Effect of graft site and gonadotrophin treatment on follicular development of canine ovarian grafts transplanted to NOD-SCID mice
Abstract
Purpose
To investigate the effect of graft site and gonadotrophins administration on the number and survival rate of follicles of canine ovarian grafts transplanted to NOD-severe combined immune deficiency (SCID) mice.
Methods
Fresh ovarian cortex slices obtained from immature bitches were grafted subcutaneously (SC), under kidney capsule (KC) or into ovarian bursa (OB) in NOD-SCID mice. Two months after surgery, the mice allocated into non-treated and treated gonadotrophins groups that injected with porcine follicle stimulating hormone during 7 days and human chorionic gonadotrophin 48 h later. Ovarian grafts were collected after 10 h of last injection and processed for histology.
Results
The number of transitional and preantral follicles under KC and into OB was significantly higher in gonadotrophins-treated mice than those who received saline. Furthermore, the survival rates of primary, transitional and preantral follicles under KC and into OB grafts were significantly higher than those placed SC in the treated gonadotrophins group, and in the non-treated gonadotrophins group; the proportion of primary and preantral follicle survival was significantly higher under KC and into OB than SC grafts.
Conclusions
In canine ovarian xenografting, administration of gonadotrophin could be effective for improvement of survival of transplanted ovary. Furthermore, the grafting into OB appeared to be better than grafting under KC, which in turn is better than SC.
Introduction
Transplantation of reproductive organs, including ovarian tissues, started over 100 years ago using both human and animal models [1]. Xenografting a tissue involves harvesting the tissue from a donor of a given species and inserting it into a recipient belonging to a different species. In the case of ovarian tissue, the animal serves as an “incubator” for follicle and gamete maturation. However, while xenografts might appear to be an interesting alternative to mature follicles, the problem of dealing with xenograft rejection has to be kept in mind [2]. Typically, severe combined immune deficiency (SCID) mice are used as xenograft recipients owing to their T- and B-cell deficiency, which allows revascularization and survival of the transplanted grafts without a rejection reaction [3].
Ever since, the ovarian xenografting has made it feasible for ovarian cryobanking for woman receiving chemotherapy, especially with alkylating agents, which are known to be gonadotoxic [4–7], and grafting of ovarian slices enables the remaining ovarian tissue to be stored for use in the future. This may allow the reproductive lifespan of the female to be prolonged or the tissue to be grafted at times [3].
Moreover, as the number of species and genetic variability within species (biodiversity) is decreasing at an alarming rate, and preservation of endangered species would augment captive breeding programs aimed at addressing this loss [8], so ovarian tissue transplantation proves to be a potentially promising method for preserving endangered and threatened animals as well as for the preservation of genetic resources (genome resource bank) of guide dogs for blind people, since one of the most conductive features of the shortage of guide dogs is the castration of both male and female in advance of the beginning of training. Thereafter, once the newly trained dogs are recognized as superior guide dogs, they can never reproduce [9–11].
Numerous studies have documented follicular development in ovarian tissue of human [12–14], monkey [15], elephant [8], cow [16], sheep [17], wombat [18] and cat [19] when transplanted to immunodeficient mice. Furthermore, it was demonstrated that xenografted ovarian tissue of a range of species, including human, can have the potential to give rise to antral follicles containing oocytes. The generation of first live offspring to originate from mouse xenografted ovarian tissue into a rat recipient has been obtained [20], and the first successful transplantation of cryopreserved ovarian tissue resulted in a pregnancy and live birth in human [21], monkey [22], rabbit [23] and sheep [24], but the efficiency of ovarian xenografting needs more effort to be greatly improved.
One problem is that, although ovarian grafts contain many primordial follicles, these cortical pieces cannot be directly reanastomosed to a blood supply and, therefore, remain hypoxic until new blood vessels grow into them. Consequently, the survival of the graft and the follicles it involves will in part depend on the rate of vascular ingrowth into the graft. The time needed to achieve an adequate perfusion of the transplanted tissue with new blood vessels is critical for the follicular survival and the functional longevity of the graft [25]. More importantly, vascular anastomosis is likely to be influenced by angiogenic growth factors [26]. The mRNA for vascular endothelial growth factor (VEGF) and transforming growth factor beta (TGF-β), a very potent angiogenic factor, are normally expressed in the ovary [27] and are rapidly upregulated by hypoxia as well as by gonadotropins [28]. Thus, one of the reasons why ovarian grafts are more successful in bilaterally oophorectomized than intact mice [29]. Imthurn et al. [26] showed that exogenous gonadotrophins, given to either the donor or the recipient, could increase the number of developing follicles but the magnitude of this effect was influenced by the timing of the injections relative to the time of grafting. However, the effect of gonadotrophins either before or after graft on further follicular development or fertility restoration on human and animal ovarian tissue grafting to mice is still controversial [25]. The administration of gonadotrophins to recipient mice after transplantation under kidney capsule (KC) [30] or abdominal wall [26], or in ovarian pedicle [15] showed no difference in follicular survival. In contrast, several reports showed an increase in the total number of growing follicles and can initiate follicular growth in the graft with administration of gonadotrophins [31–33]. Different gonadotrophins stimulation schedules and doses have been used in an attempt to improve follicular development in grafted ovarian tissue in different animals [26, 34, 35]. However, there are no reports showing the effects of the graft sites and gonadotrophins administration in canine ovarian graft.
The aim of the current study was to investigate prospectively the effect of graft site and gonadotrophins administration on the number and survival rate of follicles in different developmental stages of fresh canine ovarian grafts transplanted to NOD-SCID mice.
Materials and methods
Animals and xenotransplantation
Female NOD-SCID mice (8- to 10-week-old, n = 44; CLEA Japan, Tokyo, Japan) were used as recipients for xenografts. All mice were housed in polycarbonate cages and were maintained in a specific pathogen-free environment in light-controlled (lights-on from 0700 to 1900 hours) and air-conditioned rooms (temperature 24 ± 1°C, humidity 50 ± 10%). They had access to standard laboratory chow (CE-2; CLEA Japan, Tokyo, Japan) and water ad libitum.
Canine ovaries of different breeds were obtained from immature bitches (n = 9) undergoing routine ovariohysterectomy in a veterinary clinic. The age ranged from 4 to 8 months. Both ovaries were transported to the laboratory in a thermo flask containing sterile saline at 4°C. After transportation, the fat, ligaments and medulla were trimmed off carefully and removed. Following this, the ovarian cortex was micro-dissected and further cut into small pieces approximately (1 mm × 1 mm × 1 mm) in HTF media [10]. Then the ovarian pieces were washed three times and held at 37°C in the same medium.
Mice were anesthetized by an intraperitoneal injection of 5 mg/ml of sodium pentobarbital (Dainippon Pharmaceutical Co., Ltd., Osaka, Japan). The surgical field was prepared for aseptic surgery and a single dorsal longitudinal incision through the skin gave access to the flanks on both sides. Once both of the recipientsˈ own ovaries were oophorectomized bilaterally, the recipients received either six ovarian grafts placed subcutaneously (SC) onto the back of the mouse, or two ovarian grafts under each KC (the kidney was exteriorized by an incision performed behind the last rib, then the capsule was torn with small forceps), or one ovarian graft into each ovarian bursal cavity (OB). The reproductive tract was returned to the abdominal cavity and the skin incision was closed with a clip (9-mm auto clip, 427631, Becton Dickinson, Tokyo, Japan), then the mice were located on a warm plate until sufficient recovery had obtained.
Sixty days after surgery, the recipient mice were allocated into two groups: non-treated control group (SC n = 6; OB n = 8; KC n = 8) and gonadotrophins-treated group (SC n = 6; OB n = 8; KC n = 8). The treated mice received intraperitoneal injections of porcine follicle stimulating hormone (FSH) (2 IU/day; Sigma, St. Louis, MO, USA) for 7 days, and 5 IU human chorionic gonadotrophin (hCG) (Sankyo, Tokyo, Japan) was injected after 48 h. The controls received saline. Then 10 h later mice were euthanized under anesthesia to recover the grafts.
Histology
The recovered ovarian grafts were placed in 10% phosphate buffered formaldehyde fixative, embedded in paraffin, cut into thin 5 μm serial sections and stained with hematoxylin and eosin. Follicles were counted in sections of both transplanted tissue (gonadotrophins-treated and non-treated) and in control tissue taken from the biopsy before transplantation.
The follicles at the level of the nucleus of the oocyte in all serial sections were counted according to classifications of Kutluk et al. [36] as shown in Table 1. The distance between sections was 50 μm. Five sections (5 μm in thickness) were prepared for tissue specimen. The number of follicles in a circle of 900 μm in diameter (view field of 0.64 mm2), containing the highest number of follicles in each section was counted in a total of 5 fields of view. The survival rates of the follicles were calculated as the number of follicles in grafted-control/number of follicles in fresh ovarian tissue samples × 100, and the number of follicles in grafted-treated/number of follicles in fresh ovarian tissue samples × 100.
| Stage | Description |
|---|---|
| Primordial | Oocytes partially or completely encapsulated by squamous pregranulosa cells |
| Early primary | At least one of pregranulosa cells become columnar (enlarged) |
| Primary | All granulose enlarged, a single granulosa cell layer |
| Transitional | 1–2 granulosa cell layer, distinct zona pellucida |
| Preantral | Oocytes encapsulated by more than 2 layers of granulosa cells, no antrum formation |
| Antral | Oocytes encapsulated by more than 2 layers of granulosa cells, with antrum formation |
- Kutluk et al. [36]
The animals used in this study were treated and cared in accordance with the Guiding Principles for the Care and Use of Research Animals established by Obihiro University of Agriculture and Veterinary Medicine.
Statistical analysis
All data were subjected to analysis of variance (ANOVA), and the significance of the difference among means was determined by Wilcoxon/Kruskal–Wallis test. The numbers of follicles are presented as mean ± standard deviations. Results were considered significant when p values were <0.05.
Results
Effect of gonadotrophins
Effect of gonadotrophins after SC xenograft
Table 2 shows the number of follicles in fresh ovarian tissue, non-treated control group and gonadotrophins-treated group in SC xenotransplantation. There was no significant difference in the number of primordial, early primary and primary follicles between gonadotrophins-treated and non-gonadotrophin-treated mice. Likewise, there was no significant difference in the number of transitional and preantral follicles between two groups. However, the survival rates of transitional and preantral follicles improved, but not significantly (p > 0.05), from 8.2 and 7.2% in those received saline to 28.4 and 20.8% in gonadotrophins-treated mice (Fig. 1).
| Exp. group (no. of grafts) | No. of mice | Primordial | Early primary | Primary | Transitional | Preantral | Antral |
|---|---|---|---|---|---|---|---|
| Non-grafted (n = 32) | – | 4.06 ± 2.19a | 3.93 ± 1.89a | 2.87 ± 1.00a | 0.59 ± 0.66a | 0.40 ± 0.55a | 0.0 ± 0.0 |
| Grafted-control (n = 31) | 6 | 2.12 ± 1.82b | 2.31 ± 0.99b | 1.28 ± 0.66b | 0.11 ± 0.32b | 0.08 ± 0.28b | 0.0 ± 0.0 |
| Grafted-treated (n = 32) | 6 | 2.06 ± 1.54b | 2.46 ± 1.26b | 1.34 ± 0.97b | 0.18 ± 0.39b | 0.09 ± 0.29b | 0.0 ± 0.0 |
- Different superscripts within columns indicate significant difference (p < 0.05)
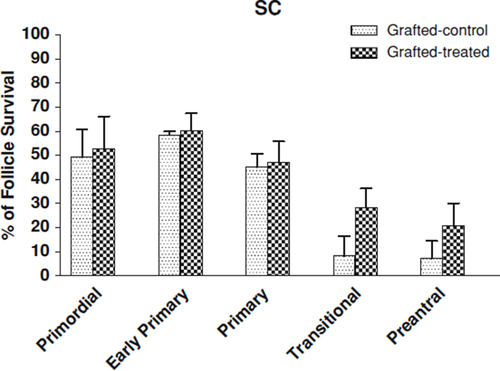
Graph showing the percentage of follicular survival after grafting subcutaneously (SC). The survival rates of the follicles were calculated as the number of follicles in grafted-control/number of follicles in fresh ovarian tissue samples × 100, and the number of follicles in grafted-treated/number of follicles in fresh ovarian tissue samples × 100
Effect of gonadotrophins after KC xenograft
Table 3 shows the follicular growth in transplants between fresh ovarian tissue, non-treated control group and gonadotrophins-treated group after KC xenograft. The number of transitional and preantral follicles in gonadotrophins-treated mice was significantly higher than those received saline (p < 0.05). The survival rates of transitional and preantral follicles were significantly improved by gonadotrophin treatment (p < 0.05, Fig. 2).
| Exp. group (no. of grafts) (n = 32) | No. of mice | Primordial | Early primary | Primary | Transitional | Preantral | Antral |
|---|---|---|---|---|---|---|---|
| Non-grafted | – | 4.15 ± 2.21a | 4.09 ± 1.94a | 2.40 ± 1.41a | 0.46 ± 0.67a | 0.31 ± 0.59a | 0.0 ± 0.0 |
| Grafted-control | 8 | 2.37 ± 1.96b | 2.96 ± 1.42b | 1.71 ± 1.05b | 0.06 ± 0.24b | 0.03 ± 0.17b | 0.0 ± 0.0 |
| Grafted-treated | 8 | 2.46 ± 1.68b | 2.81 ± 1.44b | 1.68 ± 1.14b | 0.28 ± 0.45a | 0.21 ± 0.55a | 0.0 ± 0.0 |
- Different superscripts within columns indicate significant difference (p < 0.05)
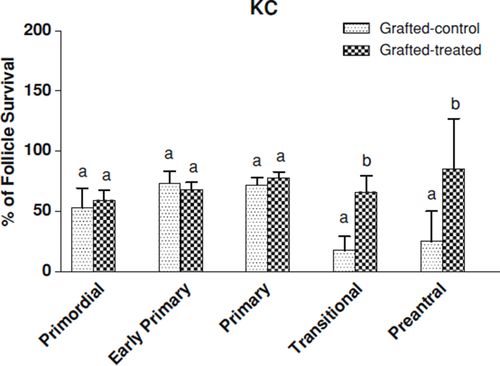
Graph showing the percentage of follicular survival after grafting under kidney capsule (KC). The survival rates of the follicles were calculated as the number of follicles in grafted-control/number of follicles in fresh ovarian tissue samples × 100, and the number of follicles in grafted-treated/number of follicles in fresh ovarian tissue samples × 100. Values with different superscripts (a, b) are significantly different (p < 0.05)
Effect of gonadotrophins after OB xenograft
As shown in Table 4, there was no significant difference in the number of primordial, early primary, primary between gonadotrophins-treated mice and non-treated mice. However, the number of transitional and preantral follicles in gonadotrophins-treated mice was significantly higher (p < 0.05) than non-treated mice. Furthermore, the percentage of survival transitional and preantral follicles in gonadotrophins-treated mice was significantly higher than non-treated group (p < 0.05, Fig. 3).
| Exp. group (no. of grafts) (n = 16) | No. of mice | Primordial | Early primary | Primary | Transitional | Preantral | Antral |
|---|---|---|---|---|---|---|---|
| Non-grafted | – | 3.62 ± 2.02a | 3.25 ± 2.40a | 2.18 ± 1.27a | 0.62 ± 0.88a | 0.56 ± 0.51a | 0.0 ± 0.0 |
| Grafted-control | 8 | 2.62 ± 0.61a | 1.62 ± 1.14b | 1.93 ± 0.68a | 0.12 ± 0.34b | 0.06 ± 0.25b | 0.0 ± 0.0 |
| Grafted-treated | 8 | 2.56 ± 0.96a | 1.5 ± 1.46b | 2.06 ± 0.68a | 0.50 ± 0.51a | 0.43 ± 0.51a | 0.0 ± 0.0 |
- Different superscripts within columns indicate significant difference (p < 0.05)
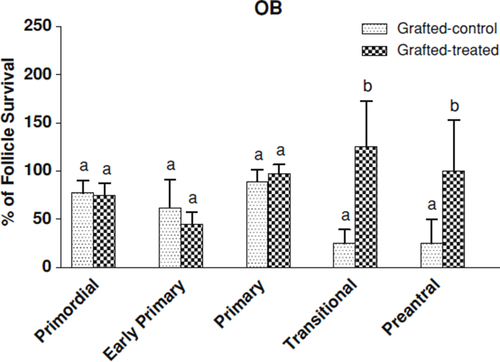
Graph showing the percentage of follicular survival after grafting into ovarian bursa (OB). The survival rates of the follicles were calculated as the number of follicles in grafted-control/number of follicles in fresh ovarian tissue samples × 100, and the number of follicles in grafted-treated/number of follicles in fresh ovarian tissue samples × 100. Values with different superscripts (a, b) are significantly different (p < 0.05)
Site of transplantation and follicular development in xenografts
In grafted controls without gonadotrophins treatment, as shown in Table 5, the survival rates of primary and preantral follicles under KC and into OB grafts were significantly higher than those placed SC. Furthermore, the proportions of transitional follicle survival under OB were significantly higher than SC grafts. In gonadotrophins-treated group (Table 6), the proportion of transitional and preantral follicle survival were significantly higher under KC and into OB than SC grafts. These results indicate that gonadotrophin treatments are much more effective for KC and OB than for SC grafts.
| Graft site | Primordial | Early primary | Primary | Transitional | Preantral |
|---|---|---|---|---|---|
| SC | 49.2 ± 25.81 | 58.4 ± 3.36 | 45.2 ± 12.15a | 8.2.4 ± 18.33a | 7.2 ± 16.09a |
| KC | 52.75 ± 32.97 | 73.25 ± 20.43 | 71.75 ± 12.81b | 17.5 ± 23.62ab | 25.0 ± 50.0b |
| OB | 77.25 ± 26.52 | 62.0 ± 59.25 | 89.25 ± 24.54b | 25.0 ± 28.86b | 25.0 ± 50.0b |
- SC subcutaneous, KC kidney capsule, OB ovarian bursa
- Different superscripts within columns indicate significant difference (p < 0.05)
| Graft site | Primordial | Early primary | Primary | Transitional | Preantral |
|---|---|---|---|---|---|
| SC | 52.8 ± 30.08 | 60.2 ± 16.03 | 47.2.6 ± 19.14a | 28.4 ± 17.50a | 20.8 ± 20.86a |
| KC | 59.25 ± 17.15 | 67.75 ± 12.65 | 77.75 ± 10.42b | 66.0 ± 26.78b | 85.25 ± 83.45b |
| OB | 74.5 ± 26.26 | 44.75 ± 24.95 | 97.0 ± 19.61b | 125.0 ± 95.74b | 100.0 ± 106.06b |
- SC subcutaneous, KC kidney capsule, OB ovarian bursa
- Different superscripts within columns indicate significant difference (p < 0.05)
Discussion
Results of the present study indicate that the follicular development of xenotransplanted ovarian tissue was influenced by the site of transplantation. The proportions of follicular survival under KC and into OB xenografts were significantly higher than those placed SC (Tables 5, 6). Nisolle et al. [37] also reported higher follicular survival rate in human grafts placed under KC than the peritoneum and SC sites. Cleary et al. [32] showed that increased graft recovery rates and follicle survival were more evident after grafting under the KC compared with grafting to SC sites in xenografted ovarian tissues of common wombat. Hernandez-Fonseca et al. [38] showed that human ovarian cortical grafts transplanted under the KC had significantly higher proportions of growing follicles compared with those recovered from the SC. Furthermore, Yang et al. [39] showed that graft recovery rate and oocyte yield were significantly less at the SC graft site with only 56–58% of the grafts recovered compared with the OB and KC which had 98–100% and 93–95% of the grafts retrieved, respectively. The number of two-cell embryos produced was significantly higher with oocytes from grafts to the OB, compared with KC and SC graft sites [39].
Our present study also showed that the effect of gonadotrophins on follicular development after xenografting was influenced by the site of transplantation. Although there was no significant difference in the number of follicles between gonadotrophins-treated and non-gonadotrophins-treated mice after SC graft, the number of transitional and preantral follicles in gonadotrophins-treated mice was significantly higher than those received saline after KC and OB (Tables 3, 4). Previous reports have shown that the effect of gonadotrophins either before or after graft on further follicular development or fertility restoration on human and animal ovarian tissue grafting to mice is still controversial. Kaneko et al. [33] reported that the treatment of host mice with FSH enhanced the growth of porcine antral follicles, increased dramatically the number of full-sized oocytes, much greater numbers of oocytes had the ability to resume meiosis to the mature stage, and development of oocytes after in vitro fertilization (IVF) reached the blastocyst stage. Cleary et al. [32] obtained similar results in common wombats after the host mice were given FSH for 4 or 7 days a greater numbers of morphologically normal oocytes were recovered from ovarian grafts. Likewise, treatment of host mice with FSH for over 20 weeks [6] or for 12 weeks [40] or for 6 weeks [13] can initiate follicular growth and increased the number of antral follicles within human ovarian xenografts, and some antral follicles formed early corpora lutea in response to hCG [41] with resumption of meiosis of the oocytes [6]. Treatment of the mice with human menopausal gonadotrophin (hMG) for 14 days [31], or beginning 4 days (hMG, 5 IU/d) before the transplantation [34], or equine chorionic gonadotrophin (eCG) for 4 weeks [41] can initiate follicular growth and increased the number of antral follicles within human ovarian xenografts.
On the contrary, the administration of gonadotrophins to recipient mice after transplantation of mice ovarian tissue under KC [30] or abdominal wall [26] showed no difference in follicular survival. Bosch et al. [19] indicated that the gonadotropin treatment of cryopreserved cat ovarian tissue grafts did not alter the proportion of resting to growing follicles or mean follicle diameter in comparison with controls from untreated mice; however, luteinization, but not ovulation, of antral follicles was observed only in grafts from treated mice. The gonadotrophin releasing hormone (GnRH) agonist treatment was not able to prevent the primordial follicle depletion after the xenografting of cryopreserved human ovarian tissue in neck muscle of NOD-SCID mice. Furthermore, it causes an additional loss of follicles if administered during the critical neovascularization period after the transplantation [42], and significant loss of primordial follicles after prolonged gonadotropin stimulation in xenografts [43]. However, Imthurn et al. [26] showed an increase in the total number of growing follicles in the graft with administration of recombinant human FSH and LH.
The main cause for follicular loss of graft in the process of ovarian transplantation is believed to be post-transplantation hypoxic ischemia. The duration of ischemia prior to revascularization of transplanted ovarian tissue depends on several factors, such as the site of graft, the size of tissue and presence and amount of angiogenic factors [34]. In mice, Liu et al. [44] observed the transplantation accounts for approximately 42% of the loss in follicle population. Similar substantial loss of follicles was obtained also in larger animal models [45]. Since subcutaneous grafts in our experiment not respond to exogenous gonadotrophins (Table 2), it may be possible that subcutaneous grafts have a poor blood supply and altered vascular integrity, in turn, does not allow adequate delivery of the gonadotrophin to the grafts.
Because the cortical pieces cannot be directly reanastomosed to a blood supply and, therefore, remain hypoxic until perfusion of the tissue with new blood vessels. Consequently, the survival of the graft and the follicles it involves will in part depend on the rate of vascular ingrowth into the graft. The time needed to achieve an adequate perfusion of the transplanted tissue with new blood vessels is critical for the follicular survival and the functional longevity of the graft [25]. In humans, neovascularization and stromal cell proliferation occur on day 3 [46], mice after 3 h [30]. Kim et al. [47] elucidated that the ovarian cortex could tolerate ischemia at least for 3 h and stromal cells were more vulnerable to ischemic damage compared to primordial follicles. So the survival of ovarian implants was improved under conditions that support vascular maintenance. As well, the extensive capillary bed of KC naturally favours rapid graft revascularization [26].
As shown in Tables 2, 3 and 4, the gonadotrophins did not increase the number of early stages of follicles (from primordial stage to primary stage). This may be explained by the fact that, in mammals, unknown factors trigger activation of primordial follicles to grow to transitional stages in a gonadotropin-independent manner [48]. From primary stages to ovulation, follicular development is dependent on FSH and LH [49]. Additionally, once the follicles initiate growth, they are more likely to survive and continue growth in the presence of gonadotrophin. Kaneko et al. [33] suggested that follicular growth is stimulated or maintained largely by exogenous gonadotrophins, not by endogenous mouse FSH. In the present study antral follicle not obtained, this may be due to low dosage of gonadotrophins that used causing limited positive effect on the developmental ability of follicles. Beyond what has been said, the role of gonadotrophin in regulation of follicle growth, as well as ovarian stimulation, needs more investigation.




