Anaemia and renal function in heart failure due to idiopathic dilated cardiomyopathy
Abstract
Background:
Anaemia and renal dysfunction are common in patients with heart failure (HF). Most studies involve western cohorts with ischaemic aetiology receiving treatment likely to impair renal function.
Aims:
To investigate the frequency of anaemia and renal dysfunction and the relationship between the two within a cohort of 163 newly diagnosed Black African idiopathic cardiomyopathy patients prior to commencing HF treatments and compare those findings to those of western HF cohorts.
Methods:
Single-centre retrospective analysis. Anaemia defined as haemoglobin concentration <13.0 g/dL for males (n=85) and <12 g/dL for females (n=78). Probable renal dysfunction defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2, using serum creatinine concentrations.
Results:
The mean age was 48±11 years, 52% were male. Overall, 13.5% of patients were anaemic and 11.8% had evidence of renal dysfunction, while 1.2% had both. Renal dysfunction was significantly more common in older patients (mean age 58±13 vs. 47±10 years: p<0.001).
Conclusion:
The frequency of anaemia and renal dysfunction in this cohort was lower than that reported in western HF cohorts. These data infer a more limited relationship between HF, anaemia and renal dysfunction in patients without atherothrombotic disease; hence extrapolation of HF data from the western world to other populations should be interpreted cautiously.
1. Introduction
Anaemia and renal dysfunction are common in patients with heart failure (HF). Neither is benign in this clinical setting. Chronic kidney disease is very common in patients with HF, and for those patients the prognosis is often quite poor 1. Similarly, strong epidemiological associations between anaemia in HF patients and severity of HF symptoms and prognosis have been noted 2. Given that both are common in HF and poor prognostic markers, a key clinical question in recent years has been whether these two conditions are linked? Moreover, if they are linked, do they represent a particularly poor prognostic marker or simply a reflection of progressively worsening cardiac and neurohormonal function or, indeed, reflect the common use HF treatments (e.g. ACE inhibitors, AII receptor blockers and aldosterone antagonists) that interfere with the neurohormonal activation system and therefore renal function? Determining the underlying causes (particularly independent of one another) of renal dysfunction and anaemia in patients with HF has, therefore, many clinical implications.
The prevalence of both anaemia 2 and renal dysfunction 1 and the relationship between these two clinical conditions within HF cohorts has been reported 3,4. However, most studies involve western world HF cohorts with primary ischaemic aetiology, often receiving treatment likely to impair renal function and therefore confound any attempt to investigate their underlying cause(s) and relationships between the two. Alternatively, in the developing world setting, the aetiology of HF is often non-ischaemic and affected patients rarely receive standard HF treatments prior to specialist assessment and management.
1.1. Study aims
It is within this context we had the unique opportunity to examine the frequency of anaemia and renal dysfunction and the relationship between the two within a developing world cohort of Black African patients. These patients presented with HF due to idiopathic dilated cardiomyopathy and data was recorded prior to commencing HF specific treatments. We compared our findings to those of western HF cohorts to determine if: a) the underlying prevalence and relationship between anaemia and renal dysfunction is different in developing world vs. developed world cohorts and b) if so, whether this provides any insights into the relationship and prognostic importance of these conditions in patients with HF.
2. Methods
2.1. Study cohort
This study was a retrospective analysis of a single-centre cohort study, from which other health outcome and treatment data has been previously reported 5. The study population consisted of newly diagnosed Black African patients with idiopathic dilated cardiomyopathy in New York Heart Association (NYHA) functional class I to IV. Importantly, baseline data were collected prior to initiation of HF-specific treatments (ACE inhibitors, AII receptor blockers, aldosterone antagonists and beta-blockers (β-blocker)). The Committee for Research in Human Subjects of the University of Witwatersrand approved the initial study protocol which conformed to the principles outlined in the Declaration of Helsinki. All subjects gave written informed consent for the collection of these data.
2.2. Study inclusion/exclusion criteria
Inclusion criteria were: 1) age≥18 and <80 years, 2) left ventricular ejection fraction <45% on echocardiography or radionuclide angiography, 3) sinus rhythm. Exclusion criteria were: 1) chronic obstructive pulmonary disease, 2) significant valvular heart disease, 3) history or evidence of ischemic heart disease, 4) systolic blood pressure >180 mmHg and/or diastolic blood pressure >100 mmHg, 5) pregnancy, and 6) severe liver disease, defined as enzymes >2 times the upper limit of normal.
2.3. Anaemic status
Consistent with other studies reporting the anaemia in HF cohorts 678910111213, anaemia was defined according to World Health Organisation criteria 14, haemoglobin (Hb) concentrations <13.0 g/dL for males and <12.0 g/dL females were classed as anaemia.
2.4. Renal function
Renal function was assessed via an estimated glomerular filtration rate (estimated GFR mL/min/1.73 m2) calculated using the Modification of Diet in Renal Disease (MDRD) abbreviated formula (estimated GFR mL/min/1.73m2=186.3×(serum creatinine mg/dL)−1.154×(age)−0.203×(0.742 female sex)×(1.212 if African American) 15 using serum creatinine concentrations (μmol/L) converted to mg/dL. An estimated GFR is preferred for the assessment of renal function over assessment of serum creatinine 16. The MDRD formulas have been validated in several studies in different patient populations and have been used to estimate GFR in patients with HF 3,4,8,10,12,17 and include an adjustment for Black race 15, given the propensity for greater total muscle mass in this population 16. The presence of probable renal dysfunction was identified according to a threshold of <60 mL/min/1.73 m2, a definition used in many clinical trials in HF 3,18 and other studies reporting renal function within HF cohorts 10,24,25 and a threshold below which complications of renal impairment appear 3.
2.5. Cardiac function
An average left ventricular ejection fraction (LVEF) was calculated, using several calculations of LVEF obtained by standard clinical methods (multi-gated equilibrium cardiac blood pool scintigraphic technique and two dimensional targeted M-mode echocardiography with Doppler color flow mapping using both manual and machine calculations of LVEF) 5. The functional capacity of each patient was determined according to NYHA classification. All patients above the age of 40 years had a coronary angiogram performed to rule out coronary artery disease.
2.6. Statistical analysis
All data analyses were performed using SPSS version 12.0.1. Data are presented as percentage (%) or mean values±standard deviation (SD). Between group differences were determined using an analysis of variance (ANOVA), or by independent samples t-test. χ2 was used for analysis of categorical variables. A Pearson correlation was used to evaluate the relationship between continuous variables. Testing was two-sided, and p values<0.05 were considered significant. Multiple logistic regression models were used to determine the independent predictors of anaemia and probable renal dysfunction: to examine potential differences in Hb concentration or frequency of anaemia according to age, renal function or LVEF, the cohort was grouped into tertiles based on each of these variables.
3. Results
The study cohort of 163 Black African patients comprised 85 males (52%) and 78 females with a mean age 48±11 years. The clinical characteristics of this cohort are summarised in Table 1. All patients had left ventricular systolic dysfunction (mean LVEF 25±9%). Functionally, the majority of patients were classified as NYHA Class II or III. As newly diagnosed patients prior to substantive treatment, no patients had been pre-treated with an ACE inhibitor or beta-blocker at the time of assessment, although 150 patients (92%) were being treated with furosemide (mean dose of 137±46 mg/day).
| All patients n=163 | |
|---|---|
| Demographics | |
| Male/female (%) | 52/48 |
| Age, years | 48±11 |
| Medication (mean±SD) | |
| Furosemide (mg/day) | 137±46 |
| Anthropometrics (mean±SD) | |
| Weight (kg) | 69±15 |
| BMI (kg/m2) | |
| Male | 24±4 |
| Female | 27±6 |
| Cardiac parameters (mean±SD) | |
| Average LVEF (%) | 25±9 |
| Systolic BP (mmHg) | 117±20 |
| Diastolic BP (mmHg) | 75±18 |
| NYHA Class (n) | I (11), II (69), III (54), IV (12) |
| Haematological and biochemical parameters (mean±SD) | |
| Haemoglobin (g/dL) | |
| Male | 14.9±1.7 |
| Female | 13.7±1.9 |
| est GFR (mL/min/1.73m2) | 88.2±26.9 |
| Se creatinine (μmol/L) | 97.5±31.5 |
| Se sodium (mmol/L) | 139.5±4.2 |
| Se uric acid (μmol/L) | |
| Male | 492.3±124.2 |
| Female | 479.5±110.0 |
| Se urea (mmol/L) | 15.3±29.1 |
| Se potassium (mmol/L) | 4.1±0.6 |
| Se glucose (mmol/L) | 5.2±1.8 |
- a BMI, body mass index; Average LVEF, Left Ventricular Ejection Fraction, averaged for each patient from a number of methods of calculation; NYHA Class, New York Heart Association Classification; est GFR, estimated glomerular filtration rate; Se, serum.
3.1. Anaemia
Overall, 13.5% of patients were anaemic at the time of assessment, with greater frequency in females (14.1%, mean Hb 13.7±1.9 g/dL) compared to males (12.9%, mean Hb of 14.9±1.7 g/dL) although the proportion of anaemic patients was not significantly different between the sexes (p=1.0). Anaemic females (n=11) were significantly younger than non-anaemic females (42±13 years vs. 50±12 years: p=0.04), although no significant difference in age was detected between anaemic males (n=11) and non-anaemic males (52±11 years vs. 48±11 years: p>0.05). Mean averaged LVEF was significantly lower in anaemic females than non-anaemic females (23.5±4.2% vs. 27.6±8.9%: p=0.025). Mean serum urea concentrations were significantly lower in anaemic males than non-anaemic males (6.9±1.6 mmol/L vs. 16.7±30.8 mmol/L: p=0.009). Anaemic males and females had significantly lower mean Hb, as was to be expected, than their non-anaemic counterparts (p<0.001). No significant difference in the mean Hb or frequency of anaemia was detected according to averaged LVEF tertiles or NYHA Class.
A weak positive correlation was detected between Hb and LVEF with borderline significance in females (r=0.224: p=0.057), but not in males (r=0.118: p=0.290).
There was no difference in the mean Hb or frequency of anaemia according to age tertiles for the cohort as a whole, however when considered according to sex, the mean Hb decreased significantly between the youngest and the oldest tertiles (<42 vs. ≥53 years; 15.5±1.6 g/dL vs. 14.2±1.7 g/dL: p=0.008) and the middle and the oldest tertiles (42–52 years vs. ≥53 years; 15.2±1.5 g/dL vs. 14.2±1.7 g/dL: p=0.033) for males only. A small positive correlation was detected between Hb and age in females (r=0.223: p=0.05), and a weak negative correlation between Hb and age in males (r=−0.322: p=0.003) was identified.
On multiple logistic regression analysis (males and females combined) none of the following variables were significant predictors of anaemia (age, sex, serum creatinine, estimated GFR, NYHA Class or LVEF).
Overall, the frequency of anaemia found in this cohort (13.5%) was lower than that reported in western HF cohorts which have employed the same definition of anaemia 6,7,8.
3.2. Renal function
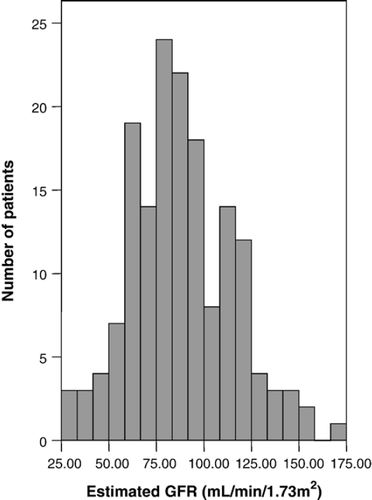
Approximately 11.8% of the cohort (8 males and 11 females) demonstrated signs of probable renal dysfunction as evidenced by estimated GFR <60 mL/min/1.73 m2 (see Table 2). The mean serum creatinine concentration for the whole cohort was 97.5±31.5 μmol/L, the value varied significantly (p<0.001) according to sex, (106.5±33.2 for males vs. 87.7±26.4 μmol/L for females). The mean estimated GFR was 88.1±26.9 mL/min/1.73 m2 and was slightly higher in males (90.9±26.4 mL/min/1.73 m2) compared to females (85.2±27.2 mL/min/1.73 m2) though not significantly so (p=0.180). Fig. 1 and Table 2 summarise these data, of note, patients with an estimated GFR <60 mL/min/1.73 m2 were significantly older, had significantly greater mean serum creatinine, potassium and uric acid concentrations relative to those with a higher estimated GFR (≥60 mL/min/1.73 m2).
| est GFR <60 mL/min/1.73 m2 | est GFR ≥60 mL/min/1.73 m2 | p | |
|---|---|---|---|
| Demographics | |||
| Number of patients (% of cohort) | 19 (12) | 142 (88) | |
| Male/female (%) | 42/58 | 54/46 | ns |
| Age (years) | 58±13 | 47±10 | <0.001 |
| Medication (mean±SD) | |||
| Furosemide (mg/day) | 146±63 | 137±44 | ns |
| Cardiac measures (mean±SD) | |||
| LVEF averaged (%) | 26.2±11.2 | 25.3±8.8 | ns |
| NYHA class (n) | I (3), II (6), III (6), IV (3) | I (7), II (63), III (48), IV (9) | |
| Systolic blood pressure (mmHg) | 123±18 | 117±20 | ns |
| Diastolic blood pressure (mmHg) | 80±12 | 74±13 | <0.05 |
| Haematological and biochemical parameters (mean±SD) | |||
| est GFR (mL/min/1.73m2) | 46.7±10.8 | 93.7±23.3 | <0.001 |
| Se creatinine (μmol/L) | 154.6±42.1 | 89.9±20.0 | <0.001 |
| Se sodium (mmol/L) | 138.4±5.7 | 139.6±3.9 | ns |
| Se uric acid (μmol/L) | |||
| Male | 602.0±130.3 | 476.2±116.7 | <0.05 |
| Female | 586.7±187.2 | 457.1±78.7 | ns |
| Se urea (mmol/L) | 11.0±7.9 | 15.8±30.9 | ns |
| Se potassium (mmol/L) | 4.5±0.9 | 4.1±0.5 | <0.05 |
| Haemoglobin (g/dL) | 14.2±1.8 | 14.3±1.9 | ns |
| Male | 14.6±1.9 | 14.9±1.7 | ns |
| Female | 13.9±1.8 | 13.6±1.9 | ns |
| Anaemia (%) | 10.5 | 14.1 | |
| Male<13 g/dL (n) | 1 | 10 | ns |
| Female<12 g/dL (n) | 1 | 10 | ns |
- a BMI, body mass index; Average LVEF, Left Ventricular Ejection Fraction, averaged for each patient from a number of methods of calculation; NYHA Class, New York Heart Association Classification; est GFR, estimated glomerular filtration rate; Se, serum.
On multiple logistic regression analysis (males and females combined) an estimated GFR <60 mL/min/1.73 m2 was more common in older patients (odds ratio [OR] 1.140 per year, 95% CI 1.052 to 1.234: p=0.001); patients with higher serum potassium concentrations (OR 4.363 per mmol/L, 95% CI 1.407 to 13.528: p=0.011) and higher serum urea concentrations (OR 1.069 per mmol/L, 95% CI 1.020 to 1.121: p=0.005), but not serum Hb concentration nor the presence of anaemia.
The frequency of patients with probable renal dysfunction (estimated GFR <60 mL/min/1.73 m2) is not consistent across all reported HF cohorts in the literature (see Table 3). Overall 11.8% of the cohort had an estimated GFR of <60 mL/min/1.73 m2.
| Trial (test treatment) | Number of patients | Creatinine exclusion (μmol/L) | Mean age (y) | Mean creatinine (μmol/L) | Mean est GFR (mL/min/1.73m2) | % est GFR <60 mL/min/1.73 m2 |
|---|---|---|---|---|---|---|
| Current cohort | 161a | NA | 48 | 97.5 | 88.2 | 11.8 |
| CONSENSUS 20 (Enalapril) | 243 | >330 | 71 | 133 | 45b | - |
| SOLVD (Enalapril) 3 | 6630 | >177 | 60 | 106 | 70.4 | 32 |
| DIG (Digoxin) 19 | 6800 | >265 | 63 | 112 | 61.6 (median) | 46 |
| CIBIS-2 (Bisoprolol) 22 | 2647 | ≥300 | 61 | 103 | 77.5b | 33 |
| COMET (carvedilol vs. metoprolol) 21 | 3029 | Not reported | 62 | 99 (median) | 67.2 | - |
| CHARM (candesartan) 18 | 2680 | 265 | 66 | 97 (median) | 69 (median) | 35 |
| CARE-HF (Cardiac resynchronisation therapy) 23 | 813 | Not reported | 66 (median) | 106 (median) | 60 (median) | ~50 |
- a 161 patients with serum creatinine concentration data.
- b Calculated using Cockcroft-Gault formula.
3.3. Anaemia and renal function
Although no statistically significant differences in the frequency of anaemia according to renal function were detected, (see Table 2), the frequency of anaemia in those patients with an estimated GFR <60 mL/min/1.73 m2, was 10.5%, compared to 14.1% of those with an estimated GFR ≥60 mL/min/1.73 m2 only 2 patients (1.2%) of the cohort (n=161 with a calculated estimated GFR) had both anaemia and probable renal dysfunction.
When the cohort was divided into tertiles based on estimated GFR, no significance in the mean Hb or the frequency of anaemia was detected, between the groups when the cohort was considered as a whole or by sex.
A small negative correlation was evident (r=−0.221, p=0.513), between estimated GFR and Hb concentration in anaemic females. Conversely, a modest positive correlation was detected in anaemic males between estimated GFR and Hb concentration (r=0.601, p=0.051). There was no correlation between estimated GFR and Hb when the cohort was considered as a whole irrespective of anaemia status (r=0.046, p=0.562).
4. Discussion
This is the first study of anaemia and renal dysfunction in a cohort of patients with HF from a developing world setting. Based on potentially important differences in the age and gender balance, race, underlying aetiology of HF and treatment history, these data provide potentially important insights into similar reports from the western world. As such, we found a lower frequency of underlying anaemia and renal dysfunction in this cohort of HF patients (13.5% and 11.8% respectively) when compared to typical reports from western HF cohorts.
4.1. Anaemia
The frequency of anaemia in this current cohort was 13.5% overall, a figure notably different to that reported by studies examining anaemia in western HF cohorts. For example, the prevalence of anaemia reported in western world HF cohorts applying the WHO definition of anaemia 14 ranges from 15.9–57% 6,7,8. O'Meara and colleagues 12,17 have recently reported in a cohort of HF patients from the three CHARM trials that 26% of the cohort (n=2653, 33–35% female, 10–16% black) was identified with anaemia. Interestingly, a greater percent of anaemic patients (n=677) were black (16%) than non-anaemic patients (n=1976) (10% black) in this cohort 12. Of note also, in a cohort of 148 patients with HF (mean age 64.5 years, 26% female, 48% ischaemic aetiology and 30% dilated cardiomyopathy), 57% were anaemic 11. Similarly, Go et al. 8 have also recently reported in a large cohort of older HF patients (mean age 71.8 years, 46% female, 10% black) that 43% of patients were identified as anaemic according to WHO standards 8.
In the western world overall, anaemia is often reported to be associated with older age, greater HF severity and renal dysfunction with reported rates as high as 70% 2.
Not withstanding the difficulty in making firm comparisons due to the plethora of definitions used to identify underlying anaemia in this context 2 there do appear to be some important differences in the frequency and characteristics of anaemia in this Black African cohort. Clearly, the reasons are likely to be multifaceted. The most obvious difference between the cohort in the analysis and western HF cohorts considered here 6,7,8 is their age and the sex distribution; with a mean age 48 years and 48% of the population being female, this must account for a considerable part of this difference.
The underlying cause of anaemia in this Black African cohort is largely unknown. We were not able to identify whether the low Hb observed in some patients is indicative of haemodilution. However, with 150 patients prescribed a mean furosemide dose of 137 mg/day, severe fluid overload in these patients would most probably have been limited. Significantly, these patients were all new presentations of idiopathic dilated cardiomyopathy, and data were collected prior to commencement of specific HF treatments. It is reasonable to suggest that confounding factors notwithstanding (e.g. a higher proportion of menstruating females), the anaemia found in this cohort is different to that reported in greater concentrations in western chronic HF cohorts, where low haemoglobin may be influenced by underlying vascular (atherothrombotic), in particular renovascular disease and chronic disease status; reflective of systemic organ dysfunction due to deterioration of the HF syndrome 27.
4.2. Renal function
We also found potentially important differences in renal function in this Black African cohort compared to typical western HF cohorts. Whilst the mean serum creatinine and mean serum sodium concentrations of the whole cohort were within the accepted normal range, the mean serum urea concentration was notably elevated above the accepted normal range, a finding which may possibly be evidence of some degree of pre-renal dysfunction in this cohort. On examination of the patients identified with probable renal dysfunction (estimated GFR <60 mL/min/1.73 m2), mean serum potassium concentrations were significantly higher; however, this value was within the accepted normal range. Diastolic blood pressure was also found to be significantly higher in this group, perhaps reflective of neurohormonal activity. For males, the mean serum uric acid concentration was significantly higher than that of male patients with normal renal function. Conversely, serum uric acid concentrations were elevated above the accepted normal range irrespective of estimated GFR, a finding which may be reflective of the use of furosemide in this cohort.
Twelve percent of patients in this current cohort had an estimated GFR <60 mL/min/1.73 m2, indicative of probable renal dysfunction, a rate considerably lower than that reported by large HF clinical trials 3,18. The mean estimated GFR in this current study was also higher, suggestive that this current cohort of HF patients has better renal function than patients enrolled in some of the large HF clinical trials 3,18; this is to be expected, given the young age of this population and the absence of a chronic HF profile.
4.3. Anaemia and renal function
Only 2 patients (1.2%) were identified with probable renal dysfunction as well as being anaemic. Although no differences were detected in the frequency of anaemia according to declining renal function, a modest positive relationship was detected between renal function and Hb in the presence of anaemia, for males. However this relationship was weaker and negative in nature between estimated GFR and Hb concentration in anaemic females. The negative nature of this relationship for anaemic females in conjunction with the significant difference in the mean ages of anaemic and non-anaemic women may reflect the influence of menstrual status on Hb concentration and the influence of age on estimated GFR. Hence, older women in this cohort may have a lower estimated GFR due to the influence of age on renal function, yet they are more than likely postmenopausal and have slightly greater serum Hb concentrations than their menstruating peers, who are younger and have better renal function. Considering that prevalence of anaemia in non-pregnant women of the developing world is estimated to be as high as 40–50% 14 and the prevalence of iron deficiency anaemia in European menstruating women is estimated at 1–14% 28, the anaemia seen in female patients in this cohort may not be associated with HF but rather a commonly occurring outcome associated with menstruation.
The absence of a notable positive relationship between renal function and the presence of anaemia in this HF cohort as a whole differs considerably from the typical sequelae of HF often observed in western HF cohorts, that anaemia is common, its prevalence increases with the severity of HF, declining renal function and increasing age 2.
4.4. Comparisons with other heart failure cohorts
These data reported suggest that we may not be able to rely on the data reported from western HF cohorts and extrapolate those findings to developing world HF cohorts. These data from a cohort of HF patients not yet exposed to ACE inhibitors and beta-blockers do, however, stimulate consideration that the anaemia and renal dysfunction observed commonly in western HF patients may not be the result of underlying pathological processes but perhaps merely a side effect from otherwise beneficial pharmacological treatments of HF.
4.5. Limitations
There are several limitations to this current analysis. The data is a retrospective analysis of secondary data and as such not all variables were available for the 163 patients, and no substantial conclusions may be made from these data alone. The sample size of the study is relatively small and the potential problem of Type II error in being under-powered to detect clinically significant differences based on age, sex, renal dysfunction and anaemic status was highly likely. There was also considerable difference in the number of patients in this current study compared to that of the studies for which comparisons are made. At the stage of data collection no further investigations into the cause of anaemia had been performed. Future prospective studies involving detailed follow-up soon to be undertaken in this population 29 will allow greater study of these differences between HF cohort of the western and developing worlds.
4.6. Summary
Despite these limitations, these unique data provide an important counter-point to data derived from predominantly western cohorts with HF where high rates of underlying anaemia and renal dysfunction are reported. Whilst the definition of anaemia differs significantly amongst researchers 2, it is known that within most western cohorts of HF for which the prevalence of anaemia is reported, anaemia is common, its prevalence increases with the severity of HF, declining renal function and increasing age 2. In this cohort we have found that although anaemia was present in some patients it was not particularly common. Moreover, the frequency of anaemia did not increase with the severity of HF, or with increasing age or with decreasing renal function. Overall, these data suggest a more limited relationship between HF, anaemia and renal dysfunction in Black African patients without atherothrombotic disease. As previous data were predominantly derived from western cohorts, this study suggests that any extrapolation of current HF data to non-western populations should be interpreted with caution.
5 Acknowledgments
S.I. and S.S. are supported by the National Health and Medical Research Council of Australia (NHMRC) and the National Heart Foundation of Australia (NHF). V.V. is supported by a World Heart Failure Society (WHFS) fellowship.




