Dissociation between gene and protein expression of metabolic enzymes in a rodent model of heart failure
Abstract
Studies in advanced heart failure show down-regulation of fatty acid oxidation genes, possibly due to decreased expression of the nuclear transcription factors peroxisome proliferator activated receptor α (PPARα) and retinoid X receptor α (RXRα). We assessed mRNA and protein expression of PPARα and RXRα, and for several PPAR/RXR regulated metabolic proteins at 8 and 20 weeks following myocardial infarction induced by coronary artery ligation. Infarction resulted in heart failure, as indicated by reduced LV fractional shortening and increased end diastolic area compared to sham. There was a progressive increase in LV end systolic area, myocardial ceramide content and atrial natriuretic peptide mRNA, and a deterioration in LV fractional area of shortening from 8 to 20 weeks. Protein and mRNA expression of PPARα and RXRα were not different among groups. The mRNA for PPAR/RXR regulated genes (e.g. medium chain acyl-CoA dehydrogenase (MCAD)) was down-regulated at 8 and 20 weeks post-infarction; however, neither the protein expression nor activity of MCAD was reduced compared to sham. In conclusion, reduced mRNA expression of PPAR/RXR regulated genes is not dependent on reduced PPAR/RXR protein expression.
1. Introduction
Recent studies suggest that changes in cardiac substrate metabolism affect left ventricular (LV) function and remodeling in heart failure 1. The healthy adult heart uses fatty acids as the primary substrate for the generation of ATP and, during the early stages of heart failure, the myocardium continues to derive most of its energy from fatty acids 2,3. On the other hand, in advanced heart failure, there is down-regulation of the mRNA and protein expression of fatty acid oxidation enzymes, and the myocardium switches to greater carbohydrate oxidation 4,5. The time course and mechanisms responsible for the down-regulation of the fatty acid oxidation pathway in advanced heart failure are not clear.
Recent studies suggest that decreased expression of the nuclear transcription factors peroxisome proliferator activated receptor Ρ (PPARΡ) and the retinoid X receptor Ρ (RXRΡ) may play a key role in the myocardial substrate switch observed under these conditions 6. These proteins form a heterodimer and increase transcription of genes encoding key enzymes involved in fatty acid metabolism, such as medium chain acyl-CoA dehydrogenase (MCAD), carnitine palmitoyl transferase I (CPT-I), uncoupling protein-3 (UCP3) and mitochondrial thioesterase-1 (MTE-1), and the inhibitory kinase of pyruvate dehydrogenase, pyruvate dehydrogenase kinase-4 (PDK-4) 6789. Studies in patients 10 and animals 11,12 with advanced end stage heart failure show that there is a significant reduction of PPARΡ and/or RXRΡ mRNA and/or protein levels compared to non-failing hearts. It remains unclear, however, if the down-regulation of PPARΡ and/or RXRΡ and fatty acid oxidation enzymes occurs early in the development of heart failure, or if it is observed only in the late-stage.
The primary goal of the present study was to test the hypothesis that the mRNA and protein levels of PPARΡ, RXRΡ and the mRNA and protein expression of key fatty acid oxidation enzymes would progressively decrease in the failing heart. Studies were performed using the well-established rat infarct model of heart failure 13, and measurements of LV function, and the mRNA and protein levels of PPARΡ, RXRΡ and enzymes involved in fatty acid metabolism were made at 8 and 20 weeks following induction of heart failure by coronary artery ligation or sham surgery.
Elevated myocardial ceramide levels are associated with cardiac dilatation and reduced contractility 141516; however, it is unclear if ceramide contributes to cardiac dysfunction in heart failure. Ceramide is a toxic lipid intermediate that can impair mitochondrial function and trigger apoptosis, and is associated with cardiac dysfunction and LV chamber dilation in rat and mice models of increased myocardial fatty acid metabolism 141516. Thus, we hypothesized that heart failure would results in a progressive increase in myocardial ceramide content compared to sham animals.
2. Methods
2.1. Study design and induction of myocardial infarction
This study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication number 85–23) and the Institutional Animal Care and Use Committee at Case Western Reserve University. All cardiovascular and biochemical measurements were conducted in an investigator blinded fashion. Animals were maintained on a reverse 12-h:12-h light/dark cycle (i.e. lights off at 7AM) and all procedures and tissue harvests were performed in the fed state between 3 and 6 h into the dark phase of the cycle. It has been established that the myocardial content of mRNA for metabolic genes plateau during this time period 17.
Heart failure was induced by myocardial infarction as previously described 18. Briefly, 7-week-old male Wistar-Kyoto rats weighing ~250 g were anesthetized with 1.5–2.0% isoflurane, intubated and ventilated. An infarct was induced by ligation of the left coronary artery and sham animals were subjected to the same surgical procedure without coronary artery ligation. Sham and ligated rats were assigned to either an 8-week (11 sham and 11 ligated) or 20-week post-infarction (10 sham and 10 ligated) study group, at which times echocardiography was performed and LV pressures were measured as described below.
2.2. Echocardiography
LV function was evaluated by echocardiography using a Sequoia C256 System (Siemens Medical) with a 15-MHz linear array transducer as previously described 18. Briefly, at either 8 or 20 weeks post-surgery, rats were anesthetized with 1.5–2.0% isoflurane by mask, the chest was shaved, the animal was situated in the supine position on a warming pad and ECG limb electrodes were placed. Two-dimensional (2D), 2D-guided M-mode and Doppler echocardiographic studies of aortic and transmitral flows were performed from parasternal and foreshortened apical windows. End diastolic and end systolic areas (EDA and ESA) were measured using software resident on the ultrasonograph, and fractional area shortening, myocardial performance index (MPI) and cardiac index (CI) were calculated as previously described 18.
2.3. Hemodynamic measurements
At either 8 or 20 weeks post-surgery, rats were intubated, ventilated and anesthetized (1.5–2.0% isoflurane) as previously described 18. A 3.5-Fr microtip pressure transducer catheter (Millar Instruments) was introduced into the LV via the right carotid artery. Heart rate (HR), maximum LV pressure, peak end diastolic pressure (EDP), peak ±dP/dt and the time constant of isovolumic LV relaxation (Tau, the time constant in milliseconds of the best-fit monoexponential pressure decay from the pressure at maximum −dP/dt to a positive pressure above the previous LV end diastolic pressure by 10 mm Hg or a duration of 20 ms, whichever happens first) were recorded using a Digi-Med® Heart Performance Analyzer-τ™ over a 30-s period. Following the measurement of LV pressure, the heart was excised, the scar tissue was visually identified and removed, and LV tissue was frozen in liquid nitrogen and stored at −80 °C for biochemical analysis.
2.4. RNA extraction and quantitative RT-PCR
RNA extraction and quantitative RT-PCR were performed on frozen powdered non-infarcted LV tissue (isolated either 8 or 20 weeks post-surgery) using previously described methods (Chomczynski, 1987, 25/id; Heid, 1996, 1224/id; Gibson, 1996, 2/id). Specific quantitative assays were designed from rat sequences available in GenBank for expression of PPARΡ, RXRΡ, citrate synthase (CS), uncoupling protein 2 (UCP2), atrial natriuretic peptide (ANP) and genes that are known to be regulated by PPARΡ: MCAD, CPT-I, PDK-4, UCP3 and MTE-1 8,9,17,19. Standard RNA was made for all assays by the T7 polymerase method (Ambion), using total RNA isolated from rat hearts. The correlation between the Ct (the number of PCR cycles required for the fluorescent signal to reach a detection threshold) and the amount of standard was linear over at least a 5-log range of RNA for all assays. To control for sample-to-sample differences in RNA concentration, the mRNA level for cyclophilin was quantitatively measured in each sample. Expression of cyclophilin was not different among the experimental groups; therefore, the PCR data are reported as the number of transcripts per number of cyclophilin molecules.
2.5. Western immunoblot analysis
Protein was extracted from frozen powdered LV tissue as previously described 12. Either 50 or 75 μg of total protein was separated by electrophoresis in 10% SDS-PAGE gels and transferred onto a PVDF membrane. Membranes were incubated with specific antibodies to PPARΡ, RXRΡ (1:100; Santa Cruz Biotechnology, Inc.) or MCAD (1:2000; Caymen Chemical). After conjugation with the secondary antibody, the membranes were developed in a chemiluminescence substrate solution (Santa Cruz Biotechnology, Inc.) and bands were quantified using commercially available software. All samples were run in duplicate and normalized to a standard sample of normal male Wistar-Kyoto rats that was loaded in duplicate on each gel.
2.6. Metabolites and enzyme activities
Plasma-free fatty acids and triglycerides were determined using spectrophotometric assays, and the maximal activities of MCAD and CS were measured in frozen powdered LV tissue (isolated either 8 or 20 weeks post-surgery), as previously described 12,23,24. C16-ceramide content in non-infarcted LV tissue was measured by a capillary gas chromatographic procedure with a flame ionization detector using C17-ceramide as an internal standard, as previously described 25.
2.7. Statistical analysis
Data are expressed as group means±S.E. Groups were compared by two-way ANOVA followed by Bonferroni t-test for multiple comparisons. For all statistical analysis, significance was accepted at P<0.05.
3. Results
3.1. Body and heart mass
Body mass increased significantly from 8 to 20 weeks in both groups, with no differences between sham and heart failure groups (Table 1). Total LV mass (LV mass+scar tissue mass) was significantly higher in the 20-week groups compared to the 8-week groups, and there were no differences between sham and heart failure groups. The mean scar tissue mass was the same for the 8- and 20-week heart failure groups. The LV mass/body mass ratio was lower in the 20-week sham group compared to the 8-week shams and was higher in the 20-week heart failure group compared to the 20-week shams. There was no difference in the LV mass/body mass ratio between the 8- and 20-week heart failure groups. RV mass and the RV mass/body mass ratio were both significantly higher in the heart failure animals compared to shams at both 8 and 20 weeks (Table 1).
| Sham | Heart failure | |||
|---|---|---|---|---|
| 8 weeks | 20 weeks | 8 weeks | 20 weeks | |
| Body mass (g) | 501±21 | 643±24a | 519±15 | 591±42 |
| Total LV mass (g) | 1.12±0.04 | 1.32±0.04a | 1.14±0.04 | 1.33±0.08b |
| Scar mass (g) | - | - | 0.21±0.02 | 0.21±0.03 |
| Total LV mass/body mass (mg/g) | 2.29±0.10 | 2.06±0.06a | 2.16±0.07 | 2.29±0.10** |
| RV mass (g) | 0.30±0.02 | 0.32±01 | 0.41±0.03a,c | 0.48±0.06c,** |
| RV mass/body mass (mg/g) | 0.60±0.04 | 0.50±0.02 | 0.82±0.06a,c | 0.83±0.12c,** |
- a Values are mean±S.E.M.
- a P<0.05 compared to 8-week sham.
- b P<0.05 compared to 8-week heart failure.
- c P<0.001 compared to sham group.
- ** P<0.05 compared to 20-week sham.
3.2. Cardiac function
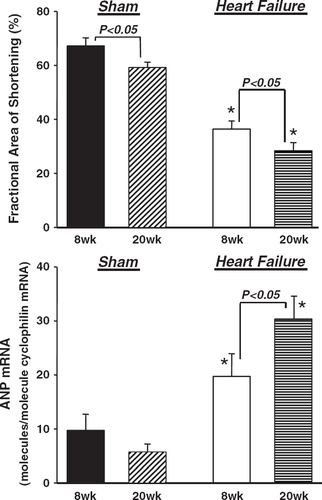
Rats with heart failure had reduced LV fractional area of shortening and greater LV end diastolic and end systolic cross sectional areas than sham animals at both 8 and 20 weeks (Fig. 1, Table 2). There was a significant increase in LV end systolic area and a decrease in fractional area of shortening from 8 to 20 weeks, suggesting a progressive deterioration in LV systolic function (Fig. 1, Table 2). Myocardial performance index and cardiac index were significantly reduced in the heart failure animals compared to sham at 8 and 20 weeks, with no further reduction from 8 to 20 weeks (Table 2).
| Sham | Heart failure | |||
|---|---|---|---|---|
| 8 weeks | 20 weeks | 8 weeks | 20 weeks | |
| Heart rate (beats min−1) | 337±13 | 319±18 | 330±13 | 318±17 |
| Peak LV pressure (mm Hg) | 106±5 | 112±7 | 97±6 | 107±6 |
| Peak LV end diastolic pressure (mm Hg) | 8±1 | 9±1 | 10±1* | 15±3a,* |
| Peak LV +dP/dt (mm Hg s−1) | 6510±380 | 6850±620 | 5150±440* | 5710 ±640* |
| Peak LV −dP/dt (mm Hg s−1) | −4800±370 | −5170±760 | −3890±390 | −4230±460 |
| Tau (ms) | 15±1 | 14±1 | 18±1* | 19±2a,* |
| LV end diastolic area (cm2) | 0.72±0.06 | 0.83±0.08 | 0.98±0.08* | 1.21±0.17a,* |
| LV end systolic area (cm2) | 0.24±0.04 | 0.35±0.05 | 0.63±0.06b,* | 0.90±0.16a,c,* |
| Myocardial performance index | 0.31±0.02 | 0.33±0.03 | 0.66±0.06b,* | 0.60±0.05a,* |
| Cardiac index (ml.min−1.kg−1) | 111±13 | 118±11 | 63±9b,* | 64±8a,* |
- a Values are mean±S.E.M.
- a P<0.05 compared to 20-week sham.
- b P<0.05 compared to 8-week sham.
- c P<0.05 compared to 8-week heart failure.
- * P<0.05 compared to sham group (main effect for treatment).
The LV dysfunction that resulted from coronary ligation was not accompanied by changes in heart rate or peak LV pressure at 8 or 20 weeks (Table 2). LV EDP and Tau were elevated in the heart failure groups at both 8 and 20 weeks. Peak +dP/dt was significantly reduced in the heart failure rats and −dP/dt tended to be reduced but the difference did not reach statistical significance (P=0.064). There were no differences in LV pressure data between the 8- and 20-week heart failure groups.
3.3. mRNA expression
mRNA expression was normalized to the gene cyclophilin, which did not differ between groups (data not shown). ANP was elevated approximately 200% and 580% in the 8- and 20-week heart failure animals, respectively, compared to shams (Fig. 1). There was a significant increase in ANP expression from 8 to 20 weeks in the heart failure groups (Fig. 1).
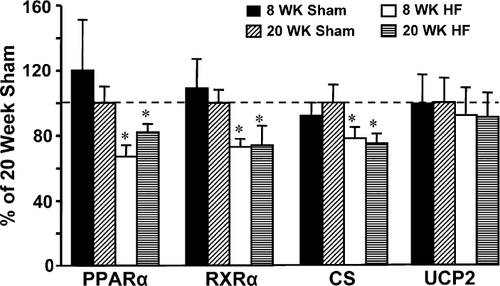
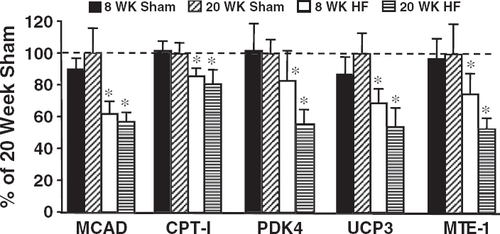
Expression of PPARΡ, RXRΡ, CS and genes that are known to be regulated by PPARΡ (MCAD, CPT-I, PDK-4, UCP3 and MTE-1) were significantly reduced by heart failure, but were not different between the 8- and 20-week time points (Table 3, Figs. 2 and 3). UCP2 gene expression was not altered following infarction.
| Sham | Heart failure | |||
|---|---|---|---|---|
| 8 weeks (n=9) | 20 weeks (n=9) | 8 weeks (n=9) | 20 weeks (n=9) | |
| PPAR-Ρ | 0.234±0.063† | 0.205±0.021 | 0.137±0.014* | 0.159±0.010* |
| RXR-Ρ | 0.293±0.044 | 0.291±0.031 | 0.212±0.016* | 0.206±0.035a,* |
| CS | 1.93±0.17 | 2.21±0.23 | 1.72±0.17* | 1.60±0.13a,* |
| UCP2 | 6.57±0.95 | 7.32±1.07 | 6.75±1.24 | 6.50±1.09 |
| MCAD | 8.26±0.69 | 9.65±1.52 | 5.98±0.77* | 5.48±0.53a,* |
| mCPT I | 1.83±0.08 | 1.90±0.14 | 1.63±0.09* | 1.47111±0.15a,* |
| PDK4 | 2.46±0.44 | 2.51±0.23 | 2.07±0.47* | 1.40±0.19a,* |
| UCP3 | 0.029±0.004 | 0.034±0.005 | 0.024±0.003* | 0.018±0.004a,* |
| MTE1 | 0.792±0.114 | 0.847±0.161 | 0.634±0.111* | 0.450±0.064a,* |
- a Values are mean±S.E.M. †P<0.05 compared to 8-week heart failure.
- a P<0.001 compared to 20-week sham.
- * P<0.05 compared to sham group (main effect for treatment).
3.4. Enzyme activity and protein expression
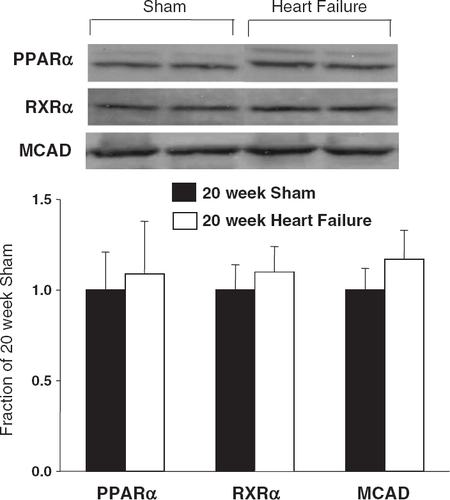
Protein expression for PPAR-Ρ, RXR-Ρ and MCAD was not different among groups (Fig. 4). CS activity was significantly reduced in the heart failure animals compared to shams. In contrast, MCAD activity (either normalized to grams wet weight tissue or normalized to CS activity) was not different between the groups (Table 4). The ~15% reduction in CS activity and the lack of a change in the ratio of MCAD/CS suggests a modest reduction in mitochondrial content with heart failure.
| Sham | Heart failure | |||
|---|---|---|---|---|
| 8 weeks | 20 weeks | 8 weeks | 20 weeks | |
| MCAD (μmol g ww−1 min−1) | 9.26±1.04 | 8.20±0.48 | 7.44±0.49 | 7.78±0.68 |
| CS (μmol g ww−1 min−1) | 135±9 | 130±8 | 117±4* | 116±6* |
| MCAD/CS | 0.067±0.004 | 0.063±0.002 | 0.063±0.003 | 0.066±0.003 |
- a Values are mean±S.E.M.
- * P<0.05 compared to sham (main effect for treatment).
3.5. Metabolite levels
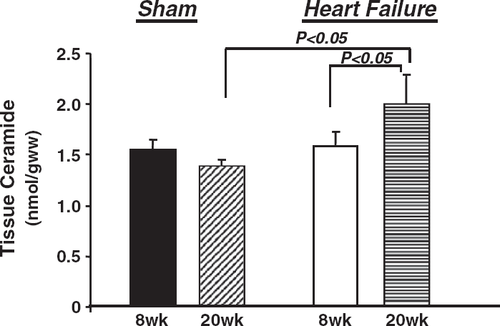
Plasma-free fatty acids were increased in the 20-week animals compared to the 8-week groups, but there were no differences between sham and heart failure groups (Table 5). Plasma triglyceride content did not differ among the groups (Table 5). Tissue levels of C16-ceramide were not elevated in the 8-week heart failure group compared to sham, but were significantly higher in the 20-week heart failure group compared to the 8-week heart failure animals and the 20-week shams (Fig. 5).
| Sham | Heart failure | |||
|---|---|---|---|---|
| 8 weeks | 20 weeks | 8 weeks | 20 weeks | |
| Plasma-free fatty acids (μmol/ml) | 0.30±0.04 | 0.41±0.07* | 0.20±0.02 | 0.33±0.05* |
| Plasma triglycerides (mg/ml) | 0.74±0.05 | 0.93±0.06 | 0.85±0.07 | 0.90±0.08 |
- a Values are mean±S.E.M.
- * P=0.004 compared to 8-week group.
4. Discussion
The present study showed that there were no alterations in PPARΡ, RXRΡ or MCAD protein levels in rats with infarct-induced heart failure. There was a clear down-regulation of the mRNA for the nuclear receptors PPARΡ and RXRΡ, and for PPARΡ regulated genes; however, this was not translated into reduced protein expression. Despite a progressive deterioration in fractional area of shortening and increases in LV end systolic area and ANP mRNA from 8 to 20 weeks post-infarction, there was not a corresponding fall in the expression of genes encoding proteins involved in fatty acid metabolism. A modest but significant ~15% decrease in CS activity at both 8 and 20 weeks post-infarct suggests that there was a reduction in mitochondrial content with heart failure, without a selective down-regulation of fatty acid oxidation. These results are consistent with the concept that down-regulation of fatty acid oxidation enzymes is a late stage occurrence in heart failure.
Previous work in patients 4,5,10 and animals 11,23,26 found reduced mRNA expression for fatty acid oxidation enzymes, PPARΡ and/or RXRΡ in severe late-stage heart failure; however, little is known about the time course of this effect over the progression of failure. In studies utilizing the rat coronary artery ligation model of heart failure, mRNA levels of PPARΡ, CPT-I and of medium chain, long chain and very long chain acyl-CoA dehydrogenase were shown to be reduced at 20 or 24 weeks post-ligation 11,26. The findings of the present study agree well with the previously observed changes in mRNA in late stage heart failure. Furthermore, we find that repression of these genes occurs 8 weeks following the induction of heart failure and is not associated with reduced PPARΡ or RXRΡ protein expression.
In contrast with previous studies in patients and animals with late stage heart failure, the results of this investigation demonstrate that protein levels and activity of MCAD were not reduced despite down-regulation of the mRNA. Sack et al. showed that protein levels of both MCAD and long chain acyl-CoA dehydrogenase are reduced in patients with end stage heart failure 4. Myocardial protein levels of MCAD, RXRΡ (but not PPARΡ) and MCAD activity are reduced in end stage pacing-induced heart failure in dogs 12. Rosenblatt-Velin et al. reported a significant decrease in MCAD protein content in a subset of coronary artery ligated rat hearts isolated 20 weeks after ligation. For inclusion in this group, the rats had to exhibit five signs of severe heart failure (assessed by the Feldman criteria for heart failure); of the 16 rats ligated, only 6 met this requirement 26. In the present study, MCAD protein content and activity were determined from LV tissue taken from all infarcted rat hearts, and this difference in inclusion criteria may explain the disparity between the MCAD protein results of this study and those reported previously.
Elevated myocardial ceramide levels are associated with cardiac dilatation and reduced contractility 141516; however, it is unclear if ceramide contributes to cardiac dysfunction in heart failure. In this study, there was no increase in myocardial C16-ceramide levels 8 weeks after coronary artery ligation, despite significantly reduced LV function and chamber dilation. At 20 weeks post-ligation, however, tissue levels of ceramide increased by 40% in heart failure rats relative to shams (Fig. 4), which corresponded with a significant increase in LV end systolic area and a decrease in fractional area of shortening from 8 to 20 weeks post-infarction (Fig. 1). While it is intriguing to speculate that greater ceramide accumulation may contribute to progressive cardiac dysfunction in heart failure, a causal link remains to be demonstrated.
In summary, there is mRNA down-regulation of PPARΡ regulated genes in rats with infarct-induced heart failure despite no decrease in the protein expression of PPARΡ or RXRΡ. Although there was a progressive increase in LV end systolic area and ANP mRNA expression and deterioration in fractional area of shortening, there were no differences in mRNA levels for metabolic proteins between 8 and 20 weeks post-infarction. In addition, MCAD protein levels and activity were not altered despite down-regulation of MCAD mRNA. Thus, reduced mRNA expression of PPARΡ/RXRΡ regulated fatty acid oxidation enzymes is not dependent on reduced protein expression of PPARΡ and/or RXRΡ.
5 Acknowledgements
This work was supported by National Heart, Lung and Blood Institute grants HL-74237 (WCS) and HL-074259 (MEY), and the Diabetes Association of Greater Cleveland (MPC). Eric E. Morgan was supported by a Predoctoral Fellowship from the American Heart Association (Ohio Valley Chapter). The authors thank David J. Durgan and Margaret A. Hotze (University of Texas Health Science Center at Houston) for their assistance with the mRNA isolation and real-time quantitative-PCR procedures and Dr. Timothy Musch (Kansas State University) for assistance and advice in the development of the rat infarction model.




