Clinical profile, contemporary management and one-year mortality in patients with severe acute heart failure syndromes: The EFICA study☆
Abstract
Background:
Little is known about the epidemiology of acute decompensated heart failure (ADHF) in patients admitted to intensive and coronary care units (ICU/CCU). Observational data may improve disease management and guide the design of clinical trials.
Aims:
EFICA is an observational study of the clinical profile, management and survival of ADHF patients admitted to ICU/CCU.
Methods:
The study included 599 patients admitted to 60 ICU/CCUs across France. Relevant data was recorded during hospitalisation. Survival was assessed at 4 weeks and 1 year.
Results:
The main cause of ADHF was ischaemic heart disease (61%); 29% of patients had cardiogenic shock. Mortality was 27.4% at 4 weeks and 46.5% at 1 year, increasing to 43.2% and 62.5%, respectively, when including pre-admission deaths. Shock patients had the highest [57.8% vs. 15.2% without shock (p<0.001)] and patients with hypertension and pulmonary oedema had the lowest 4-week mortality: (7%). Pre-admission NYHA class III–IV heart failure, not initial clinical presentation, influenced 1-year mortality.
Conclusion:
ADHF is a heterogeneous syndrome. Based on initial clinical presentation, three entities with distinct features and outcome may be described: cardiogenic shock, pulmonary oedema with hypertension, and ‘decompensated’ chronic heart failure. This should be taken into account in future observational studies, guidelines and clinical trials.
1. Introduction
Each year, heart failure accounts for 6.5 million days spent in hospital in the US 1 and 1.4 million in France 2. This is nearly twice the number of hospital days attributed to admissions for acute myocardial infarction 1,2. Among patients admitted with the general diagnosis of heart failure, those presenting to the emergency department with acute and/or decompensated heart failure (ADHF) are often haemodynamically unstable, with disabling and life-threatening symptoms. Following evaluation and stabilization in the emergency department, most patients will usually require admission into intensive care units (ICU) or coronary care units (CCU).
Unlike chronic heart failure (CHF), for which a wealth of information is available, specific epidemiological data on ADHF are currently lacking. Indeed, available data are non-specific, and usually derived from hospital discharge records corresponding to the general heart failure code [ICM 184] 345 as well as from a limited number of recent large clinical trials in patients with worsening heart failure 6789.
ADHF encompasses a broad spectrum of conditions, and although the terms ‘acute’ and ‘decompensated’ heart failure are used interchangeably, these conditions are actually different. Conditions may also differ with regard to the initial clinical presentation, heart failure history, aetiology, pathophysiology, and subsequent disease outcome. This heterogeneity and the confusing nomenclature employed in describing this syndrome, together with the scarcity of appropriate epidemiological data, have contributed to the lack of progress made in developing new therapies and in agreeing on management guidelines for this patient population. Accordingly, the syndrome remains poorly defined and poorly understood.
To answer some of these questions, we designed the EFICA (Etude Française de l'Insuffisance Cardiaque Aiguë) study, a 1-year observational follow-up study of severe ADHF patients admitted to a representative sample of 60 French ICU/CCUs. Our aims were to investigate the clinical and aetiologic features of severe ADHF, to measure disease impact in terms of length of hospital stay, short- and long-term mortality rates, and to evaluate determinants of short- and long-term survival. Since ADHF in the setting of acute coronary syndromes has unique pathophysiology and has been the subject of a number of recent observational and interventional studies, we decided not to consider this category in the present study.
2. Methods
2.1. Hospitals and patients
Participating centres were randomly selected from a national registry of adult ICUs and CCUs (about 500) in public, semi-private and private hospitals in France, commonly admitting severe ADHF patients 10. Centres which declined to participate were replaced using an ad hoc procedure of re-sampling. The size and medical activity of centres varied significantly, depending on their location and the size of population in their respective geographical area. Therefore the contribution of centres to the patient population of the present study varied accordingly.
From April to October 2001, participating centres enrolled consecutive patients meeting the following criteria: aged ≥18 years, and admitted to an ICU or CCU following initial mobile care unit transportation and/or emergency department admission, with signs and/or symptoms compatible with the diagnosis of ADHF according to the opinion of the attending investigator. Patients admitted directly to a general ward were excluded to help ensure selection of patients with severe forms of ADHF.
2.2. Data collection and follow-up
Data was collected from medical records by the investigators using a structured case record form. Patient demographics, cardiovascular risk factors, comorbidities, history of cardiac disease, heart failure and surgical intervention as well as results of clinical, ECG, haemodynamic, X-ray, echocardiographic and laboratory tests were collected upon admission to the ICU/CCU. Data on the aetiology and the main precipitating cause of ADHF, drug prescriptions before and following admission, as well as data on concurrent medication and main therapeutic measures performed during hospitalisation in the ICU/CCU were collected from medical records upon discharge. Given the very poor prognosis associated with cardiogenic shock, clinical presentation of patients was categorized according to the presence of cardiogenic shock by the primary investigator. Data concerning length of ICU/CCU stay and total hospital stay, and mortality at 4 weeks and at 1 year were obtained by phoning patients and carers and liaising with the patient's physicians.
2.3. Steering committee
All CRFs were reviewed for ADHF diagnosis, clinical presentation and aetiology by a steering committee composed of five senior cardiologists, two senior intensive care specialists and three epidemiologists. No specific definition was used. Adjudication was based on a consensus between the steering committee members. If an agreement was not reached, cases were classified as “unknown”.
2.4. Emergency department registries
In order to estimate total in-hospital ADHF mortality, patients with ADHF diagnosis who died before ICU/CCU admission were registered in 34 emergency departments among the 60 participating centres. These 34 centres agreed voluntarily to record data on all patients referred to and admitted in these centres. Characteristics of ADHF patients (sex, age, cardiovascular history and risk factors, ADHF aetiology and precipitating factors, symptoms and clinical signs on admission) included in these 34 registry centres were not statistically different from those of patients included in the 26 other centres.
2.5. Sample size and statistical analysis
Based on the assumption of 50% mortality at 1 year, we needed to enrol approximately 600 patients to achieve a 4% level of precision of proportions within the 95% confidence interval (CI). This was also expected to provide adequate statistical power for multivariate regression analyses.
Mortality risk was estimated using Kaplan-Meier method. Survival analysis was performed using the logistic model for 4-week mortality and the Cox proportional hazard model for 1-year mortality. Variables with a univariate p value below 0.10 were included in an ascending stepwise model. Continuous variables were assessed using the quartile distribution and a dichotomous distribution with a clinical cut-off. Patients with missing values were excluded. For the variables included in the final model, all interactions between two variables were studied. Analyses were performed using SAS© software version 8.2. A p value<0.05 was considered statistically significant.
2.6. Ethical and regulatory considerations
Data were anonymised, stored electronically and analysed. National regulatory boards (National Scientific Committee for Data Processing and Freedom and National Medicine Council) approved the database format and the anonymisation procedure.
2.7. Role of the funding source
Orion Laboratories provided financial support for steering committee meetings. Although Orion personnel were present as observers during steering committee meetings, data entry and analyses were done entirely independently of the company. The steering committee acted independently in planning and designing the study, and developing the study report and manuscript.
3. Results
3.1. Hospitals and patients
The 60 participating centres consisted of 26 ICUs and 34 CCUs from 18 university hospitals, 31 general hospitals and 11 semi-private and private hospitals.
From April to October 2001, 599 patients with complete records were enrolled, of which 18 were excluded from analysis after adjudication by the steering committee (for either shock of non-cardiogenic origin or acute myocardial infarction with no ADHF). Baseline characteristics for the remaining 581 patients are summarised in Table 1.
| All patients | Cardiogenic shock | pa | ||
|---|---|---|---|---|
| (n=581) | Yes (n=166) | No (n=415) | ||
| Sex (%, M/F) | 59/41 | 66/34 | 56/44 | 0.02 |
| Age (years, mean [SD]) | 73 [13] | 70 [14] | 73 [12] | 0.01 |
| CCU/ICU (%) | 51/49 | 31/69 | 59/41 | 0.00 |
| Cardiovascular risk factors (%) | ||||
| None | 11 | 15 | 9 | 0.05 |
| Hypertension | 60 | 53 | 62 | 0.04 |
| Hypercholesterolaemia | 30 | 28 | 32 | 0.36 |
| Diabetes | 27 | 26 | 27 | 0.95 |
| Obesity | 22 | 19 | 23 | 0.27 |
| Smoking | 34 | 39 | 33 | 0.19 |
| Alcoholism | 12 | 17 | 10 | 0.02 |
| Cardiovascular history (%) | ||||
| None | 19 | 28 | 16 | 0.001 |
| IHD | 46 | 42 | 48 | 0.18 |
| Myocardial infarction | 22 | 19 | 23 | 0.28 |
| Non-IHD† | 46 | 46 | 45 | 0.85 |
| Cardiac surgery and/or PCI | 33 | 29 | 35 | 0.15 |
| History of CHF‡ (%) | 0.003 | |||
| None | 34 | 42 | 30 | |
| NYHA class I-II | 36 | 24 | 40 | |
| NYHA class III | 22 | 24 | 21 | |
| NYHA class IV | 9 | 10 | 9 | |
| History of hospitalisation for AHF (%) | 35 | 37 | 35 | 0.65 |
| Chronic comorbidity§ (%) | 51 | 52 | 50 | 0.58 |
- † Non-ischaemic heart disease including valvulopathy and/or lower limb arteritis and/or stroke;
- ‡ Severity of CHF within the last month before the index hospitalization;
- § Chronic obstructive pulmonary disease, renal failure (serum creatinine >200 μmol/L), malignancy, cirrhosis/liver dysfunction, type I diabetes, immunosuppression.
- d M, male; F, female; SD, standard deviation; CCU, coronary care unit; ICU, intensive care unit; IHD, ischaemic heart disease; PCI, percutaneous coronary intervention; CHF, chronic heart failure.
- a Cardiogenic shock vs. no cardiogenic shock.
The mean age of the study population was 73 years (SD 13), and 59% were male. Hypertension was the main cardiovascular risk factor (60%). An equal percentage of participants presented with a history of either ischaemic heart disease (IHD) or non-ischaemic heart disease (e.g. valvulopathy, lower limb arteritis, stroke). The majority of study participants had a history of either no or mild (NYHA class I-II) CHF (34% and 36%, respectively). Half (51%) of the population presented with a chronic comorbid condition, including chronic obstructive pulmonary disease (COPD), renal failure, malignancy, cirrhosis, type I diabetes and immunosuppression.
A high proportion of participants presented with pulmonary oedema (82%), and a total of 29% showed signs of cardiogenic shock (Table 2). The most common precipitating factor for patients with cardiogenic shock, and for those without, was ischaemia (Table 2).
| All patients | Cardiogenic shock | pa | ||
|---|---|---|---|---|
| (n=581) | Yes (n=166) | No (n=415) | ||
| Aetiology of ADHF (%) | ||||
| IHD | 61 | 64 | 59 | 0.31 |
| Dilated cardiomyopathy | 15 | 19 | 14 | 0.09 |
| Hypertensive and/or hypertrophic cardiomyopathy | 15 | 10 | 18 | 0.01 |
| Valvular heart disease | 21 | 17 | 23 | 0.17 |
| Other aetiology | 4 | 5 | 4 | 0.66 |
| More than one aetiology | 17 | 15 | 17 | 0.92 |
| Unknown | 0.2 | 0.6 | 0 | 0.29 |
| Precipitating factors (%) | ||||
| Ischaemia | 42 | 49 | 39 | 0.02 |
| Arrhythmias | 25 | 26 | 24 | 0.50 |
| Infection | 20 | 22 | 19 | 0.47 |
| Hypertensive crisis | 8 | 2 | 10 | 0.003 |
| Drug compliance | 7 | 5 | 8 | 0.21 |
| Other† | 25 | 35 | 21 | 0.001 |
| None | 8 | 4 | 9 | 0.04 |
| Symptoms on admission (%) | ||||
| Cardiogenic shock | 29 | 100 | 0 | <0.0001 |
| Pulmonary oedema | 82 | 60 | 91 | <0.0001 |
| Peripheral oedema | 27 | 20 | 30 | 0.02 |
| Angina | 14 | 17 | 13 | 0.29 |
| Hepatomegaly | 20 | 24 | 18 | 0.09 |
| Syncope | 4 | 9 | 2 | 0.0002 |
| Arrhythmia | 23 | 26 | 21 | 0.17 |
| Stroke | 1 | 1 | 1 | 1.00 |
| Clinical signs | ||||
| SBP (mmHg, mean [SD]) | 126 [39] | 93 [30] | 139 [34] | <0.0001 |
| DBP (mmHg, mean [SD]) | 71 [22] | 54 [19] | 77 [20] | <0.0001 |
| X-ray pulmonary congestion (%) | 92 | 83 | 95 | <0.0001 |
| ECG on admission (%) | ||||
| Normal | 13 | 8 | 14 | 0.05 |
| Ischaemia | 29 | 39 | 25 | 0.001 |
| Atrial fibrillation | 25 | 26 | 25 | 0.83 |
| LV hypertrophy | 13 | 10 | 14 | 0.12 |
| Ventricular tachycardia | 2 | 2 | 2 | 1.00 |
| Left bundle branch block | 17 | 19 | 17 | 0.45 |
| Other arrhythmias | 12 | 13 | 11 | 0.46 |
| Other conduction abnormalities | 16 | 18 | 16 | 0.48 |
| Echocardiography (%) | ||||
| Available | 76 | 66 | 80 | 0.001 |
| LVEF (%, mean (SD)) | 38 (15) | 34 (16) | 40 (15) | 0.0001 |
| LVEF >45% | 27 | 18 | 30 | 0.03 |
| Laboratory tests (%) | ||||
| Hyponatraemia | 29 | 39 | 25 | 0.005 |
| Abnormal serum K+ | 33 | 45 | 28 | <0.0001 |
| Creatinine (>200 μmol/L) | 53 | 71 | 45 | <0.0001 |
| Liver dysfunction | 61 | 83 | 51 | <0.0001 |
| Biological marker of cardiac injury (%) | ||||
| At least one available‡ | 87 | 89 | 87 | 0.98 |
| At least one raised | 42 | 56 | 36 | <0.0001 |
- † Endocarditis, drug adverse effect, COPD decompensation, pulmonary embolism, anaemia, exacerbation of renal failure, surgery (<5% each);
- ‡ troponin T, troponin I, MB-CPK.
- c ADHF, acute/decompensated heart failure; IHD, ischaemic heart disease; SBP, systolic blood pressure; SD, standard deviation; LV, left ventricular; LVEF, left ventricular ejection fraction. Hyponatraemia: Serum sodium <130 mmol/L, Dyskalaemia: serum potassium >5.5 mmol/L or <3.5 mmol/L.
- a Cardiogenic shock vs. no cardiogenic shock.
Only a small proportion of participants (12%) was not taking medication during the month prior to hospital admission (Table 3). In the ICU/CCU, in accordance with the patient's condition, ventilatory support was provided in 59% of cases (invasive ventilation, 35%; non-invasive positive pressure ventilation 24%). Central venous catheterization was performed in 33% of cases and catecholamines administered in 53% of cases.
| Within 1 month before admission (n=581) (%) | During ICU/CCU hospitalisation (n=581) (%) | On discharge from ICU/CCU (n=452) (%) | |
|---|---|---|---|
| None | 12 | 1 | 0 |
| ACE inhibitor | 42 | 42 | 57 |
| Angiotensin II antagonist | 9 | 3 | 3 |
| Beta-blocker | 26 | 13 | 21 |
| Digitalis | 19 | 16 | 17 |
| Loop diuretic | 52 | 87 | 79 |
| Thiazide diuretic | 8 | 3 | 3 |
| Spironolactone | 14 | 18 | 19 |
| Other K+-sparing diuretic | 1 | 1 | 7 |
| Nitrates | 33 | 50 | 35 |
| Dihydropyridine calcium antagonist | 15 | 6 | 9 |
| Non-dihydropyridine calcium antagonist | 9 | 4 | 5 |
| Aspirin/antiplatelet agent | 35 | 43 | 45 |
| Low molecular heparin | 6 | 38 | 35 |
| Unfractionated heparin | 5 | 43 | 27 |
| Oral anticoagulant | 21 | 12 | 16 |
| Amiodarone | 21 | 27 | 27 |
| Class I antiarrhythmic | 2 | 2 | 0 |
| Statin | 16 | 13 | 18 |
| NSAID | 2 | 0 | 0 |
| Corticosteroid | 3 | 6 | 3 |
3.2. Length of stay
Total length of stay (LOS) was similar in patients both with and without cardiogenic shock (15.1 days [SD 23.8] vs. 14.5 days [SD 15.1], p=0.71). Length of stay in ICU/CCU was slightly longer in patients suffering from cardiogenic shock than those without this complication (7.6 [SD 10.2] days vs. 5.7 days [SD 9.4], p=0.04).
3.3. Mortality
The mortality follow-up was obtained in 100% of patients at 4 weeks and 99% of patients at 1 year. Four-week mortality was 27.4% and the 1-year mortality was 46.5%. An additional 93 deaths related to ADHF were notified by the 34 emergency department registries as deaths that occurred before ICU/CCU admission. These 93 patients were slightly older than the study population (77 years [SD 13] vs. 72 years [SD 13], p=0.12), but had similar sex and ADHF aetiology profiles. When including these deaths, the estimated total 4-week and 1-year mortality rates rose to 43.2% and 62.5%, respectively.
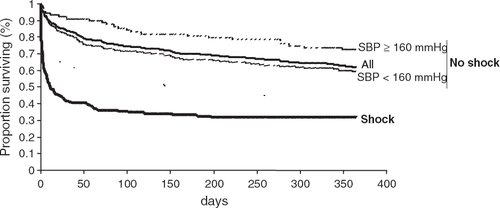
Patients with cardiogenic shock were more likely to die during the first 4 weeks after admission than those without cardiogenic shock (57.8% vs. 15.2%, p<0.0001), and within the first year after admission (68.2% vs. 37.9%, p<0.0001) (Fig. 1). However, if 1-year mortality was calculated solely from those patients alive after 4 weeks, then mortality was similar for both cardiogenic shock and no cardiogenic shock patients (24.6% vs. 26.7%, p=0.88).
3.4. Determinants of mortality
Stepwise multiple regression analysis included all the variables listed in Tables 12–3. Independent variables associated with 4-week mortality for patients with cardiogenic shock and without cardiogenic shock are summarised in Table 4.
| OR | (95% CI) | p | |
|---|---|---|---|
| Cardiogenic shock group (n=166) | |||
| Age 65–74 vs. <65 (years) | 4.17 | (1.55–11.8) | 0.04 |
| Arrhythmia | 3.06 | (1.28–7.85) | 0.02 |
| Comorbidity | 2.56 | (1.21–5.60) | 0.02 |
| Smoking | 0.30 | (0.13–0.66) | 0.003 |
| LVH signs on ECG | 0.15 | (0.03–0.59) | 0.01 |
| Hypertension and/or hypertrophic cardiomyopathy | 0.06 | (0.01–0.23) | 0.0003 |
| No cardiogenic shock group (n=392†) | |||
| Renal dysfunction‡ | 3.25 | (1.69–6.44) | 0.0005 |
| Angina and/or ECG ischaemia and/or raised cardiac enzymes | 2.47 | (1.29–4.78) | 0.006 |
| Liver dysfunction | 2.11 | (1.09–4.21) | 0.03 |
| History of previous ADHF episode | 1.94 | (1.01–3.73) | 0.05 |
| Chronic comorbidity | 1.87 | (0.99–3.56) | 0.05 |
| SBP≥160 vs. <160 (mmHg) | 0.40 | (0.14–0.97) | 0.05 |
| Pulmonary oedema | 0.33 | (0.14–0.78) | 0.01 |
- a LVH, left ventricular hypertrophy; ECG, electrocardiogram.
- † 23 missing values;
- ‡ History of chronic renal failure and/or raised creatinine on admission.
- d SBP, systolic blood pressure; ECG, electrocardiogram; ADHF, acute/decompensated heart failure.
For the cardiogenic shock group, risk factors included age 65–74 years, arrhythmia and comorbidity. Variables associated with a protective effect included smoking, LVH signs on ECG, and hypertension and/or hypertrophic cardiomyopathy.
For the group without cardiogenic shock (Table 4), risk factors included renal dysfunction, angina and/or ECG ischaemia and/or raised cardiac enzymes, liver dysfunction, a history of ADHF and chronic comorbidity. Variables associated with a protective effect included pulmonary oedema and SBP≥160 mmHg. LV systolic function, at least assessed by LVEF, did not independently influence short-term outcome.
Patients without cardiogenic shock were further categorized into two subgroups according to systolic blood pressure (SBP) on admission either above or below the arbitrary cut-off value of 160 mmHg. Patients with SBP≥160 mmHg on admission (n=90) had a lower 4-week mortality rate than patients with SBP<160 mmHg on admission (n=325) (7% vs. 17%, p=0.03), despite a tendency to be older (75 years [SD 12] vs. 73 years [SD 12], p=0.09). Of note, patients with SBP≥160 mmHg on admission were more likely to have a history of hypertension, ECG signs of LV hypertrophy and LVEF >45% compared to patients with SBP<160 mmHg on admission (all p<0.05). Kaplan-Meier survival curves for the two subgroups of patients without cardiogenic shock with SBP ≥ or <160 mmHg on admission are presented in Fig. 1.
As 29–365-day mortality did not differ between patients with and without cardiogenic shock, an analysis was performed in all patients alive at 4 weeks (n=418) to determine variables associated with mortality at 1 year. Variables independently associated with 1-year mortality included history of CHF (vs. no CHF, relative risk (RR) 1.96 [95% CI 1.17–3.27], p=0.01). Mortality rates (29–365 days) were 19.3% (95% CI 12.6–26.0) in 141 patients without a history of CHF; 23.3% (95% CI 16.6–30.1) in 166 patients previously diagnosed with CHF class NYHA I-II; and 38.6% (95% CI 19.8–47.5) in 111 patients previously diagnosed with CHF class NYHA III-IV.
Other determinants of mortality at one year in patients alive at 4 weeks included hyponatraemia (RR 1.85 [95% CI 1.22–2.82], p=0.004), malignancy (RR 2.31 [95% CI 1.35–3.96], p=0.002), renal dysfunction (RR 1.84 [95% CI 1.21–2.80], p=0.005), arteritis (RR 1.76 [95% CI 1.03–2.98], p=0.04) and smoking (RR 0.55 [95% CI 0.34–0.9], p=0.02).
4. Discussion
EFICA is the first multicentre observational epidemiological study of ADHF patients admitted to ICUs and/or CCUs, with 1-year follow-up data. The results confirm that ADHF is a heterogeneous clinical syndrome that carries a poor prognosis. More importantly, our study demonstrated that 4-week mortality was strongly influenced by initial clinical presentation. However, after the acute phase, the presence of pre-admission CHF, but not the initial clinical presentation, contributed most to 1-year mortality in patients alive after 4 weeks.
4.1. Short- and long-term mortality
Little information is available on short-term mortality in patients admitted with ADHF. In studies of hospitalised patients with worsening CHF, 4-week or in-hospital mortality was consistently below 20% 1,11,12. Other data are derived from registries or clinical trials in patients with acute coronary syndrome and acute heart failure 1,13. In such patients, mortality varied substantially according to the clinical presentation, i.e. with cardiogenic shock 14151617181920 or without cardiogenic shock 212223.
In our study, 4-week mortality was 27.4%, which is higher than previously reported. This is almost certainly because we included only patients with severe ADHF who all needed admission to cardiac or general ICUs, and the majority required ventilatory support and catecholamine administration. An additional estimate of 4-week mortality, accounting for patients with ADHF who died before they could be admitted to the ICU/CCU, provided the worrying figure of 43.4%.
Previously published studies included patients with ADHF admitted to general or cardiology wards and not necessarily in ICUs 24. This may explain the substantially lower mortality rate reported by the EuroHeart Failure Survey (13% mortality at 12 weeks) 24,25, which presents a far more optimistic picture compared with our own results. An important feature of our study is that it recruited consecutive unselected patients in the most acute situation, regardless of underlying disease and clinical presentation. One-year mortality rate in the study population was 46.5%, rising to 62.5% when additional pre-admission deaths were included. ADHF was associated with a prolonged hospitalisation period, longer than those related to acute myocardial infarction in French Hospitals 2.
4.2. Heterogeneity of ADHF and determinants of mortality
Four-week mortality was strongly influenced by initial clinical presentation. The presence of cardiogenic shock contributed most to mortality at 57.8% compared to 15.2% for patients without cardiogenic shock. Among patients without cardiogenic shock, those presenting with SBP over 160 mmHg and almost always pulmonary oedema had a relatively better short-term prognosis. Although LV systolic function did not independently influence short-term outcome, the presence of pre-admission CHF or comorbidities such as arteritis, malignancy and renal failure markedly influenced 1-year mortality in patients alive after 4 weeks.
ADHF cannot be regarded as one homogeneous entity.
In previous studies, authors have attempted to stratify patients according to pathophysiology, based on clinical signs and symptoms present upon admission. Our study allowed stratification of patients with ADHF into three categories according to clinical presentation upon admission 1 patients with cardiogenic shock 2, patients with high blood pressure (SBP≥160 mmHg) and pulmonary oedema without cardiogenic shock; and 3 patients with ‘normal’ blood pressure (SBP<160 mmHg) and no cardiogenic shock. These three categories are easily recognizable based on clinical signs and symptoms, and have dramatically different short-term prognoses.
4.2.1. Patients with cardiogenic shock
Cardiogenic shock was present in 29% of ADHF patients admitted to ICU or CCU with 27.4% mortality at 4 weeks. Except for studies of heart failure complicating acute myocardial infarction 14,15,16,17, this subgroup is usually disregarded in epidemiological studies of ADHF. In the present study, ischaemic heart disease was the predominant aetiologic factor in ADHF patients, featuring equally in both cardiogenic shock and no cardiogenic shock ADHF groups (64% vs. 59%, p=0.31). This demonstrates that cardiogenic shock does not result exclusively as a complication of cardiac ischaemia. However, although the cardiogenic shock subgroup carried the worst prognosis in our study, this rate is lower than that previously reported in patients with acute coronary syndromes with shock, which was around 65% at 30 days 19.
4.2.2. Patients with high blood pressure and pulmonary oedema and without cardiogenic shock
The second subgroup was present in 15% of ADHF patients with 7% mortality at 4 weeks. Other authors have described the condition of ADHF combined with pulmonary oedema and hypertension, but have not reported on its frequency or outcome 27,28. In our study, this profile was associated with the lowest 4-week mortality.
Of interest, a history of hypertension, high BP upon admission and history and/or ECG signs of LVH were also associated with reduced mortality in the cardiogenic shock group. Therefore, this clinical entity, combining hypertension and pulmonary oedema upon admission, clearly carried the best prognosis among all forms of ADHF. This is most likely related to the higher prevalence of preserved LV systolic function when SBP is high.
4.2.3. Patients with ‘normal’ blood pressure (SBP<160 mmHg) and without cardiogenic shock
This third subgroup was present in 56% of the total patient group with 17% mortality at 4 weeks. ADHF in these patients was frequently associated with ischaemic heart disease and poor ventricular function. This probably equates to the patients classed as ADHF patients in previous observational studies such as the French Survey on heart failure 4 or the EuroHeart Failure Survey 25. We suggest that this subgroup should be described as having ‘decompensated’ heart failure. In our study, this condition is the most common, accounting for more than half of the patients admitted with ADHF. Although it is less severe than cardiogenic shock, in our study it was still associated with a high mortality at 4 weeks.
Despite being the most powerful determinant of short-term mortality, initial clinical cardiovascular presentation had no influence on long-term (29–365 days) mortality. Mortality, after the acute phase, in patients who survived the first 4 weeks, was similar whether patients were admitted with shock, with hypertension or with worsening heart failure.
4.3. Other determinants of mortality
Myocardial ischaemia and a history of hospitalisation for ADHF were both independently associated with a higher mortality in our patients without cardiogenic shock. This is in accordance with other CHF studies showing that mortality is consistently higher in patients with ischaemic aetiology than in patients with non-ischaemic aetiology 12,29,30. However, as stated above, compared to other CHF studies, history of CHF had no independent influence on short-term mortality in our study. This might be related to the sizeable proportion of patients admitted with ADHF and preserved ventricular function. By contrast, after the acute phase, the presence of NYHA class III-IV symptoms within the month before admission was associated with a doubling of long-term mortality from 19.3% to 38.6% in patients alive at 4 weeks.
Similar to studies of patients with CHF, observational studies of ADHF indicate that patients are generally elderly, and older than patients hospitalised for acute myocardial infarction 17,19 or other acute disease states such as septic shock 31. Consistent with the findings of studies in patients with shock and CHF 32, the present study confirms the negative influence of aging on outcome, at least in patients with cardiogenic shock.
In patients surviving the presenting episode of ADHF, factors associated with subsequent mortality were consistent with those described in previously published findings of studies in hospitalized CHF patients 11,25,33. Most notably, renal dysfunction was associated with an increased risk of death. Consequently, preservation of kidney function in the treatment of ADHF may be an important issue to address 34.
4.4. Management of patients
Guidelines for the management of ADHF became available only recently and were published after this study was performed 35. So far, no report has described the contemporary management of patients with ADHF. The EFICA study provides important information on the current management of ADHF in representative CCUs/ICUs in a developed country. This information is expected to contribute to the design of future clinical trials and the development of guidelines for managing patients with ADHF. For instance, the use of catecholamines in this study is surprisingly high despite accumulating evidence of their questionable effects on outcome 36.
4.5. Study limitations
Our study selected 60 centres that had an ICU or CCU connected to an emergency department. Because we aimed to enrol patients with life-threatening symptoms that required rapid intervention, we included only patients admitted to ICU/CCUs. This excluded patients admitted to general wards, whose clinical profiles, management and outcome may be conceivably different.
5. Conclusion
Our data confirm that ADHF is a heterogeneous syndrome, and that initial clinical presentation was the most powerful determinant of short-term management and outcome. Specifically, we describe three distinct entities: cardiogenic shock, pulmonary oedema with hypertension, and ‘decompensated’ heart failure. Although characterised by distinct degrees of severity, all three ADHF syndromes were associated with a very poor prognosis and led to long hospital stays. Previous reports restricted to patients admitted to general wards and cardiology departments seriously underestimate the mortality rate in patients with severe ADHF admitted to emergency departments, intensive care and coronary care units.
So far, very little progress has been made in developing new, effective therapies and implementing management guidelines in this patient population. These findings highlight the need for further research in these areas. Future clinical trial endpoints should include early mortality and hospital LOS. Our study provides detailed data, critical for the design of such trials.
6 Acknowledgements
We thank the study investigators for their contribution to the study and Orion for funding this study. Members of the steering committee all received honoraria from Orion for their involvement in the project. A. Mebazaa and A. Cohen_Solal are respectively chairman and member of the steering committee for a randomised controlled trial comparing levosimendan and dobutamine in patients with acute heart failure.
Contributors: F. Zannad, A. Mebazaa, Y. Juillère, A. Cohen_Solal, L. Guize, L. Paolozzi, P. Rougé, K. Samii, P. Blin and M. Desnos were responsible for the design, preparation and execution of the study. M.H. Barlet and C. Vincent were responsible for data management and P. Blin and F. Alla for statistical analyses. F. Zannad, A. Mebazaa, Y. Juillière and F. Alla prepared the draft report and all co_authors contributed to its revision.
Study investigators: M.C. Aumont (Hôpital Bichat Claude Bernard, Paris), N. Baille (Hôpital Sainte Blandine, Metz), M. Barboteu (Centre Médical Cardiologique, Evecqmont), D. Belletante (Centre Hospitalier Germon et Gauthier, Beuvry), M. Benhamou (Clinique des Hauts de Seine, Chatenay Malabry), F. Bernard H.I.A. du Val de Grâce, Paris), C. Beuscart (Hôpital Yves Le Foll, Saint Brieux), P.E. Bollaert (Hôpital Central, Nancy), J.F. Camilleri (Centre Hospitalier, Draguignan), A. Carasse (CHI des Portes de L’Oise, Beaumont sur Oise), Y. Castaing (Hôpital Pellegrin, Bordeaux), J.P. Colin (Centre Hospitalier, Dourdan), C. Crombe (Hôpital Joseph Inbert, Arles), B. de Breyne (Hôpital Cardiologique Louis Pradel, Bron), N. Djilali (Centre Hospitalier, Vendome), J. Dolo (Centre Hospitalier Général, Abbeville), D. Dubois (Centre Hospitalier, Arras), M. Ducoudre (Polyclinique Aguilera, Biarritz), J. Durand Gasselin (Hôpital de Font Pré, Toulon), H. Faure (Centre Hospitalier Victor Dupuy, Argenteuil), M. Feissel (Centre Hospitalier Belfort Montbelliard, Belfort), J.P. Fosse (Hôpital Avicenne, Bobigny), M. Galinier (Hôpital Rangueil, Toulouse), H. Garnier (Centre Hospitalier des Pays de Morlaix, Morlaix), H. Gayet (Centre Hospitalier Général, Abbeville), N. Ghanem (CHI Eubonne Montmorency, Montmorency), E. Glaser (Hôpital Départemental Stell, Rueil Malmaison), G. Grollier (Hôpital Côte de Nacre, Caen), V. Haddad (Clinique La Casamance, Aubagne), J.M. Joussen (Clinique MGEN, Maison Laffitte), P. Kalfon (Hôpital des Diaconesses, Paris), R. Kawatli (Centre Hospitalier des Courses, Maison Laffitte), G. Laplatte (CHG Louis Pasteur, Colmar), S. Lasserre (Hôpital de la Croix Rousse, Lyon), Q.V. Le (Centre Hospitalier William Morey, Chalon sur Saone), H. Le Marec (Hôpital Nord Laennec, Nantes), F. Lemesle (CHAM, Amilly Montargis), A. Leon (Hôpital Robert Debré, Reims), O. Leroy (Hôpital Guy Chatiliez, Tourcoing), J. Lipiecki (Hôpital Gabriel Montpied, Clermond Ferrand), C. Louis (CHG Paul Morel, Vesoul), J.P. Martelli (CHI du Bassin de Thau, Sete), A. Merouani (Centre Hospitalier, Alençon), Y. Meurisse (Hôpital Trousseau, Tours), H.O. Michel (Hôpital Jacques Coeur, Bourges), R. Mossaz (Centre Hospitalier Intercommunal, Frejus), D. Mouhoud (Centre Hospitalier Départemental Les Oudairies, La Roche sur Yon), A. Moumen (Centre Hospitalier Nord Mayenne, Mayenne), P. Noel (Centre Hospitalier Le Bon, Beaune), C. Prost (Centre Hospitalier Lucien Hussel, Vienne), J. C. Quiret (Hôpital Sud, Amiens), D. Rafier (Centre Hospitalier Général, Auch), V. Raymond (Hôpital Saint Antoine, Paris), G. Roualt (Hôpital Ponchaillou, Rennes), B. Stimesse (Clinique Mutualiste, Saint Etienne), P. Sultan (Centre Hospitalier Maréchal Joffre, Nemours), J.P. Usdin (American Hospital of Paris, Neuilly sur Seine), M. Vercel (Centre Hospitalier Alphonse Guérin, Ploermel), P. Vignon (Hôpital Dupuytren, Limoges), R. Zelinski (Clinique Saint Sauveur, Mulhouse).




