p38 MAP-kinase in cultured adult rat ventricular cardiomyocytes: expression and involvement in hypertrophic signalling
Abstract
Both α-adrenoceptor- and β-adrenoceptor-stimulation lead to hypertrophic growth of the myocardium. But only β-adrenoceptor-stimulation requires the pre-cultivation of cells with active TGF-α. In order to define signalling molecules that are specifically involved in α-adrenoceptor-dependent hypertrophy, changes in expression and hypertrophic responsiveness during pre-cultivation with TGF-α were investigated. Isolated adult ventricular cardiomyocytes from rats were either cultured in 20% (v/v) foetal calf serum (FCS) to activate autocrine released TGF-β or used without pre-treatment. Protein synthesis was analysed by 14C-phenylalanine incorporation. Expression of signalling molecules was determined by immunoblotting. During cultivation of cardiomyocytes with active TGF-β only the expression of p38 MAP-kinase increased. Subsequent stimulation of β-adrenoceptors induced protein synthesis in a p38 MAP-kinase-dependent way. However, stimulation of β-adrenoceptors activated p38 MAP-kinase irrespective of pre-treatment with TGF-β. In the absence of this cytokine, hyperosmolarity or reconstitution of mechanical activity increased protein synthesis via p38 MAP-kinase activation in freshly isolated cells. In conclusion, activation of p38 MAP-kinase is a newly identified necessary signalling step required for β-adrenoceptor induced hypertrophic growth. Like activation of adenyl cyclase, activation of p38 MAP-kinase is up-stream of the TGF-β-induced coupling to the regulation of protein synthesis. Reconstitution of mechanical activity mimics the co-activation required and induced by TGF-β.
1. Introduction
Plasma levels of catecholamines are found to be increased under conditions leading to myocardial hypertrophy and heart failure in vivo 1. In vitro, α-adrenoceptor stimulation of freshly isolated adult ventricular cardiomyocytes causes hypertrophy in a protein kinase C (PKC), PI 3-kinase and p70S6 kinase-dependent manner 2 3 4 5. These cells do not show any hypertrophic response to selective β-adrenoceptor stimulation, in contrast to the in vivo situation, where β-adrenergic stimulation can also cause myocardial hypertrophy 6. In vitro pre-cultivation of cardiomyocytes in the presence of foetal calf serum restores their in vivo-like behaviour 7. This culture system therefore permits analysis of the cellular mechanisms by which β2-adrenoceptor stimulation induces myocardial hypertrophy. Previous studies using this culture system showed that TGF-β, which is released by the cells and activated by proteases present in foetal calf serum, is necessary for the hypertrophic response to β-adrenergic stimulation 7,8. In contrast to the β-adrenoceptor-dependent pathway, α-adrenoceptor-dependent hypertrophy is not different between cells pre-cultivated with TGF-β or not. The priming effect of TGF-β for cardiac growth is likely to play a role in vivo, since cardiac expression of TGF-β has been found to be increased in patients with idiopathic hypertrophic cardiomyopathy 9 or in spontaneously hypertensive rats during the transition to heart failure 10. It may therefore be suggested that under these pathological conditions β-adrenergic stimulation contributes to the dysregulation of myocardial growth.
Some of the signalling molecules required for β-adrenoceptor-dependent stimulation of protein synthesis are in common with those of the α-adrenoceptor-induced pathway: for example, PI 3-kinase and p70S6 kinase 2. PKC-activation is a further step that is localised upstream the above mentioned enzymes but not activated by β-adrenoceptor stimulation. It further suggests, that members of the family of mitogen activated protein kinases, like p38 MAP-kinase and p42/44 MAP-kinase (ERK), are involved in hypertrophic regulation of the heart 11,12. For example, p38 MAP-kinase activation was found to be critically involved in the induction of hypertrophy in some cases 13 but not in all cases 14. In the same cell system under investigation, angiotensin II was characterized as a strong activator of p38 MAP-kinase, but increases protein synthesis via p42/p44 MAP-kinase rather than p38 MAP-kinase 15. This focussed our interest on members of the MAP-kinase family.
In this study, we hypothesized that some of the above mentioned signalling molecules change their expression level during exposure to active TGF-β. If so, such changes in expression might contribute to the priming effect of TGF-β. Therefore, we further investigated whether such factors are linked to β-adrenoceptor-dependent regulation of protein synthesis and whether their regulation depends on TGF-β. In this manuscript we show that p38 MAP-kinase expression increases during the pre-cultivating time in the presence of TGF-β. Subsequently we investigated whether p38 MAP-kinase is causally involved in β-adrenoceptor-dependent protein synthesis requiring such pre-incubation. Finally, we investigated whether the expression level of p38 MAP-kinase is a limiting factor for hypertrophy coupling of p38 MAP-kinase in protein synthesis.
2. Methods
All animal studies were performed in accordance with guidelines described in the NIH Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH, publication no. 85-23, revised 1996).
2.1. Cell isolation, short-term, long-term cultures
Ventricular cardiomyocytes isolated from 200–250 g male Wistar rats were suspended in basal culture medium and plated on 60 mm culture dishes. The culture dishes had been pre-incubated overnight with 4% (v/v) foetal calf serum (FCS) in medium 199. The basal culture medium (CCT) was modified medium 199 including Earl's salts, 2 mM l-carnitine, 5 mM taurine, 100 IU/ml penicillin and 100 μg/ml streptomycin. To prevent growth of nonmyocytes, the media were also supplemented with 10 μM cytosine-β-d-arabinofuranoside (pH 7,4).
Four hours after plating, cultures were washed twice with CCT medium. As a result of the medium change, broken cells were removed resulting in cultures of 93±2% quiescent rod-shaped cells. These cells were either directly stimulated (short-term cultures) or the cardiomyocytes were supplied with CCT medium containing 20% (v/v) FCS and incubated for up to 6 days without change of media (TGF-β pre-treatment protocol). In both cases the experiments were carried out in basic culture medium under serum free conditions (control) or with addition of respective substances.
2.2. Incorporation of 14C-phenylalanine
The rate of protein synthesis was determined by exposing cultures to l-14C-phenylalanine (0.1 μCi/ml) for 24 h. Incorporation of radioactivity into acid insoluble cell mass was determined as described before 7 and normalized to the precursor pool (acid soluble cell mass).
2.3. SDS-gel electrophoresis
Protein extracts from adult ventricular cardiomyocytes for SDS-gel electrophoresis were prepared as described before 16. Protein extracts (100 μg) were loaded on a 12% (w/v) SDS-page (acryl amide/bisacryl amide 30:1). After electrophoresis, proteins were transferred onto reinforced nitrocellulose by semidry blotting. The sheets were saturated with 2% (w/v) bovine serum albumin and incubated for 2 h with rabbit polyclonal anti rat p42 MAP-kinase, rabbit polyclonal anti protein kinase B, and rabbit polyconal anti rat protein kinase C α and δ. After washing, the sheets were re-incubated with an alkaline phosphatase-labeled goat anti-rabbit IgG. Finally, bands were visualized by alkaline phosphatase activity using 5-bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium. p38 MAP-kinase activation was calculated from the amount of phosphorylated p38 MAP-kinase and the total amount of p38 MAP-kinase. Therefore two blots were performed and two different first antibodies were used. First a rabbit polyclonal anti-rat p38 MAP-kinase and second a polyclonal anti-rat phosphorylated p38 MAP-kinase antibody (0.2 μg/ml, RBI). SB202190 (1 μM) was used as a p38 MAP-kinase inhibitor.
2.4. Hyperosmolarity
In order to cause hyperosmolarity, cells were exposed to culture medium containing 150 mM sucrose for 24 h. Following p38 MAP-kinase, activation and hypertrophy were measured as described before.
2.5. Electrical activity
Regular mechanical activity was achieved by electrical stimulation. Cardiomyocytes were stimulated by 0.5 Hz for 2, 4 and 24 h by two wire loops which were arranged parallel lying on the top of the cells and cultivated for up to 24 h with 14C-phenylalanine. The cells were then either used for measuring protein synthesis or p38 MAP-kinase activation. In order to differentiate between electrical stimulation and mechanical activity, cells were co-incubated with butanedione monoxime (BDM, 20 mM) which prevents cell contraction by inhibiting the actin and myosin interaction induced by electrical stimulation. Controls were incubated for 24 h without electrical stimulation.
3. Statistics
Data are given as means±S.E.M. Statistical comparisons were performed by one-way analysis of variance and use of the Student–Newman–Keuls test for post hoc analysis. In cases in which two groups were compared, conventional t-tests were performed. Differences with p<0.05 were regarded as statistically significant. All data analyses were performed using SAS software, version 6.11 (SAS Institute, Cary, NC, USA).
4. Results
4.1. Changes in cellular expression of hypertrophy associated signalling molecules during cultivation of adult ventricular cardiomyocytes
In order to determine signalling molecules that are specifically involved in the hypertrophic response of cells pre-treated with TGF-β, cardiomyocyte cultures were used in which the cells were incubated in the presence of 20% foetal calf serum for up to 6 days. As shown previously, the cells release substantial levels of TGF-β into the medium, which is activated by proteases present in the foetal calf serum 17. p38 MAP-kinase, p42/44 MAP-kinase, protein kinase B (PKB) and protein kinase C (PKC) α and δ were analysed by immunoblotting. Data were normalized to actin expression. Among the signalling molecules under investigation only p38 MAP-kinase expression was increased during pre-treatment. It was elevated after 3 days of cultivation and remained increased thereafter (fig. Fig. 1A,B).
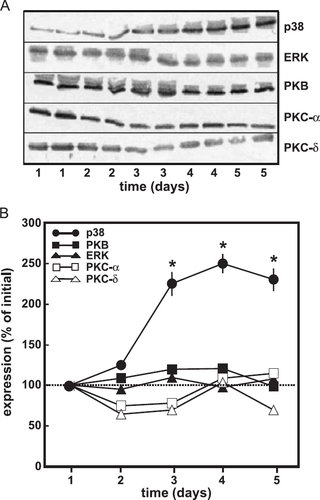
4.2. p38 MAP-kinase activation by isoprenaline in cardiomyocytes pre-treated with TGF-β and its implication for the regulation of protein synthesis
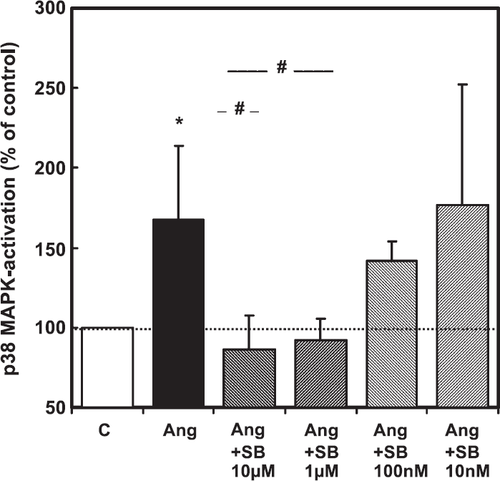
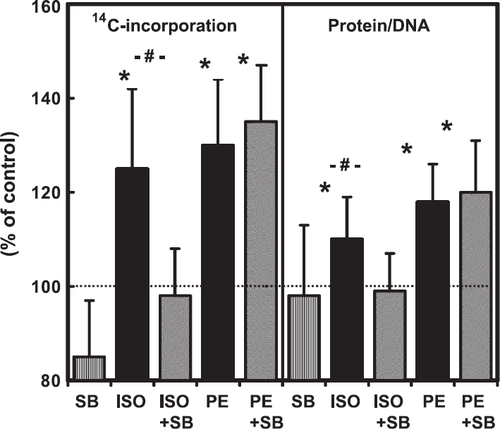
The experiments performed so far have shown a correlation between upregulation of p38 MAP-kinase (this study) and the induction of hypertrophic responsiveness to β-adrenoceptor stimulation 18,19. This suggests a causal relationship between both. In order to show a causal link between p38 MAP-kinase activation and the β-adrenoceptor-dependent hypertrophy, cells were used 6 days after cultivation and the hypertrophic responsiveness to β-adrenoceptor stimulation was investigated thereafter by addition of 100 nM isoprenaline. This was done in the presence or absence of the p38 MAP-kinase inhibitor SB202190. This inhibitor, which is known to inhibit angiotensin II induced activation of p38 MAP-kinase in adult ventricular cardiomyocytes 20, was used in a concentration-dependent way (fig. Fig. 2). Based on this concentration–response curve, SB202190 was used at a concentration of 1 μM throughout the subsequent experiments. Cells were also stimulated by phenylephrine (10 μM), an α-adrenoceptor agonist in order to exclude any unspecific effects of SB202190 on stimulation of protein synthesis. The results, which confirm earlier observations, showed that isoprenaline and phenylephrine increased protein synthesis, as determined by 14C-phenylalanine incorporation and total protein content (fig. Fig. 3). However, SB202190 was sufficient to inhibit the hypertrophic response to isoprenaline but did not impair the response to phenylephrine (fig. Fig. 3).
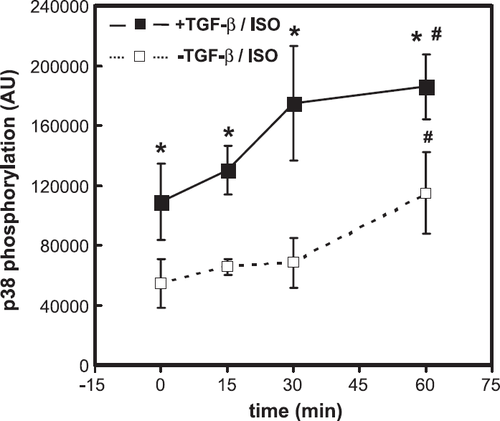
In the next set of experiments we confirmed that isoprenaline activates p38 MAP-kinase. p38 MAP-kinase activation was analysed by demonstration of p38 MAP-kinase phosphorylation. Isoprenaline raised the phosphorylation of p38 MAP-kinase within 60 min (fig. Fig. 4). However, activation of p38 MAP-kinase was not restricted to cardiomyocytes pre-incubated with TGF-β (fig. Fig. 4). In contrast to isoprenaline, phenylephrine did not activate p38 MAP-kinase (data not shown).
4.3. Influence of TGF-β on the induction of p38 MAP-kinase expression in cultured cardiomyocytes
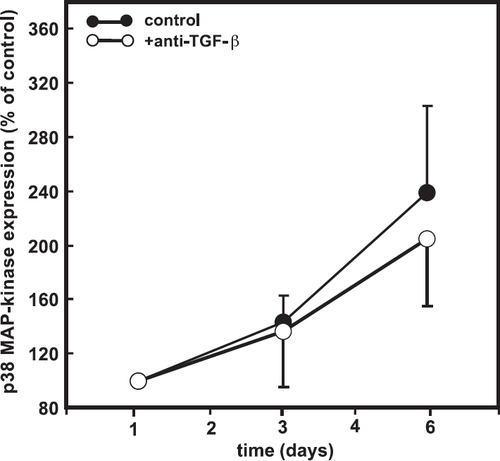
The aforementioned experiments support our hypothesis, if one assumes that expression of p38 MAP kinase is too low in cardiomyocytes not pre-treated with TGF-β to trigger protein synthesis and that TGF-β is responsible for the induction of p38 MAP-kinase expression in this system. To confirm this, cells were incubated with a neutralizing antibody against active TGF-β for 6 days. However, the presence of this neutralizing antibody did not prevent the upregulation of p38 MAP-kinase (fig. Fig. 5).
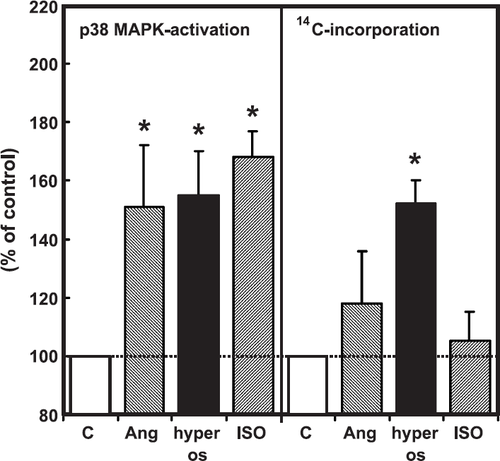
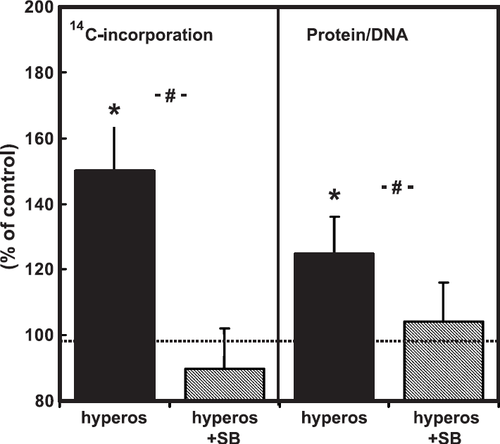
In order to show that the lower expression level of p38 MAP-kinase in freshly isolated cardiomyocytes is not a limiting factor, we performed experiments with three different stimuli for p38 MAP-kinase activation, namely hyperosmolarity, isoprenaline, and angiotensin II. All three agonists activated p38 MAP-kinase (fig. Fig. 6), however, with the exception of hyperosmolarity, induced by 150 mM sucrose added to the medium, none of the other agonists raised 14C-phenylalanine incorporation (fig. Fig. 6). In order to investigate whether hypertrophic growth caused by hyperosmolarity depends on p38 MAP-kinase, cells were co-incubated again with SB202190. SB202190 was able to reduce protein synthesis and protein mass after addition of 150 mM sucrose to the cell medium (fig. Fig. 7).
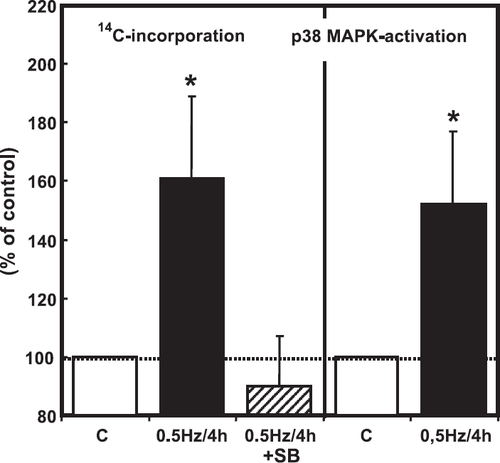
4.4. Reconstitution of mechanical activity increases protein synthesis via p38 MAP-kinase
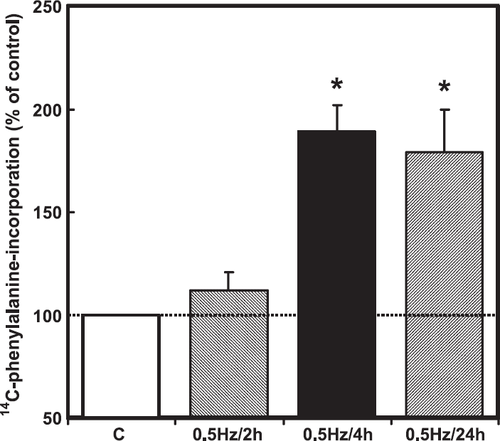
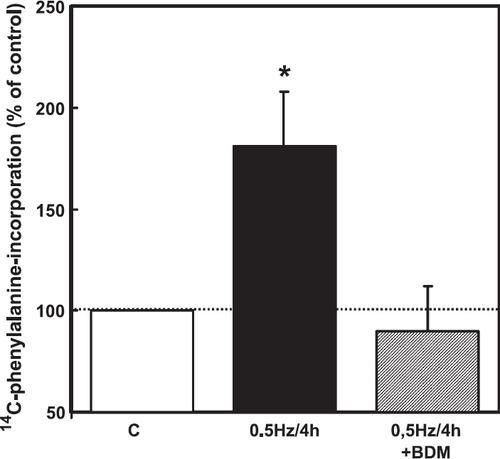
Although the experiments on cardiomyocytes exposed to hyperosmolarity indicated that p38 MAP-kinase expression level in freshly isolated cardiomyocytes is sufficient to induce hypertrophic growth via this pathway, it remained unclear whether this pathway was related to the regulation of protein synthesis under more physiological or pathophysiological conditions. We hypothesized that mechanical forces generated by hyperosmolarity trigger p38 MAP-kinase-activation and the hypertrophic response. If this is true, mechanical stress, like cell contraction, may also induce hypertrophic growth via this pathway. Therefore, in order to demonstrate a more physiological mechanical intervention than hyperosmolarity, we performed experiments to reconstitute mechanical activity. Reconstitution of the mechanical activity of cardiomyocytes was achieved by electrical stimulation of the cardiomyocytes at 0.5 Hz for 2, 4 and 24 h (fig. Fig. 8). Reconstitution of mechanical activity for 4 h activated p38 MAP-kinase (fig. Fig. 9). In addition, it raised protein synthesis in a p38 MAP-kinase-dependent way (fig. Fig. 9). To provide evidence that mechanical contraction rather than electrical stimulation is responsible for the observed p38-dependent increase in protein synthesis, the interactions of actin and myosin were inhibited by pre-incubation of cells with BDM (20 mM). In the presence of BDM, electrical pacing no longer caused an increase in protein synthesis (fig. Fig. 10). BDM alone decreased protein synthesis by 21±6% (p<0.05) in non-stimulated cardiomyocytes.
5. Discussion
In the present study we hypothesized that cultivation of cardiomyocytes in the presence of active TGF-β changes the expression of key signalling molecules involved in the regulation of protein synthesis. This hypothesis was based on our previous findings, that cardiomyocytes pre-cultured with TGF-β develop hypertrophic responsiveness to β-adrenoceptor stimulation that cannot be found in newly isolated cells 8. The main finding of our study is that under these conditions p38 MAP-kinase expression raises significantly. Upregulation of p38 MAP-kinase has not been investigated in cardiomyocytes under specific conditions before. However, a recent report has shown that mechanical unloading of the heart in patients with a left ventricular assist device increased p38 MAP-kinase expression 21. This finding supports our report on isolated cells, as these cells do not beat spontaneously. Thus, they have, by virtue of this lack of mechanical activity, a certain type of mechanical unloading. In contrast, the expression value of key signalling molecules involved in the hypertrophic response to other hypertrophic stimuli, i.e. that of α-adrenoceptor stimulation, did not change. In agreement with these observations we did not find any differences in the amount of α-adrenoceptor stimulation or the signalling cascades by which α-adrenoceptor stimulation increases protein synthesis in adult cardiomyocytes. The upregulation of p38 MAP-kinase prompted us to investigate more specifically the role of p38 MAP-kinase activation in β-adrenoceptor-dependent stimulation of protein synthesis.
Data from the present study clearly demonstrates that p38 MAP-kinase activation is permissive for the hypertrophic responsiveness of cardiomyocytes to β-adrenoceptor stimulation. Thus, our study confirms data on mouse cardiomyocytes that also reported that p38 MAP-kinase is permissive but not necessary for the induction of hypertrophy in case of β-adrenoceptor stimulation 18. It is also clear that p38 MAP-kinase activation is not a prerequisite for induction of hypertrophic responsiveness, as α-adrenoceptor-dependent stimulation is independent from p38 MAP-kinase activation. In fact, signalling molecules of the hypertrophic pathway which are either specifically involved in the PKC-dependent pathway induced by α-adrenoceptor stimulation or which are common for α- or β-adrenoceptor-dependent induction of protein synthesis are not changed during cultivation. This includes different PKC isoforms, the p42/44 MAP-kinase, and PKB. In line with these observations, we could not find any differences in the signalling pathway linked to α-adrenoceptor stimulation in cultured versus freshly isolated cells in several previous studies. The finding that phenylephrine neither activated p38 MAP-kinase nor increased protein synthesis in a p38 MAP-kinase-dependent way is in agreement with these other reports 22,23.
The finding that β-adrenoceptor-dependent stimulation of hypertrophy is dependant on p38 MAP-kinase activation, is new. Although the upregulation of this kinase during cultivation in parallel with the development of hypertrophic responsiveness to β-adrenoceptor stimulation strongly suggested that the induction of hypertrophic responsiveness to β-adrenoceptor stimulation by TGF-β is mediated by an increased expression induced by the cytokine, our further experiments in this study demonstrated that this is not the case. This is indicated by the following results: First, the upregulation of p38 MAP-kinase is independent from TGF-β, as a neutralizing antibody directed against TGF-β did not prevent upregulation. The same type of antibody has been shown previously to inhibit induction of hypertrophic responsiveness 8. Second, β-adrenoceptor stimulation activates p38 MAP-kinase in both, freshly isolated cells and pre-treated cells. Third, hypertrophic stimuli generated by electrical stimulation are sufficient to induce hypertrophic stimulation via p38 MAP-kinase activation. All these experiments indicate that the expression level of p38 MAP-kinase in newly isolated cells is not too low for induction of hypertrophic responsiveness. Our study also confirmed earlier studies on neonatal cardiomyocytes that had already shown that electrical pacing induced p38 MAP-kinase phosphorylation 24. We conclude that β-adrenoceptor-dependent hypertrophy requires co-activation of other factors in addition to p38 MAP-kinase. Similar, morphological changes of cardiomyocytes caused by IL-1 were found to require co-activation of p42/p42 MAP-kinase and p38 MAP-kinase, indicating that p38 MAP-kinase exerts some of its effects in concert with other factors 25. Therefore, although the present study has identified p38 MAP-kinase activation as a new signalling step specific for β-adrenoceptor-dependent hypertrophy, this step is located up-stream of the TGF-β induced changes in hypertrophic signalling molecules.
The question of whether p38 MAP-kinase activation is part of the hypertrophic response of the heart is still a matter for debate. Recent studies on transgenic mice with cardiac specific expression of dominant negative forms of p38 MAP-kinases have shown that p38 MAP-kinase activation is not necessary to trigger hypertrophy in response to pressure overload 26. On the other hand, mechanical stretch-induced increase in protein synthesis was suppressed by p38 MAP-kinase inhibition on cardiomyocytes from neonatal and adult rats 27 28 29. Our in vitro data on reconstitution of mechanical activity strongly suggest that p38 MAP-kinase is part of a direct mechanical transduction of hypertrophic responses. It seems not to be involved in many cases of receptor-dependent hypertrophic signals, i.e. α-adrenoceptor stimulation (this study and Refs. 18 and 23), endothelin 30, angiotensin II (this study and Ref. 29), cocaine 31 or leukemic inhibitory factor 25.
In addition to the adrenoceptor-dependent activation of p38 MAP kinase, this stress activated kinase was also activated by mechanical stress of the cardiomyocytes. This was performed in our experiments either by hyperosmolarity or, alternatively, by reconstitution of mechanical activity by electrical pacing. As the latter approach does not allow us to differentiate between electrical activation and mechanical activation we repeated these experiments in the presence of butanedione monoxime. Using the same approach Kubisch and colleagues, demonstrated that induction of immediate-early response genes linked to cardiac hypertrophy, i.e. egr-1, are induced by reconstitution of pacing induced activity. As shown in these experiments, butanedione monoxime did not alter membrane potential 32. However, the use of butanedione monoxime may interfere with calcium currents and phosphatases as well, which raises the question of whether BDM differentiates between electrical stimulation and mechanical stimulation 33. However, our conclusion that mechanical stress activates p38 MAP kinase phosphorylation is based on the combined use of hyperosmolarity and electrical reconstitution.
In summary, this study identifies p38 MAP-kinase activation as a crucial step in the hypertrophic response of rat ventricular cardiomyocytes to β-adrenoceptor stimulation. Since this type of hypertrophy mimics characteristic features of the hypertensive heart at the transition to heart failure, this suggests that the above mentioned step is critical in the transition from compensated hypertrophy to decompensated hypertrophy. It is in line with this suggestion that hypertensive end-organ damage and premature mortality in a rat model of cardiac hypertrophy was found to depend on p38 MAP-kinase activation 34.
Acknowledgements
The authors thank Daniela Schreiber and Nadine Lorenz for excellent technical assistance. Financial support of this study by the Deutsche Forschungsgemeinschaft, grant Schl324/4-1, is gratefully acknowledged. This study was part of the thesis of C. Müller.




