delay, ESD) from the Doppler spectrum were recorded in apical 4-chamber and 2-chamber views at the level of the basal and medium segments of inter-ventricular septum, lateral, anterior and inferior wall and all the possible differences between the basal and medium segments were calculated. Maximal absolute difference (Δ) greater than 50 ms between two segments, either in EMD or ESD was considered as the marker of intra-ventricular dyssynchrony [10,11].
The pacemaker settings were optimized by echocardiography at randomisation and at the cross-over visit. Atrio-ventricular delay was set to ensure, with full ventricular capture, the longest DFT without truncation of the A wave by closure of the mitral valve [8] and inter-ventricular (V-V) delay by choosing the maximal aortic velocity-time integral at different V-V settings [12].
2.3. Surgical procedure
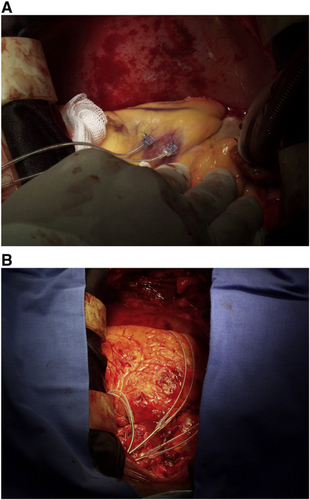
All patients underwent a standardized on-pump CABG procedure, with the use of left internal thoracic artery (LITA) sewn to the left anterior descending artery and saphenous vein grafts anastomosed to the remaining coronary vessels. The pacing parameters (pacing and sensing thresholds, pacing impedance, slew rate) were verified after institution of cardiopulmonary bypass (CPB) by placing the lead tip on the epicardium of the left ventricular wall at several sites within the segment of latest activation as identified during the pre-operative echocardiographic study, defined as the segment where the longest ESD and/or EMD values were observed. The site with best pacing parameters was marked by means of a single 7–0 suture. The implantation of the epicardial bipolar leads (CapsureEpi 4968, 35 cm, Medtronic, Minneapolis, MN) was performed after administration of blood cardioplegia. The atrial lead was fixed on the right atrium (between the appendage and the sinus node), and the ventricular leads were fixed on the right ventricle (anterior wall) and left ventricle (on the site previously marked) by means of the 5–0 sutures (Fig. 2). After release of the cross-clamp, in the reperfusion period, the pacing and sensing parameters of the leads were verified. Subsequently the leads were passed through the second left intercostal space and connected to the biventricular pacemaker (InSync III 8042, Medtronic Minneapolis, MN) located in a pocket in the left subclavicular portion.
2.4. Randomisation
Patients were randomised into two groups: CRT− and CRT+. In the CRT− group devices were programmed to be inactive with a minimum pacing rate of 40 bpm in the VVI mode. In the CRT+ group the devices were programmed to be active by selecting the atrio-biventricular mode with the AV delay optimized by the echocardiographic evaluation. After 3 months, CRT programming was crossed-over so that patients in the CRT− group were changed to CRT+ and vice versa.
2.5. Clinical evaluation
The efficacy of treatment was analyzed by measuring the following three clinical parameters at baseline and at the end of each phase of the cross-over period.
Heart failure severity was assessed from NYHA functional class. Exercise performance was measured using the 6-minute walk test distance, based on the method recommended by Lipkin et al. [13]. Quality of life was evaluated by means of the Minnesota Living With Heart Failure Questionnaire (MLwHF) score [14].
2.6. End-points
The primary end-points were NYHA functional class, the distance walked in 6 min and quality of life.
The secondary end-points were echocardiographic parameters of remodelling including LVEF, end-systolic volume (ESV) and the amount of MR.
2.7. Statistical analysis
The sample size was established, based on the assumption of 80% power and 5% significance. For detection of a 60 m difference in the 6-minute walk test distance a sample of 32 (2×16) patients is needed. For detection of a 13 point difference in the MLwHF score at least 36 (2×18) patients are required. These values were established on the basis of the Altman's nomogram. Taking into account the potential study drop-outs due to mortality, failure of pacing, missing data or lack of compliance, we decided to increase the calculated sample size by about 25%, thus the enrolled study group consisted of 23 patients, producing 46 full cross-over data sets.
An independent statistician performed all data analyses. Values are expressed as the mean±standard deviation unless otherwise indicated. All continuous variables were evaluated by Shapiro-Wilks test for normality. If data distribution was not normal a Mann-Whitney test or Cochran test was used. Normally distributed variables were compared by Student's test. Categorical variables were compared by Pearson chi-squared test or in case of small samples by Pearson chi-squared test with Yates correction or Fisher's exact test.
3. Results
3.1. Study population
A total of 23 consecutive patients meeting the inclusion criteria were enrolled in the study. All patients were in NYHA class III despite optimal pharmacological treatment which consisted of beta-blockers in 95%, ACE-inhibitors in 90% and diuretics in 80%. Intra-ventricular asynchrony was diagnosed in all 23 patients (100%) whereas inter-ventricular asynchrony was present in 17 patients (70%). Four patients with CCS≤2 angina pectoris underwent dopamine stress echocardiography, which confirmed the presence of viable myocardium. The baseline characteristics of patients are presented in Table 1.
| Characteristic | All patients at baseline (n=23) | At randomisation (n=22) | ||
|---|---|---|---|---|
| CRT+ First (n=11) | CRT− First (n=11) | P value (CRT+ vs. CRT− first) | ||
| Age (yrs) | 64.7±7 | 66±6 | 62±7 | 0.18 |
| Male (% of pts) | 87 | 91 | 91 | 1.0 |
| 6-minute walk test distance (m) | 293±58 | 278±54 | 316±52 | 0.14 |
| Quality of life (Minnesota Score) | 61±20 | 60±22 | 60±20 | 1.0 |
| QRS (ms) | 138±32 | 152±40 | 133±14 | 0.18 |
| LBBB (%) | 78 | 82 | 73 | 0.82 |
| CCS class | 2.7±0.6 | 2.8±0.7 | 2.7±0.5 | 0.86 |
| NYHA III (% of pts) | 100 | 100 | 100 | 1.0 |
| LVEF (%) | 30±2.6 | 29±3.7 | 31±3.1 | 0.25 |
| EDD (mm) | 66±6 | 68±6 | 65±6 | 0.2 |
| ESD (mm) | 52±8 | 55±8 | 50±7 | 0.14 |
| MR grade | 1.95±0.5 | 1.9±0.5 | 2±0.5 | 0.62 |
| MR area ratio (%) | 27±13 | 26±10 | 28±13 | 0.7 |
| LPEI (ms) | 153±33 | 164±36 | 148±29 | 0.32 |
| IVD (ms) | 47±27 | 55±24 | 45±28 | 0.38 |
| DFT (% of the cardiac cycle) | 43±6 | 41±7 | 44±5 | 0.45 |
| ΔEMD (ms) | 78±32 | 88±33 | 71±28 | 0.27 |
| ΔESD (ms) | 84±37 | 90±29 | 83±43 | 0.7 |
| EuroSCORE (points) | 3.4±2.2 | 4.25±2.0 | 2.5±1.9 | 0.08 |
- a LBBB — left bundle branch block, CCS — Canadian Cardiac Society, NYHA — New York Heart Association, LVEF — left ventricular ejection fraction, EDD — end-diastolic diameter, ESD — end-systolic diameter, MR — mitral regurgitation, MR area ratio — mitral regurgitation area to left atrium area ratio, LPEI — left pre-ejection interval, IVD — intra-ventricular delay, DFT — diastolic filling time, ΔEMD — maximal absolute difference in electro-mechanical delays between two segments, ΔESD — maximal absolute difference in electro-systolic delays between two segments.
3.2. Pharmacological treatment
There were no differences in pharmacological treatment of HF between the time of enrollment and randomisation except for modification of the dose of diuretics and discontinuation of nitrates.
3.3. Surgical procedure
All enrolled patients underwent CABG concomitant with epicardial CRT system implantation according to the study protocol. High operative risk (EuroSCORE≥6) was confirmed in 7 patients (30%) and in these cases IABP support was started prior to surgery.
Complete revascularization was achieved in all patients and no angina symptoms were observed in any patient throughout the post-operative follow-up period. The mean cross-clamp time was 50±11 min, and the mean CPB time was 113±21 min. The mean time needed for implantation of ventricular leads was 6.3±1.3 min, this prolonged the cross-clamp time by 14.4%. The mean total time for CRT system implantation was 16.5±3.1 min, this prolonged the CPB time by 17%.
In all cases the left ventricular lead was implanted at the optimal site within the segment of latest activation according to the pre-operative echocardiographic study.
The sensing and pacing parameters obtained directly after implantation of the CRT system are presented in Table 2.
| Parameter | At implantation | 6 months | 12 months | 6 months vs. 12 months |
|---|---|---|---|---|
| LV pacing threshold (V) | 0.90±0.8 | 0.53±0.1 | 0.56±0.1 | NS |
| LV sensing threshold (mV) | 16.0±8 | 9.4±3 | 9.1±2 | NS |
| LV impedance (Ω) | 1205±472 | 643±175 | 615±172 | NS |
| RV pacing threshold (V) | 1.10±0.9 | 0.53±0.1 | 0.69±0.2 | NS |
| RV sensing threshold (mV) | 12.6±5 | 10.7±1 | 11.0±1 | NS |
| RV impedance (Ω) | 1172±378 | 632±138 | 617±141 | NS |
| RA pacing threshold (V) | 1.3±0.7 | <0.5 | 0.69±0.2 | NS |
| RA sensing threshold (mV) | 4.5±2 | >2.8 | >2.8 | NS |
| RA impedance (Ω) | 789±269.3 | 584.7±148.2 | 627.7±140.1 | NS |
- a LV — left ventricle, RV — right ventricle, RA — right atrium. Pacing thresholds and impedance measured at 0.5 ms.
The weaning from CPB was uneventful in all patients and was performed with mild inotropic support of dopamine (5 εg/kg/min) and adrenaline (0.05 εg/kg/min).
3.4. Randomisation
There was one study drop-out due to death in the early post-operative period. Consequently 22 patients underwent randomisation into the two study groups, (n=11 per group). There were no significant differences in the main baseline characteristics between the two study groups. All randomised patients completed both phases of the cross-over study. No significant carryover and period effects were observed (Fig. 3).
3.5. Clinical results
Worsening of heart failure (increase in NYHA class) was not observed in any patient. The number of patients in whom the NYHA functional status improved by two classes was significantly greater in the CRT+ group compared to CRT− (p=0.028).
The changes in NYHA class in the CRT+ and CRT− groups compared to baseline are presented in Table 3.
| Change in NYHA class in relation to baseline | CRT− n (%) | CRT+ n (%) | P |
|---|---|---|---|
| No change (class III) | 10 (45%) | 2 (9%) | NS |
| Improvement by one class | 10 (45%) | 7 (32%) | NS |
| Improvement by two classes | 2 (9%) | 13 (59%) | 0.028 |
- a NYHA — New York Heart Association, NS — not statistically significant.
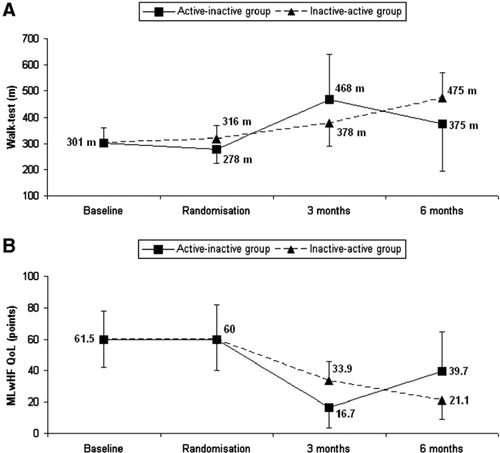
The distance walked in 6 min was significantly greater in the CRT− group compared to baseline (376.4±139.9 m vs. 300.9±56.7 m; p=0.007) and in the CRT+ group compared to the CRT− group (471.4±136.6 m vs. 376.4±139.9 m; p=0.047).
The MLwHF score decreased significantly in the CRT− group compared to baseline (36.6±19.8 points vs. 61.5±17.7 points; p<0.001) and in the CRT+ group compared to the CRT− group (18.8±18.7 points vs. 36.6±19.8 points; p=0.003).
3.6. Standard echocardiographic parameters
A statistically significant improvement in LVEF in the CRT− group compared to baseline (p<0.001) and in the CRT+ group compared to the CRT− group was noted (p<0.001). There were no significant differences in MR and ESV in the CRT− group compared to baseline values. However, a significant decrease in ESV and MR area ratio in the CRT+ group compared to the CRT− group was observed (p=0.04 and p=0.026, respectively). There was also a trend towards a decrease in MR grade in the CRT+ group compared to the CRT− group (Table 4).
| Parameter | At baseline | CRT− | CRT+ | Baseline vs. CRT− | CRT− vs. CRT+ |
|---|---|---|---|---|---|
| LVEF (%) | 30±2.6 | 34±3.1 | 40±6.1 | p<0.001 | p<0.001 |
| EDV (ml) | 228±50 | 219±47 | 207±47 | NS | NS |
| ESV (ml) | 150±29 | 143±31 | 121±35 | NS | p=0.04 |
| MR grade | 1.95±0.5 | 1.9±0.5 | 1.4±0.6 | NS | p=0.08 |
| MR area ratio (%) | 27±13 | 25±10 | 18±8 | NS | p=0.026 |
| DFT (%) | 43±6 | 44±6 | 49±5 | NS | p=0.01 |
| IVD (ms) | 47±27 | 40±26 | 18±13 | NS | p<0.01 |
| ΔEMD (ms) | 78±32 | 71±26 | 38±12 | NS | p<0.001 |
- a LVEF — left ventricular ejection fraction, EDV — end-diastolic diameter, ESV — end-systolic volume, MR — mitral regurgitation, DFT — diastolic filling time as the percentage of cardiac cycle, IVD — inter-ventricular delay, ΔEMD — maximal absolute difference in electro-mechanical delays between two segments, NS — not statistically significant.
3.7. Dyssynchrony parameters
The CABG procedure did not influence the systolic synchrony pattern — no significant differences in atrio-ventricular, inter- and intra-ventricular dyssynchrony parameters were observed in the CRT− group compared to baseline. However, there was a statistically significant improvement in dyssynchrony parameters in the CRT+ group compared to the CRT− group (Table 4).
3.8. Pacing and sensing parameters during follow-up
In all patients, pacing and sensing parameters remained stable during the follow-up period. No case of exit block was observed. The sensing and pacing parameters at 6- and 12-months follow-up are presented in Table 2.
3.9. Safety
All major adverse events (MAEs) occurred in the early post-operative period (up to 5 days post-operatively). There was one death in a female patient with high operative risk (EuroSCORE=6, LVEF=30% and small coronary vessels) due to ventricular fibrillation in the first post-operative day.
In one patient, additional surgery was needed due to bleeding. One patient required reintubation due to acute respiratory failure caused by a mucous clog obstructing the left main bronchus. The clog was removed during bronchoscopy. In one patient a false aneurysm of the femoral artery was diagnosed at the site of IABP insertion which required surgical intervention.
In total, MAEs occurred in 4 (17%) patients during short-term follow-up (30 days). No MAEs were noted during long-term follow-up (beyond 30 days).
The mean hospitalisation time for the combined procedure was 10.5±3.3 days.
During the study period one patient was hospitalised for worsening of heart failure symptoms. The patient was in the CRT+ group at that time and the reason for hospitalisation was atrial fibrillation requiring electrical cardioversion.
4. Discussion
Large multi-centre trials have demonstrated the efficacy of CRT as a method of treatment of severe heart failure with ventricular dyssynchrony [1,2]. In most patients participating in the aforementioned studies the CRT system was implanted transvenously (only in case of coronary sinus catheterization failure the LV lead could have been implanted epicardially) and the baseline characteristics do not provide detailed information about previous surgical revascularization. However, based on experience in our centre, the number of patients with severe heart failure and a recent history (less than 12 months) of CABG undergoing transvenous CRT system implantation is relatively large (15% of patients implanted in 2006). These patients could benefit from a combined procedure of surgical coronary revascularization concomitant with totally epicardial CRT system implantation. Surgical placement of pacing leads can prevent some of the problems associated with the transvenous approach: such as failure of coronary sinus catheterization, left ventricular lead dislodgement, increasing pacing threshold with time requiring reintervention, limited number of sites on the left ventricular wall which can be reached because of the anatomy of the cardiac venous system, and the risk of subclavian vein thrombosis [3,8]. In addition, the transvenous implantation procedure is usually time consuming (sometimes several attempts are needed to achieve success) and is associated with long fluoroscopic times [3,8]. On the other hand, minimally invasive epicardial lead placement is more difficult in patients with previous surgical revascularization because of pericardial adhesions and the risk of damaging grafts.
Surgical implantation of CRT concomitant with CABG is therefore beneficial for a selected group of patients, as two procedures are performed at the same time. An additional hospitalisation for transvenous CRT is not necessary, and an epicardial CRT system lead set is cheaper than a transvenous one, making this strategy cost-effective.
The value of the concept of epicardial lead implantation in patients with indications for CRT undergoing cardiac surgical procedures has recently been confirmed in the guidelines for cardiac resynchronization therapy published by the European Society of Cardiology [15]. Surgical epicardial placement is advocated for the LV lead to overcome the potential failure of transvenous implantation, but we consider that this strategy could be expanded to include all three CRT leads.
Adverse events attributable to sutured steroid-eluting epicardial leads occur significantly less frequently in comparison to coronary sinus leads [16]. An important issue to ensure good long-term lead performance is the use of an appropriate lead length, with enough slack to allow free lung movement. This prevents excessive lead tension and/or kinking, which might result in insulation damage or lead fracture. Lead displacements requiring surgical refixation are very rare [16]. It should be pointed out that the subclavicular, subcutaneous device location allows the option of transvenous pacing or defibrillation lead implantation in case of epicardial lead damage or the need for upgrade to an ICD. In our group of patients we did not notice any complications associated with epicardial leads throughout the follow-up.
Apart from a few single case reports, there are no prospective studies published in the literature to date, which assess the efficacy and safety of totally epicardial CRT system implantation concomitant with cardiac surgery, specifically CABG [17,18].
For the current study, we used a cross-over design which is suitable for initial testing of new treatment strategies. The advantage of this design is that every patient is his or her own control and therefore a relatively small sample size is needed to yield statistically significant results.
Patients enrolled to this study presented with heart failure of ischaemic origin, and were scheduled for elective CABG procedure according to ACC/AHA guidelines. DSE was performed only in patients with mild-to-moderate angina (CCS≤2). Severe stenocardia (CCS≥3) was regarded as sufficient evidence for viable myocardium. Indeed, it has been demonstrated that patients with severe angina benefit from revascularization without identification of viable myocardium with the use of imaging techniques [19].
We implanted a biventricular pacemaker in all patients included in the study as we consider that this therapy is properly validated in moderate and severe heart failure. The COMPANION trial is currently the only investigation which showed the additional benefit from implanting the more complicated and costly CRT-ICD system [20]. There are however several concerns about the methodology of this study: the absence of direct comparison between the two therapies, and the median follow-up limited to 14 months. Moreover, considering the specific subpopulation of patients with depressed LVEF undergoing CABG, additional implantation of a defibrillator does not seem to be associated with improved survival [21].
At enrollment, we used echocardiographic criteria for defining asynchrony. In most large multicenter trials of CRT published to date, as well as in the ACC/AHA and ESC guidelines for CRT, wide QRS complexes are implemented as criteria for asynchronous contraction [1,2,20]. However, there is growing evidence that electrocardiographic parameters do not always correlate with asynchrony [22]. Moreover, patients with normal QRS duration and presence of mechanical asynchrony identified in echocardiography may also benefit from CRT [23,24]. Therefore echocardiography is becoming a useful tool for assessing asynchrony in clinical practice.
In our study, we showed that in patients with heart failure and systolic asynchrony, biventricular pacing with totally epicardial system implantation during a CABG procedure is associated with additional benefit. The distance walked in 6 min was 95 m (p=0.047) longer, the quality of life improved by 18 points (p=0.003), and there was a greater number of patients with a decrease of two NYHA classes (p=0.028) in the CRT+ group compared to the CRT− group. Due to the lack of prospective investigations assessing totally epicardial CRT system implantation, we compared our findings to the results of the studies where transvenous implantation techniques were used.
In the MUSTIC study, active pacing was associated with a 73 m increase in a 6-minute walking distance (p<0.001) and a 32% improvement in quality of life (p<0.001) [25].
The MIRACLE study demonstrated that with biventricular pacing the distance walked in 6 min was 39 m longer (p=0.005), quality of life improved by 18 points (p=0.001) and NYHA class decreased significantly (p<0.001) compared to controls [26].
In the larger multicenter trials COMPANION and CARE-HF, which assessed the impact of CRT on mortality, clinical parameters were secondary end-points [2,20]. In COMPANION, in the CRT-D group, the distance walked in 6 min was 33 m (p<0.001) longer and the quality of life improved by 24 points (p<0.001) compared to controls. In CARE-HF, active pacing was associated with an amelioration of quality of life by 10 points (p<0.001) and decrease in NYHA class by 0.6 (p<0.001).
Our study demonstrated an improvement in clinical end-points which was similar or greater than in the above mentioned investigations. There are several potential reasons for these results. Firstly, we used echocardiography for defining asynchrony in contrast to previously published studies where QRS duration was used. Echocardiographic criteria may be more appropriate for identifying patients who will benefit from CRT. Secondly, all patients underwent complete surgical coronary revascularization. Restoration of the coronary blood supply may contribute to the better CRT results. Thirdly, in all patients the LV lead was placed at the optimal site according to the pre-operative echocardiographic study. For transvenous LV lead implantation, the optimal site is achieved in only about 88% of cases [3]. Finally, in all patients the AV and VV optimization procedure was performed, which has been shown to improve the efficacy of CRT [27,28].
Major adverse events were noted in 4 (17%) patients all occurred in the early post-operative period (up to 5 days post-surgery). The incidence of MAEs is similar to data from other studies analyzing the outcomes of coronary revascularization in patients with HF and comparable clinical and haemodynamic profiles [29,30].
The limitation of this study is the relatively small number of enrolled patients. Statistically reliable results were obtained thanks to the cross-over design; however our findings need to be confirmed in larger investigations with parallel study groups.
We conclude that the combined procedure of CRT and coronary artery bypass grafting is safe and that it improves the outcome of patients with severe heart failure and ventricular dyssynchrony requiring surgical revascularization.




