Is there a role for B lymphocyte chimerism in the monitoring of B-acute lymphoblastic leukemia patients receiving allogeneic stem cell transplantation?
Abstract
Objective
To determine the sensitivity and significance of B-cell chimerism for the detection of early engraftment, transplant rejection, and disease relapse.
Methods
The dynamic monitoring of lineage-specific cell subtypes (B, T, and NK cells) was made in 20 B-cell acute lymphoblastic leukemia (B-ALL) patients following allogeneic hematopoietic stem cell transplantation (allo-HSCT). In the early period after allo-HSCT, the latest establishment of B-cell complete chimerism (CC) was observed in a majority of patients.
Results
The percentage of donor cells of B-cell lineage was lower than the percent of T-cell lineage in most of the mixed chimerism (MC) patients. During graft rejection, the frequency of patients with decreasing MC of B-, T- and NK-cell lineage were 5/5, 2/5, and 2/5. When disease relapsed, five patients showed a faster decrease of the donor percent of B-cells than of T- or NK-cells. Only one patient displayed a more rapid decrease in NK-cells than in T- or B-cells.
Conclusion
Monitoring of B-cell chimerism after HSCT seems to be valuable for insuring complete engraftment, anticipating graft rejection, and relapse in B-ALL patients.
Acute lymphoblastic leukemia (ALL) is an acute disease that can quickly worsen. Treatment of ALL in adults remains a major challenge with overall survival rates in the past several decades limited to 30–40%. ALL can be of either T or B cell lineage, roughly 75% of cases of adult ALL are of B-cell lineage. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the most promising curative treatment for adults with ALL. However, the cure rate with transplantation has not been satisfactory. Frequent monitoring of mixed chimerism after allo-HSCT is clinically useful since patterns of chimerism may be predictive of graft-versus-host disease (GVHD), graft loss, or relapse.
The methods commonly used for chimerism analysis include fluorescence in situ hybridization (FISH), variable number of tandem repeats (VNTR) or short tandem repeat (STR), Y chromosome analysis, and quantitative real-time PCR (qPCR).1-3 Polymerase chain reaction (PCR) amplification of STR loci is currently the most commonly accepted and most widely used method for assessment of engraftment and mixed chimerism after HSCT. Although whole peripheral blood (PB) or bone marrow (BM) is most often used for chimerism analysis, it is important to realize that patients could show complete chimerism (CC) for one cell type, for example T-cells, whereas other cell types could be totally or in part recipient-derived.4, 5 This is called split chimerism (SC). Many studies have examined the prognostic value of the level of mixed chimerism in different cellular subsets, such as T-cells and NK-cells. So it is important to evaluate the kinetics of mixed chimerism (MC) in different cell lineages post-HSCT.6, 7
As engraftment is a dynamic process with variable kinetics among individuals, and the dynamics of this variability are not well understood. In previous studies, there have been quite a few reports about SC or discrepant results among various cell lineages in B-ALL patients post-HSCT. To understand these issues, we analyzed 19 adult patients and one ten-year-old boy with B-ALL who received allo-HSCT between 2007 and 2012. We compared lineage-specific cell subtypes, namely, B-cells, T-cells, and NK-cells, after allo-HSCT by using multiplex STR-PCR in order to determine the sensitivity and significance of B-cell chimerism for the detection of early engraftment, transplant rejection, and disease relapse.
Materials and methods
Patients
A total of 19 adults and one ten-year-old boy with B-ALL who received allo-HSCT between August 2007 and September 2012 were included in this study. The median age was 30 years old (16–54 years old), and the group was comprised of eleven males and nine females. Disease status at the time of transplantation was based on marrow morphology: 10 patients were in first remission, two were in second remission, and eight were in relapse. The donors were 16 human leukocyte antigen (HLA)-matched unrelated (MUD 75%), 2 HLA-matched related (MRD, 10%), one sibling-matched (the boy), and one HLA-haploidentical. Six patients were transplanted with sex-mismatched grafts. Patient characteristics are summarized in Table 1. Additionally, another three adult patients with T-ALL, T/NK-ALL, or Non-Hodgkin's lymphoma (NHL) who received myeloablative transplantation were also included in our analysis.
| Patient no. | TBI (GY) before HSCT | Disease status before HSCT | Gender/age | aGVHD/c grade | Causes of death | Graft type/Donor HLA matching | Survival (months) |
|---|---|---|---|---|---|---|---|
| 1 | 6 | NR | M/36 | –/2 | cGVHD | MUD 9/10 | 41.5 |
| 2 | 8 | CR2 | M/23 | – | – | MUD 10/10 | 58+ |
| 3 | 6 | CR1 | F/16 | III/1 | Relapse | MUD 9/10 | 22 |
| 4 | 6 | CR1 | F/54 | I/1 | – | MUD 8/10 | 52+ |
| 5 | 6 | CR1 | F/20 | I/1 | Relapse | MUD 10/10 | 32 |
| 6 | 6 | CR1 | M/20 | II/0 | Relapse | MUD 8/10 | 23 |
| 7 | 6 | NR | M/28 | II/0 | Relapse | MUD 9/10 | 6 |
| 8 | 8 | NR | F/30 | I/0 | Relapse | Haploidentical 4/6 | 3 |
| 9 | 8 | CR2 | F/42 | II/1 | – | MUD 9/10 | 37+ |
| 10 | 8 | NR | M/34 | III/0 | Fail | MUD 8/10 | 4 |
| 11 | 10 | CR1 | M/22 | III/2 | cGVHD | MUD 9/10 | 5 |
| 12 | 6 | CR1 | M/53 | – | Relapse | MRD 10/10 | 3 |
| 13 | 10 | CR1 | M/33 | I/1 | – | MUD 8/10 | 33+ |
| 14 | 10 | NR | F/32 | II/0 | Relapse | MUD 8/10 | 25 |
| 15 | 10 | CR1 | F/21 | IV/0 | aGVHD | MUD 8/10 | 3 |
| 16 | 10 | CR1 | M/21 | III/0 | – | MUD 10/10 | 24+ |
| 17 | 10 | NR | M/34 | I/0 | Relapse | MRD 10/10 | 9 |
| 18 | 10 | NR | F/29 | III/0 | aGVHD | MUD 8/10 | 4 |
| 19 | 10 | NR | M/10 | I/1 | – | Sibling 10/10 | 5+ |
| 20 | 8 | CR1 | F/46 | I/0 | – | MUD 10/10 | 4+ |
- CR1: first complete remission; CR2: second complete remission; NR: nonremission; cGVHD (according to IBMTR); 0: no cGVHD; 1: limited; 2: extensive; MUD: matched unrelated donor; MRD: matched related donor.
Intervals of analysis
For chimerism analysis, BM samples were collected from the donor and recipient before the transplantation and from the recipient on days 14 and 28 and at 1.5 and 2 months, then monthly or every two months thereafter. BM aspirates were collected in EDTA at the intervals described above. Mononuclear marrow cells were isolated by Ficoll-Hypaque density gradient centrifugation and washed twice in phosphate-buffered saline.
Conditioning therapy and GVHD prophylaxis
The conditioning regimen consisted of Teniposide (VM26, 15 mg·kg-1· d-1 from days −6 to −2 for each patient for 2–3 days), Cyclophosphamide (CTX, 60 mg/kg/d from days −6 to −3 for each patient for 2 days), Antithymocyte globulin (ATG, 2.5 mg/kg/d from days −5 to −2), and 6–10 Gy of Total body irradiation (TBI) was delivered in fractions of 2–3 Gy each for 2–3 days (days −3 to −1). All patients received prophylaxis to prevent GVHD; CSA at a dose of 2 mg/kg was administered intravenously from days −3, MTX 15 mg/m2 on Day 1, and 10 mg/m2 on Days 3 and 6.
Flow cytometric analysis and cell sorting
To evaluate lineage-specific chimerism, selection of CD3 (T cells), CD16/CD56 (NK cells), and CD19 (B cells) from BM samples were collected by four-color quantitative flow-cytometric (FACS) analysis on a FACSVantage SE (Becton, Dickinson and Company, USA). The percentage of B-cells in PB is lower than in BM, especially in the early period after HSCT, so BM samples were used to separate B from T and NK cells. Chimerism was detected at the first appearance of leukocyte counts adequate for cell sorting from days +14 following HSCT. The number of target cells sorted for subsequent PCR analysis ranged between 0.5 × 103 and 3 × 105 and the purity of each cell lineage was verified by flow cytometry (>95%).
Chimerism analysis
DNA was extracted from sorted cells using the QIAamp DNA Blood Mini Kit and QIAamp Investigator kit (Qiagen, Hilden, Germany). The final concentration of DNA was 0.02–0.2 ng per PCR. Chimerism studies were performed using the AmpF/STR Identifiler PCR Amplification Kit (Applied Biosystems, Foster City, CA, USA). The PCR products were analyzed using ABI 3130xl Genetic Analyzer (Applied Biosystems). Fluorescence was quantified, and the precise size of the DNA fragments was calculated with Genemapper software 3.2 (Applied Biosystems). The percentage of donor and recipient DNA was calculated from individual proportions of donor and recipient peak areas in relation to the summation of all signals from each pair of informative STR markers. The unique STR loci of the donor and recipient were used to evaluate chimerism. And a loci that had a stutter peak that was neither one repeat smaller (n − 1) nor one repeat larger (n + 1) than the allele peak was excluded.8-10
Definition of engraftment, chimerism status and relapse
Myeloid engraftment was defined as the first of three consecutive days with an absolute neutrophil count (ANC) above 0.5 × 109/L. Platelet engraftment was defined as the first of seven consecutive days with a platelet count exceeding 20 × 109/L without platelet transfusion.11, 12 Acute GVHD was graded from I to IV. The diagnosis of acute GVHD was based on clinical symptoms and/or biopsies from skin, oral mucosa, liver or gut. Chronic GVHD was assessed in patients alive after three months, and graded from 0 to 2.
CC was defined as the presence of only donor cells at all time points post-HSCT.13, 14 The variable change of donor% (percentage of donor) over 5% was defined as increasing or decreasing MC. Relapse was defined as the appearance of more than 5% blasts in the BM or new extramedullary leukemic lesions.
Results
Outcomes in patients
All patients received a myeloablative conditioning regimen, which included 6–10 Gy TBI, followed by infusion of allogeneic unmanipulated stem cells. The follow-up period of the 20 patients at the date of analysis ranged from 3 to 58 months. The overall survival (OS) is different between patients who received 6 and 8–10 Gy TBI (1/7 vs. 6/13). Before allo-HSCT, a complete remission (CR) was achieved in 12 patients, whereas eight patients showed progressive disease. In the patients with CR before HSCT, the OS (6/12) was better than those not in remission (NR, 1/8).
Up to the last follow-up, 13 patients had died after HSCT; 8/13 died of hematologic relapse (range 3–32 months, median 15.5 months) and four experienced transplant-related complications (range 3–41.5 months, median 4.5 months). The other patient (patient No. 10) was excluded from our analysis due to primary graft failure: donor cell hematopoiesis was not seen, B/T/NK chimerism <25% on +14 days, donor cells of the three lineages were not detected on +28 days and the patient died at +4 months.
The remaining seven patients who were alive at the date of analysis had survived a median of 33 months (ranged from 4 to 58 months). Among these seven surviving patients, patient No. 4 experienced disease relapse at +44 months and then received chemotherapy.
Except for patient No. 10, the median time to reach a white blood cell (WBC) count >0.5 × 109/L was +13 days (10–29 days). The median time to reach a platelet count >20 × 109/L was +13 days (9–34 days). Acute GVHD developed in 17 patients (Grade I–II in 11 patients and Grade III–IV in six patients). Among the 17 patients who survived more than 100 days after transplantation, eight developed chronic GVHD.
Pattern of chimerism of different lineages in the early post-transplant period
On +14 days after SCT, as mentioned above, patient No. 10 experienced graft failure. Lymphocytic subpopulations could not be sorted in patient No. 12 who lacked enough white blood cells. Among the remaining 18 patients, the median B-cell, T-cell and NK-cell chimerism was 81.6%, 93.8% and 99.2%, respectively (Table 2). The number of patients with CC in the B-, T- and NK-cell compartments was 3/18, 4/18, and 13/18, respectively. Within MC patients the percentage of donor cells of B- and T-cell lineages were much lower than those of NK-cell lineage. Among these 18 patients, the chimeric ratio of donor% is lower for B-cells than for T-cells in 12 patients. But we also found three patients that have a higher donor% of B-cells than of T-cells, and three patients who had a similar donor% of the two cell lineages.
| Lineage chimerism | B | T | NK | Totally | B later than T | B earlier than T | B and T almost equal | |||
|---|---|---|---|---|---|---|---|---|---|---|
| MC | CC | MC | CC | MC | CC | |||||
| 14 days | 15 | 3 | 14 | 4 | 5 | 13 | 18a | 12 | 3 | 3 |
| 28 days | 7 | 12 | 3 | 16 | – | 19 | 19 | 6 | 2 | 11 |
| 1.5 months | 5 | 14 | 2 | 17 | – | 19 | 19 | 4 | 1 | 14 |
| 2 months | 5 | 14 | 2 | 17 | 2 | 17 | 19 | 5 | – | 14 |
- MC: mixed chimerism; CC: complete chimerism; the ratio is donor% chimerism.
- a Patient No. 10 experienced primary graft failure, cell counts is too low to be detected on day 14 in patient No. 12, totally 18 patients are summarized on day 14.
On 28 day, in 19 patients, the median B-cell, T-cell and NK-cell chimerism was 97.3%, 98.9% and 100% (Table 2). The frequency of patients with CC in the B-, T- and NK-cell compartments were 12/19, 16/19 and 19/19, respectively. Among these 19 patients, six patients had a significantly lower donor cell percentage of B-cells than of T-cells and two patients had a higher donor cell percentage of B-cells than of T-cells. The remaining 11 patients had similar donor cell engraftment levels of B-cells and T-cells.
At +1.5 months, CC of the NK-cells was achieved in all 19 patients, but MC still could be seen in the B-cell and T-cell lineages (five patients with B-cell MC versus two patients with T-cell MC, Table 2). Four of the five SC patients showed a lower percentage of donor cells in the B-cell compartment than in the T-cell compartment, the remaining one patient showed opposite results.
At +2 months, 14 patients were observed with full donor chimerism in all fractions. The other four patients displayed decreasing MC in their B-cells, and two of them also showed concurrent donor% decreases in T-cells and NK-cells (Table 2 and Fig. 1). Only one patient (No. 20) did not show CC in the B-cell compartment until +3 months, while their T-cells and NK-cells had already developed stable CC at +1 month.
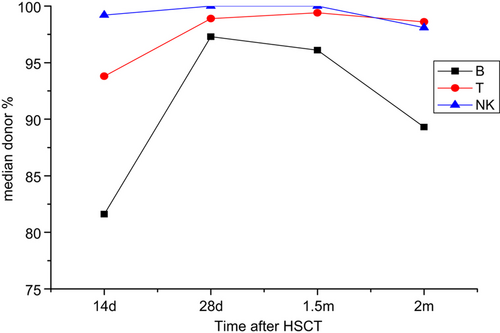
Evolution of B/T/NK cell chimerism in the early post-HSCT period.
Pattern of chimerism in graft rejection and relapse
Graft rejection was experienced in five patients. Four of them were observed with a decreasing donor% in their B-cell lineage at +2 months, and 2/4 patients also showed decreasing chimerism in the T-cell and NK-cell lineages. There was a more obvious donor% decrease in B-cells than in the other two cell lineages. In patient No.1, a decreasing donor% of B cells was seen at +3, +5.5, +10.5, +15.5 and +22 months, while T and NK cell lines retained stable CC expression.
Up to the time of analysis, eight patients experienced hematologic relapse and died. Two of them (No. 8 and No. 12) died too soon to be further monitored; both died at +3 months. A discrepancy of decreasing donor% between B and T/NK cell lineages was seen in 5/6 remaining patients. They displayed a much greater decrease in donor% in the B-cell compartment than in the T/NK-cell compartments. Three of the six patients received further therapy. Patient No. 5 received a haploidentical transplantation at +10.5 months, and relapsed again at +17.5 months and immediately received chemotherapy (Fig. 2). Patient No. 6 received three times of donor lymphocyte infusions (DLIs) (Fig. 3). Patient No. 14 received chemotherapy (Fig. 4). A different pattern of lineage dynamic chimerism after treatment were observed in these three patients. No. 5, No. 6, and No. 14 is shown in Figs. 2-4. The other three patients gave up further therapy after disease relapse (No. 3, No. 7 and No. 17).
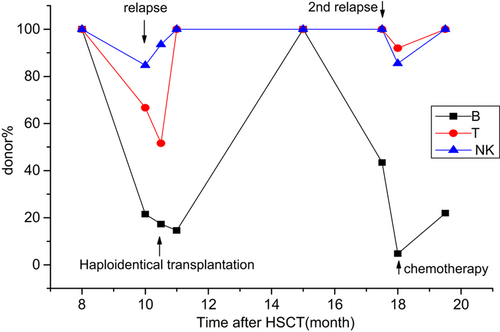
Disease evolution in patient No. 5. Relapsed on +10 months and received haploidentical transplantation at +10.5 months, second relapsed at +17.5 months and immediately received chemotherapy.
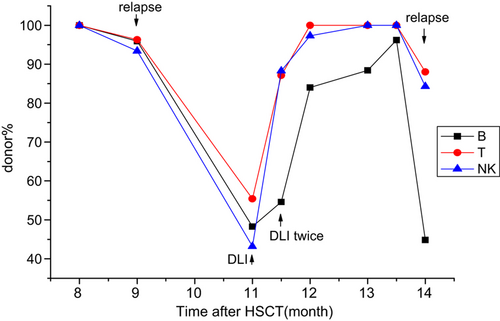
Disease evolution in patient No. 6. Relapsed at +9 months and received three times DLI between +11 and +11.5 months.
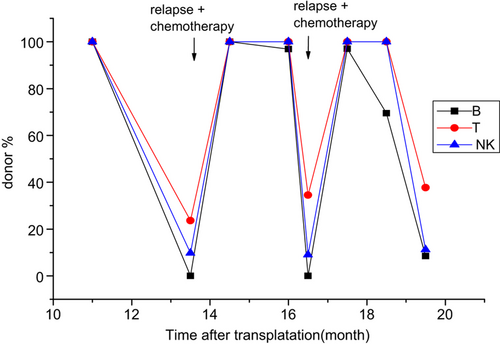
Disease evolution in patient No. 14, Relapsed at +13.5 months and +16.5 months and immediately received chemotherapy.
Another patient (No. 4) relapsed at +44 months, but was still alive at the time of writing. Compared with T and B cell lineages, chimerism analysis showed a significant decrease of the donor cell percentage of NK cells.
Pattern of chimerism in one T-ALL, one T/NK-ALL, and one NHL patient
Monitoring of lineage-specific chimerism was also made in three patients who suffered from lymphocyte-related diseases and received allo-HSCT. In the early period post HSCT, the three patients showed similar donor% of the T-, B- and NK cell lineages; <5% discrepancy between the three subsets. One patient with T/NK-ALL relapsed at four months, and a more rapid decreasing MC level in T/NK-cells than in B-cells was observed (T-cells 58.7%, NK-cells 54%, B-cells 81.7%).
Discussion
As patient management guidance, chimerism analysis plays an irreplaceable role, confirming complete engraftment and helping to prevent graft failure or relapse. In the past decade, most researchers were prone to monitor chimerism in nonmyeloablative (NMA) and reduced-intensity conditioning (RIC) HSCT patients.11, 12, 15, 16 Since most patients undergoing myeloablative (MA) HSCT rapidly achieve complete chimerism,17 it is generally believed that it is not necessary to detect chimerism in patients after myeloablative HSCT. However, in a recent study, Mickelson18 found that the median time to reach CC showed no difference between the NMA and MA groups. The results in our study verified the importance of chimerism analysis in MA patients.
In order to increase the sensitivity of chimerism analysis, many studies have examined the level of mixed chimerism of different cell subsets for early prediction of graft failure or of relapse; especially in NMA- or RIC-HCT cases. The majority of these studies showed that T-cells (from PB) are the most valuable prognostic fraction for predicting complete engraftment.6, 12-14, 16 However, one recent study reached a different conclusion.18 The possible reasons for this controversy are differences in underlying disease, conditioning regimen, and graft source. Thus, the determination of lineage-specific chimerism is very important in patients with malignancies as the original disease.19 Since the kinetics of B-cell chimerism in B-ALL has rarely been reported in prior research,19, 20 we monitored B cell (from BM) chimerism post-HSCT, compared the dynamic change in B-cells with T- and NK-cell lineages, and found some interesting results.
In the early period after allo-HSCT, the development of complete donor chimerism varied between cell lineages. In the majority of patients, CC appeared first in NK-cells followed by T-cells, while B-cells lagged behind T-cells. Although the number of patients with MC in the B-cell and T-cell compartments was similar in the early period post HSCT, the donor% of the two cell lines was notably different (Fig. 1). For instance, patient No. 20 did not achieve B-cell CC until +3 months, while she achieved T/NK-cell CC at +1 month. Moreover, a later establishment of B-cell CC was also observed in the three relapsed patients who received further therapy (Figs. 2-4). In the graft rejection phase and with recurrent disease, detection of B-cell chimerism seemed to show higher sensitivity than T and NK cell chimerism. Graft rejection was experienced by five patients. The frequency of patients with decreasing MC in B-, T-, and NK-cell subsets were 5/5, 2/5 and 2/5, respectively. When disease relapsed, five patients showed a faster decreasing donor% of B-cells than of T-cells or NK-cells. It is interesting that patient No. 4 displayed a more rapid decrease in NK-cells than in T-cells or B-cells. This observation might be partly explained by the fact that we missed the opportunity to monitor donor% decreases earlier during relapse.
During this analysis, we also monitored chimerism in one T-ALL, one T/NK-ALL and one NHL adult patient who had received myeloablative transplants. The T/NK-ALL patient relapsed at four months, and the other two patients, T-ALL and NHL, were still in disease-free state at +32 and +24 months. It was observed that in the early engraftment, donor% in B- and T-cell lineages showed no difference, CC in these two cell lineages lagged behind NK-cell CC. When disease relapsed, a faster decreasing MC was seen only in the T and NK cell lineages, but there was no difference in the donor% between the T-cells and NK-cells.
Most of previous studies focused on the association between the chimerism status in T- or NK-cells and clinical outcomes such as GVHD, relapse and survival.21, 22 Due to the small number of patients included in this pilot study, we were not able to show any relationship between them. The characterization of the chimerism status in T and NK subsets was basically in concordance with the majority of recent published studies. However, we found that in 4 of 6 relapsed patients, the donor% of NK-cells decreased more rapidly than the donor% of T-cells. The evolution of B-cell chimerism in our patients is quite interesting. Monitoring of B-cell chimerism post SCT seems to be valuable and its sensitivity may be higher than for T-cells or NK-cells in chimerism analysis. These observations support the hypothesis that we can assess B-cell chimerism to insure complete engraftment and to anticipate graft rejection and relapse in B-ALL patients. However, this notion has to be interpreted with great caution and certainly requires further assessment. For post-transplantation chimerism monitoring, the better modes are intensive and follow multiple lineages with STR or other quantitative techniques, especially in those lacking other suitable disease markers.




