Body mass index, prognosis and mode of death in chronic heart failure: Results from the Valsartan Heart Failure Trial☆
Abstract
Aims:
To assess the relationship between body mass index (BMI), mortality and mode of death in chronic heart failure (CHF) patients; to define the shape of the relationship between BMI and mortality.
Methods and results:
We performed a post-hoc analysis of 5010 patients from the Valsartan Heart Failure Trial. The end-points of the study were all-cause and cardiovascular mortality. Mortality rate was 27.2% in underweight patients (BMI<22 kg/m2), 21.7% in normal weight patients (BMI 22–24.9 kg/m2), 17.9% in overweight patients (BMI 25–29.9 kg/m2) and 16.5% in obese patients (BMI>30 kg/m2) (p<0.0001). The rates of non-cardiovascular death did not differ among groups. The risk of death due to progressive heart failure was 3.4-fold higher in the underweight than in the obese patients (p<0.0001). Normal weight, overweight and obese patients had lower risk of death as compared with underweight patients (p=0.019, HR 0.76, 95% CI 0.61–0.96; p=0.0005, HR 0.68, 95% CI 0.55–0.84; p=0.003, HR 0.67, 95% CI 0.52–0.88, respectively) independently of symptoms, ventricular function, beta-blocker use, C-reactive protein and brain natriuretic peptide levels.
Conclusions:
In CHF patients a higher BMI is associated with a better prognosis independently of other clinical variables. The relationship between mortality and BMI is monotonically decreasing.
1. Introduction
An increased body mass index (BMI) represents an important risk factor for overall mortality in the general population 1,2, development of cardiovascular disease 3 and chronic heart failure (CHF) 4. In the Framingham population, obese subjects had a doubling of the risk for CHF as compared with subjects with a normal body mass index 4. Based on these observations, the international guidelines for primary prevention of cardiovascular diseases recommend weight loss for overweight and obese patients 5,6. The strength of this concept also led to the non-evidence-based extension of this counselling to CHF patients 7.
Recent studies, nevertheless, suggest that after the onset of CHF the impact of BMI on outcome could paradoxically change. It is now well established that the occurrence of weight loss, termed cardiac cachexia, is an extremely harmful event in the natural course of CHF, being associated with a mortality rate of 50% at 18 months 8. Also, preliminary reports based on retrospective analyses have shown that patients with a higher BMI might have a higher survival rate as compared to patients with a lower BMI 91011. However, due to the lack of information on important prognostic markers such as brain natriuretic peptide levels and inflammatory status, it is not clear whether this survival difference simply reflects the severity of the disease in the underweight patients, or whether a higher BMI per se is protective in CHF.
We aimed to assess the prognostic value of BMI in a large population of CHF patients, that of the Valsartan Heart Failure Trial (Val-HeFT), independently of other strong predictors of mortality, and to assess the relationship between BMI and outcome across different categories of BMI. Finally, since a higher mortality rate in patients with lower BMI could raise doubts regarding coexisting non-cardiac diseases, we aimed to analyze the cause of death (cardiovascular vs. non-cardiovascular) and the mode of cardiovascular death in the study population.
2. Methods
2.1. Study population
The Val-HeFT study was a randomised, placebo-controlled, double-blind, parallel arm multicentre trial. Five thousand and ten patients, with a history and clinical findings of CHF due to left ventricular systolic dysfunction (ejection fraction <40% and left ventricular internal diameter >2.9 cm/m2), for at least 3 months before the screening visit; were enrolled. To be included in the study, patients had to be symptomatic (New York Heart Association functional class II-IV) and clinically stable on a stable drug regimen for 2 weeks before the first study visit and during the placebo run-in period. Weight and height were measured in every patient at the screening visit. BMI was calculated as body weight in kilograms divided by height squared in meters. We categorized patients according to BMI levels as ≤22 kg/m2 (underweight), 22–24.9 kg/m2 (normal weight), 25–29.9 kg/m2 (overweight), and ≥30 kg/m2 (obese). We used these four categories in order to allow a more detailed examination of the association between BMI and mortality across a wide range of BMI values without a priori assumption about the shape of the curve. Patients with renal failure (s-creatinine levels ≥220 μmol/L), hepatic or haematological disorders or malignancies likely to limit five-year survival were not included in the study. Anaemia was defined as haemoglobin levels <12 g/dL in men and <11 g/dL in women 12. Study design and results of the main trial have been published in detail elsewhere 13,14.
2.2. Endpoints
The primary endpoint of this analysis of the Val-HeFT database was all-cause mortality. All fatal events were recorded. An independent End Points Committee adjudicated all reports for the primary end point. Each report includes narrative summaries with supporting documentation for all events. As secondary endpoints, we analyzed the cause of death (cardiovascular versus non-cardiovascular) and the mode of cardiovascular death (sudden, progressive heart failure, stroke, or myocardial infarction).
2.3. Statistical methods
Baseline characteristics of patients across the four BMI categories were examined by the chi-square test for categorical variables. To assess whether different levels of BMI are independent predictors of mortality, a multivariate Cox proportional hazards model was performed, adjusting for the following covariates statistically significant at the univariate analysis: age (≥70 years), male sex, coronary artery disease aetiology, NYHA class (III+IV), third heart sound, systolic blood pressure (<100 mm Hg), ejection fraction (<27%), left ventricular internal diastolic diameter (≥6.8 cm), creatinine (≥107 μmol/L), bilirubin (≥10.26 μmol/L), CRP (>3.23 mg/L), use of beta-blockers, presence of anaemia or diabetes and brain natriuretic peptide (≥97 pg/mL). Randomised treatment (Valsartan vs. placebo) and use of ACE-inhibitors were not taken into account as they were not statistically significant. The results of the Cox regression model are reported in terms of hazard ratios (HR) and 95% confidence intervals, and Kaplan Meier mortality curves are shown.
The variables considered for the univariate and for the multivariate analysis were categorized according to their median value, except for age where a clinical cut-off was applied. The category of BMI defined as ‘underweight’ was considered as the reference category, therefore hazard ratios for the ‘normal’, ‘overweight’, ‘obese’ categories have been calculated. The proportional hazards assumption for the four BMI categories was checked by plotting the log(− log (S(t))) versus time, adjusting for the aforementioned covariates (where S(t) is the estimated survival distribution) and tested by means of the proportionality test. The decreasing monotonic relationship of increasing values of BMI with mortality was assessed by plotting the risk of death (expressed as hazard ratio and 95% CI) for each decile of BMI. All the analyses were performed using the SAS System version 8.02 (SAS, Cary, NC).
3. Results
All patients from the main trial population (n=5010) were included in the present study. The baseline demographic and clinical characteristics of the study population have been reported in detail previously 13. Mean age was 62.7±11.1 years (range 18–96). The majority of patients were male (80%), had CHF of ischaemic aetiology (57.2%) and were in NYHA class II (61.8%). The mean left ventricular ejection fraction was 27±7% and mean left ventricular internal diameter 6.9±0.9 cm.
Mean BMI was 26.9±4.4 kg/m2; 10.9% of patients were underweight (BMI<22 kg/m2), 23.8% normal weight (BMI 22–24.9 kg/m2), 44.8% overweight (BMI 25–29.9 kg/m2), and 20.5% obese (BMI≥30 kg/m2). The clinical characteristics of the study population divided by BMI category are reported in Table 1. Patients with lower BMI were older and had a higher NYHA class than patients with higher BMI, while left ventricular ejection fraction did not significantly differ among groups. Anaemia was more frequent in underweight and normal weight patients than in overweight and obese patients; in contrast, diabetes was more frequent in patients with higher BMI than in patients with lower BMI. There were also statistically significant differences in the use of drugs among groups, with more patients who were not treated with ACE-inhibitors and beta-blockers in the underweight group than in the obese group. Finally, brain natriuretic peptide levels were higher in the underweight and normal weight patients than in the overweight and obese patients, with a statistically significant difference among groups.
| Variable | Underweight (<22 kg/m2) n=547 | Normal weight (22–24.9 kg/m2) n=1192 | Overweight (25–29.9 kg/m2) n=2245 | Obese (≥30 kg/m2) n=1026 | p-value |
|---|---|---|---|---|---|
| Age≥70 years | 240 (44) | 421 (35) | 622 (28) | 170 (17) | <0.0001 |
| Male | 356 (65) | 963 (81) | 1879 (84) | 809 (79) | <0.0001 |
| NYHA class III-IV | 251 (46) | 462 (39) | 793 (35) | 404 (39) | <0.0001 |
| IHD | 297 (54) | 703 (59) | 1335 (59) | 530 (52) | 0.0001 |
| SBP<100 mm Hg | 97 (18) | 177 (15) | 273 (17) | 127 (12) | 0.002 |
| EF<27% | 281 (51) | 592 (50) | 1031 (46) | 483 (47) | NS |
| LVIDd≥6.8 cm | 241 (44) | 570 (48) | 1221 (54) | 656 (64) | <0.0001 |
| Third heart sound | 162 (30) | 343 (29) | 561 (25) | 230 (22) | 0.0009 |
| Bilirubin≥10.26 μmol/L | 283 (52) | 676 (57) | 1311 (59) | 567 (55) | 0.026 |
| Creatinine≥107 μmol/L | 246 (45) | 630 (53) | 1187 (53) | 481 (42) | 0.0002 |
| Anaemia (yes) | 76 (14) | 128 (11) | 167 (7) | 74 (7) | <0.0001 |
| Diabetes | 101 (18) | 245 (21) | 581 (25) | 349 (34) | <0.0001 |
| BNP≥97 pg/mL | 292 (53) | 601 (50) | 936 (42) | 342 (33) | <0.0001 |
| CRP>3.23 mg/L | 185 (42) | 473 (46) | 914 (49) | 526 (61) | <0.0001 |
| No ACE-i | 44 (8) | 111 (9) | 170 (8) | 41 (4) | <0.0001 |
| No BB | 412 (75) | 807 (68) | 1430 (64) | 611 (60) | <0.0001 |
- a Data in each body mass index category are given as number of patients (%). Abbreviations: ACE-i: angiotensin-converting enzyme inhibitor; BB: beta-blocker, BNP: brain natriuretic peptide; CRP: C-reactive protein; EF: ejection fraction; IHD: ischaemic heart disease; LVIDd: left ventricular internal diastolic diameter; NYHA: New York Heart Association Class; SBP: systolic blood pressure.
3.1. Survival analysis
During follow-up (mean 23 months, range 0–38), there were 979 events (19.5%). Table 2 reports the results of the univariate predictors of prognosis derived from the Cox regression analysis. The strongest clinical variables associated with outcome were brain natriuretic peptide levels ≥97 pg/mL (p<0.0001; HR: 2.47, 95% CI 2.13–2.87), NYHA class III and IV (p<0.0001, HR 1.90, 95% CI 1.68–2.15), and baseline creatinine ≥107 μmol/L (p<0.0001; HR: 1.82, 95% CI 1.60–2.08). When BMI was analyzed as a continuous variable, one unit increase in BMI was associated with a 4% decrease in mortality (p<0.0001, HR 0.96, 95% CI 0.95–0.98). The effect on mortality in men (HR 0.96, 95% CI 0.93–0.99, p=0.0092) was not different from that in women (HR 0.96, 95% CI 0.95–0.98, p<.0001). There was a progressive decrease in the risk of death with increasing BMI, as shown by the Cox univariate analysis on BMI as a categorical variable. Normal weight (p=0.01, HR 0.78, 95% CI 0.64–0.95), overweight (p<0.0001, HR 0.63, 95% CI 0.52–0.76) and obese patients (p<0.0001, HR 0.58, 95% CI 0.46–0.72) had a significantly lower risk of death as compared with underweight patients. Fig. 1 represents the cumulative risk of death in the four BMI categories during follow-up. Unadjusted mortality rate progressively decreased with increasing BMI category, with a rate of 27.2% in underweight patients, 21.7% in normal weight patients, 17.9% in overweight patients, and 16.5% in obese patients, showing a linear increase in mortality with decrease in baseline BMI. A similar trend was shown by plotting the risk of death for each decile of BMI (Fig. 2).
| Variable | Chi-square | HR (95% CI) | p-value |
|---|---|---|---|
| BNP≥97 pg/mL | 145.04 | 2.47 (2.13–2.87) | <0.0001 |
| NYHA class III+IV | 100.53 | 1.90 (1.68–2.15) | <0.0001 |
| Creatinine≥107 μmol/L | 82.06 | 1.83 (1.60–2.08) | <0.0001 |
| Age≥70 years | 67.04 | 1.71 (1.50–1.94) | <0.0001 |
| LVIDd≥6.8 cm | 42.98 | 1.54 (1.36–1.76) | <0.0001 |
| Anaemia | 36.82 | 1.77 (1.47–2.12) | <0.0001 |
| EF<27% | 34.48 | 1.46 (1.29–1.66) | <0.0001 |
| No BB | 33.82 | 1.53 (1.33–1.77) | <0.0001 |
| CRP>3.23 mg/L | 33.75 | 1.52 (1.32–1.75) | <0.0001 |
| IHD | 32.10 | 1.46 (1.28–1.67) | <0.0001 |
| BMI≥30 kg/m2 | 24.09 | 0.58 (0.46–0.72) | <0.0001 |
| BMI 25–29.9 kg/m2 | 23.88 | 0.63 (0.52–0.76) | <0.0001 |
| BMI 22–24.9 kg/m2 | 5.86 | 0.78 (0.64–0.95) | 0.016 |
| SBP<100 mm Hg | 12.84 | 1.36 (1.15–1.61) | 0.0003 |
| Diabetes | 11.73 | 1.27 (1.11–1.46) | 0.0006 |
| Third heart sound | 11.33 | 1.26 (1.10–1.45) | 0.0008 |
| Bilirubin≥10.26 μmol/L | 11.08 | 1.24 (1.09–1.41) | 0.0009 |
| Male | 9.62 | 1.31 (1.10–1.54) | 0.0019 |
| No ACE-I | 1.31 | 1.14 (0.91–1.43) | 0.25 |
- a Abbreviations as in Table 1.
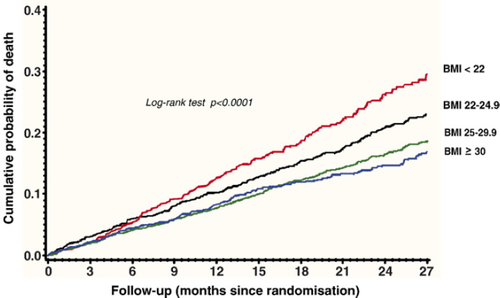
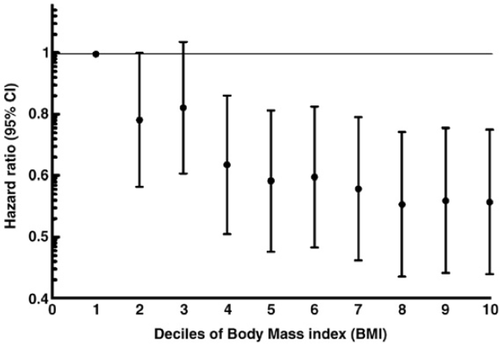
Table 3 reports the results of the multivariate Cox regression analysis. Normal weight patients (p=0.019, HR 0.76, 95% CI 0.61–0.96), overweight patients (p=0.0005, HR 0.68, 95% CI 0.55–0.84) and obese patients (p=0.003; HR 0.67, 95% CI 0.52–0.88) were at significantly lower risk of death as compared with underweight patients, independently of other strong predictors of outcome, such as NYHA class, age, creatinine, left ventricular function, diabetes, CRP and brain natriuretic peptide. Also, it is interesting to note that the hazard ratios for BMI did not change substantially after adjusting for all other variables included into the analysis (compare Tables 2 and 3).
| Variable | Chi-square | HR (95% CI) | p-value |
|---|---|---|---|
| BNP>97 pg/mL | 52.14 | 1.83 (1.55–2.15) | <0.0001 |
| BNP missing | 7.47 | 1.71 (1.16–2.50) | <0.001 |
| NYHA class III+IV | 24.78 | 1.45 (1.25–1.68) | <0.0001 |
| LVIDd≥6.8 cm | 21.49 | 1.43 (1.23–1.67) | <0.0001 |
| Creatinine≥107 μmol/L | 16.61 | 1.37 (1.18–1.60) | <0.0001 |
| Age≥70 years | 14.84 | 1.35 (1.16–1.57) | 0.0001 |
| IHD | 10.60 | 1.28 (1.10–1.49) | 0.001 |
| No BB | 9.44 | 1.28 (1.09–1.50) | 0.002 |
| BMI>30 kg/m2 | 8.81 | 0.67 (0.52–0.88) | 0.003 |
| BMI 25–29.9 kg/m2 | 12.29 | 0.68 (0.55–0.84) | 0.0005 |
| BMI 22–24.9 kg/m2 | 5.47 | 0.76 (0.61–0.96) | 0.019 |
| SBP<100 mm Hg | 6.72 | 1.29 (1.06–1.55) | 0.009 |
| EF<27% | 5.21 | 1.19 (1.02–1.37) | 0.02 |
| Third heart sound | 4.72 | 1.18 (1.02–1.38) | 0.03 |
| Male | 3.33 | 1.20 (0.99–1.47) | 0.07 |
| Diabetes | 3.01 | 1.15 (0.98–1.34) | 0.08 |
| Anaemia | 0.37 | 1.07 (0.86–1.32) | 0.54 |
| Bilirubin≥10.26 μmol/L | 0.09 | 0.98 (0.84–1.13) | 0.75 |
- a Abbreviations as in Table 1.
When a Cox regression analysis on mortality was performed, taking into account all the non-dichotomous variables as continuous measurements, the statistical significance of BMI and of the considered covariates was confirmed.
The rates of non-cardiovascular mortality were similar in the four BMI categories (3.1, 3.3, 2.2 and 1.9% respectively, overall chi-square p=0.10). The rates of death due to progressive heart failure significantly differed among the four groups, with a 3.4-fold increased risk in the underweight as compared with the obese patients. Unadjusted rate of death due to progressive heart failure was 9.5% in underweight patients, 5.5% in normal weight patients, 4.3% in overweight patients, and 2.8% in obese patients (overall chi-square p<0.0001). At the Cox univariate analysis, normal weight (p=0.0018, HR 0.56, 95% CI 0.39–0.81), overweight (p<0.0001, HR 0.43, 95% CI 0.31–0.60) and obese patients (p<0.0001, HR 0.28, 95% CI 0.18–0.44) had a significantly lower risk of death due to progressive heart failure as compared with underweight patients.
The rates of other modes of cardiovascular death (sudden death, stroke and myocardial infarction) did not significantly differ among groups (data not shown).
4. Discussion
In the present study we found that BMI is a strong predictor of all-cause mortality and death due to progressive heart failure in a large population of CHF patients; most importantly, for the first time we were able to show that the effect of BMI on prognosis is not related to more severe clinical conditions of patients with low BMI, as it is independent of other strong prognostic markers like NYHA class, brain natriuretic peptide, inflammatory status and left ventricular function. This suggests that BMI holds prognostic relevance per se. Furthermore, at the univariate analysis we observed a progressive reduction in the risk of death with increasing BMI. These results are particularly impressive, considering that more than one thousand patients in the Val-HeFT population were obese and more than two thousand were overweight, therefore minimizing the risk of a simple chance finding. Interestingly, we performed an analysis on the cause of death and found that the rate of non-cardiovascular mortality was similar in the four BMI categories. This rules out the possibility that extreme leanness could be the expression of malignant non-cardiac diseases.
Since the initial report in 1997 of an extremely high risk of death in patients with non-intentional weight loss 8, other authors have investigated the exact role of nutritional status in CHF patients. Contrary to what had been observed in the general population, i.e. a clear association between increased BMI and total and cardiovascular mortality 1,2,15,16, in patients with overt CHF this relationship seemed to be reversed. In retrospective analyses on small population samples it was found that an increasing BMI is not an adverse prognostic feature in CHF 9,17. Other authors have analyzed larger patient samples 10,11 and concluded that obesity is not associated with increased mortality and may confer a more favourable prognosis; however, in these studies few patients were obese 10, or the studies were conducted before the routine use of beta-blockers and brain natriuretic peptide assessment 11, therefore leaving this issue unresolved.
A protective effect of higher BMI has also been reported in different settings, such as ischaemic heart disease 18,19, advanced age 20, and chronic obstructive pulmonary disease 21. In the latter condition, BMI was one of the four clinical variables used to create a simple multidimensional grading system that very strongly predicted outcome. These findings suggest that in chronic diseases there are common mechanisms reflecting the severity of the illness, and particularly BMI could reflect its systemic consequences. It may be argued that in this case, a lower BMI could simply reflect a more advanced stage of the disease; in this case, the relationship between BMI and mortality would not be causal but artificial.
However, we believe that we can reasonably rule out this hypothesis, as in our multivariate model BMI predicted prognosis independently of NYHA class, ejection fraction, diabetes, brain natriuretic peptide levels, and all other clinical predictors of outcome. To our knowledge, no previous study has been able to show these data. Furthermore, the relationship between lower BMI and mortality might be influenced by other unexplored factors, like pro-inflammatory cytokines, that are closely related to outcome in patients with CHF 22. In our study, cytokine levels were not available, but the multivariate analysis performed including CRP, a potent marker of inflammatory status, showed that BMI categories were independent predictors of prognosis.
We observed a decrease in the risk of death with increasing BMI, which was progressively decreasing when univariate association was considered. Excess mortality in CHF patients with extreme leanness would be expected, but the decrease in the risk of death in overweight and obese patients as compared with underweight and normal weight patients is particularly surprising. Our findings are strengthened by the large number of patients in each BMI category.
It is not easy to explain the paradoxical protective role of higher BMI levels in CHF patients. The mechanisms underlying the association between better survival and excess weight are not entirely clear, but they could be related to an increased availability of energetic substrates. This might represent an advantage in CHF patients, who are characterized by a predominantly catabolic metabolism 23,24. We have previously shown that the amount of adipose tissue itself is protective in CHF patients 25.
Taken together, these findings open new perspectives in the understanding of the progression of CHF. Excess body weight is extremely harmful in the general population, being associated with increased risk of cardiovascular diseases and excess mortality 3,4, and we wish to underline the need to fight this health epidemic in this setting. Nevertheless, it is important to draw a distinction between the role of an elevated BMI as a risk factor for cardiovascular disease and its effect on survival after the onset of CHF. Our clinical practice should be evidence-based; so, if there is enough evidence to encourage weight loss in overweight and obese patients in the general population, there is no evidence for the same counselling in CHF patients. To our knowledge, in fact, no study has shown that a reduction of weight in patients with overt CHF is of benefit. Furthermore, it is not clear what would be the ideal BMI for CHF patients. If, in the general population, the lowest mortality rate is observed for BMI between 23.5 and 24.9 kg/m2 4 — corresponding to the so called ‘normal weight’ group — should the group of CHF patients with BMI>30 kg/m2 — that with the lowest mortality — be similarly considered as ‘normal’? Despite being highly provocative, this question should at least raise some doubts regarding some — unjustified — behaviour in our clinics. Should we encourage our patients to increase their body weight? At present there is inadequate information to answer this question; therefore, it is necessary to wait for the results of targeted clinical trials to address this point. We can only comment on the recent finding that drugs like ACE-inhibitors and beta-blockers, in addition to their effects on morbidity and mortality, have been shown to be able to prevent weight loss and even to produce a significant increase in body weight 26,27.
The present study has several limitations. First, it represents a post-hoc analysis of the Val-HeFT database, which was not initially designed to address the hypothesis of this study. Second, we observed a direct relationship between BMI levels and mortality, but a causal link between low BMI and mortality cannot be demonstrated. We cannot rule out the possibility that other unexplored factors might play a role in determining both a reduction in BMI and an increase in mortality; however, this study is focused on the clinical, not pathophysiological relevance of BMI in CHF patients. Finally, the four BMI categories had different baseline clinical characteristics, which could affect the results of our analyses. Nevertheless, our multivariate model included most of the strongest prognostic markers studied in CHF so far, therefore overcoming this limitation at least partially.
A prospective study to confirm the results of this post-hoc analysis, in order to further elucidate the apparent protective role of BMI on survival in CHF patients is now required.
5. Conflict of interest disclosures section
This study was supported by a grant from Novartis Pharma, Basel, Switzerland.
Drs. Latini, Maggioni and Anand have received honoraria for presentations; Dr. Cohn received research support through consultation arrangements.
Dr. Cicoira had full access to all of the data in concerning this paper.
Dr. Barlera takes complete responsibility for the integrity of the data and the accuracy of the data analysis.




