A Pilot Study of Fragile X Syndrome Screening in Pregnant Women and Women Planning Pregnancy: Implementation, Acceptance, Awareness, and Geographic Factors
Group for the study of FXS in the Balearic Islands: Sector Levante: María Pía, Consuelo Fernández Yagüe, Catalina Cerdà, Jerónima Amengual, Margalida Lladó, Elena Pascual de Juan, Teresa Mariscal, Pilar López Redondo, Olga Román. Hospital de Manacor: Andrés Calvo Pérez, Sabine Rehkugler, Francisco Javier Agüera. Sector Migjorn: Azucena Bande Seisdedos, Mª Jesús Fernández Castaño, Roser Gallardo Ferrer, Margalida Alomar Castell, Concha Manzanares Mir, Carmen Carrascosa Martín, Mª Teresa Sánchez-Puga Crusat, Joana Crespí Rullan, Catalina Serra Calafat, Catalina Artigues Mascaró, Antonia Cladera Riera, Joana Guardiola Martínez, Margalida Pujol Ferragut, Rocío Aguilar González, Hospital Son Llatzer: Miguel Juan Clar, Albert Tubau, Maria Antonia Ferragut. Sector Inca: Elena Portells Miralles, Carmen Ribera Gómez, Jessica Holster, Elena Montes de Oca, Dolores García, Carmen Vallejo Burgada. Hospital Inca: Agustín Hernández, Juan Trias Rojas; Ines Pomar, Joana Boyeras. Sector Poniente: Eva Navarro, Yolanda Vives Fuster, María Maimó Vaquer, Mariana Garces Más, Matteu Taylor, Silvia Miralles Corrales, Marian García Baratas, María A. Blanco García, Catalina Torrens Grau, Francisca Llinàs Alzina, Regina Sastre Arbós, Verónica Molina Gilfillan, Mª Paz Nadal Casasnovas. Sequencing service : Mª Carmen Vidal Llampurdanes, Carmen Santos Pons. Menorca/Hospital Mateu Orfila: Juan Carlos Hermoso García, Jorge Casal Moro, Miriam Ribes Redondo, Marcial Moreno Pons. Ibiza/Hospital Can Misses: Montserrat Pastor, Raquel Gastón, Rodolfo Moreno Mira, Marta Cortés.
Electronic supplementary material
The online version of this article (doi:10.1007/s10897-016-0005-3) contains supplementary material, which is available to authorized users.
Abstract
We report herein results of a study performed in the Balearic Islands which had the following goals: 1) Determine the proportion of pregnant or non-pregnant women planning pregnancy, who would choose to undergo a screening test for Fragile X Syndrome (FXS), if it is accompanied by the appropriate information; 2) Assess satisfaction and any increase in stress among women who participate in screening; 3) Collect epidemiological information about the incidence of the disease in our population; and 4) Collect demographic and health history data and assess participants’ awareness of the disease. Screening was performed on 3,731 pregnant and non-pregnant women of childbearing age and the results indicate: a very high voluntary rate of participation; a high level of self-reported satisfaction and low levels of stress because of the test; a very high incidence of premutation (1/106) in our population; and a low level of awareness about the existence of FXS (25 %). Additional findings indicate no significant correlation between self-reported health history and premutation detection, and the high premutation incidence does not seem to be specific to the indigenous Balearic population. Based on these results, we discuss the pros and cons of an implementation of preconception and pregnant women screening for FXS within a public health screening program.
Introduction
Fragile X syndrome (FXS) is caused by an expansion of a CGG triplet repeat in the 5′-untranslated region of the fragile X intellectual disability 1 (FMR1) gene. The FMR1 gene encodes the FMR1 protein (FMRP), which is essential for normal brain development. Individuals with over 200 CGG repeats are carriers of a full mutation (FM) and suffer from different degrees of intellectual disability, while 5–44 repeats are considered within the normal range. Carriers of the premutation allele (PM), which is characterized by 55 to 200 CGG repeats, or intermediate (IM) alleles 45 to 54 CGG repeats, are not affected by intellectual disability, but there is an increased risk for expansion to FM and PM, respectively, in the next generation.
FXS is the most common inherited form of intellectual disability, with an estimated incidence of the full mutation (FM) of 1 in 7000 males and 1 in 11,000 females (Hunter et al. 2014). In contrast, females have been found to be more frequent carriers of the PM allele than males. Reported female PM carrier frequencies range between 1/113 and 1/788, depending on the population (Jang et al. 2014; Toledano-Alhadef et al. 2001). Premutation alleles are important because they are associated not only with increased risk for expansion to FM, but also with a neurodegenerative condition, called fragile X-associated tremor and ataxia (FXTAS) and fragile X-associated primary ovarian insufficiency (FXPOI) (Biancalana et al. 2015).
There is ongoing debate regarding the utility of an FXS population-based screening for women of reproductive age (preconception or prenatal), although unquestionably, knowledge of being a FXS PM carrier would alert families about the possibility of having a child with FXS and provide options for consultation, family planning and reproductive choice (Abrams et al. 2012; Gutierrez et al. 2013; Kuppermann et al. 2011; Metcalfe 2012). In summary, arguments that have been brought forward in favor of population screening are: 1) The FXS disorder fulfills criteria for a disease suitable for a public health screening program, because it shows a high carrier incidence and it has serious effects on health; 2) Recent technological advances allow for accurate molecular testing in a fast and relatively cost-economical manner; and 3) There is need for public screening in a population-wide setting to avoid unsupervised, profit-driven testing that benefits the most affluent to the detriment of the less fortunate. On the other hand, arguments against screening are: 1) Screening may not be cost effective; 2) Disclosure of repeat numbers at the PM level in fetuses can cause a number of ethical concerns; and 3). There is need for pretest informed consent and adequate post-test genetic counseling (Abrams et al. 2012; Gutierrez et al. 2013; Kuppermann et al. 2011; Metcalfe 2012).
There are few reports, however, on the implementation of population-wide prenatal or neonatal screening for this disease within structured public health screening programs. Such reports could provide useful information for the debate (Berkenstadt et al. 2007). The highly controversial nature of FXS screening stands in sharp contrast to widely accepted and implemented public health screening programs such as prenatal biochemical screening tests to estimate Down syndrome risks, or neonatal screens to detect other genetic diseases, such as Cystic Fibrosis, Thalassemia and Tay-Sachs. Early detection of FXS has advantages because affected offspring can benefit from early intervention and, in the future, even therapy, if ongoing clinical trials for a pharmacological treatment are successful (Berry-Kravis 2014).
There are many arguments in favor of specifically screening for FXS in a female population, including: women carriers of a premutation are 2–3 times more frequent than males (~1/150 ♀ vs. ~1/400♂); expansion from premutation to full mutation only occurs in mother to child transmissions; during a woman's reproductive life premutations do not produce an obvious phenotype that makes them easily detectable; and once a premutation is detected, prenatal diagnosis and cascade screening can be offered, and different reproductive options will be available for future pregnancies (Abrams et al. 2012; Gutierrez et al. 2013; Kuppermann et al. 2011; Metcalfe 2012).
Purpose of the Study
The high ease of use and level of detection of current techniques (>99 %), have led us to consider that a population-based screening in women of reproductive age is feasible and could be the most efficient of population screening strategies. In addition, the defined boundaries of the study area (islands) and limited population size are favorable factors for conducting a population screening pilot program. Furthermore, in the Balearic Islands, there is good health coverage for antenatal care, and more than 95 % of pregnant women undergo the biochemical screening test for aneuploidy risk calculation. Thus, blood drawn for this test could be used for FXS testing which makes it cost efficient and avoids supplementary medical appointments and invasive procedures to participants. Finally, because Genetic Services are centralized, adequate genetic counseling can be offered pre and post-test to participants in a single location.
The molecular results of this study have been addressed in another manuscript (Alfaro Arenas et al. 2016). Herein, we present the results of an evaluation of the feasibility and desirability of screening in pregnant women and women planning pregnancy within the public health system. This study had the following objectives: 1) Determine the proportion of pregnant or non-pregnant women planning pregnancy, who would choose to undergo a screening test for Fragile X Syndrome (FXS), if it is accompanied by the appropriate information; 2) Assess satisfaction and any increase in stress among women who participate in screening; 3) Collect epidemiological information about the incidence of the disease in our population; and 4) Collect demographic and health history data and assess participants’ awareness of the disease.
Methods
Design and Participant Recruitment
Informed consent was obtained from all individual participants included in the study. The study was designed to be integrated into the routine antenatal care provided by the Genetics Department and received IRB approval from the Son Espases University Hospital ethical committee for clinical research (CEA). In brief, at week 10–12 of pregnancy, women routinely (>95 %) have a consultation with the gynecologist, undergo an ultrasound scan of the fetus, and blood is drawn for a biochemical screening test for aneuploidy risk calculation (see Fig. 1 for complete workflow). We took advantage of the same blood drawing performed for the biochemical screening test, to draw an additional 5 ml of EDTA blood for FXS testing. During the same consultation, each woman received oral information provided by the midwife and/or gynecologist about the FXS disorder together with a written leaflet (Figure S1). The provided information was a short oral explanation and a leaflet with the following information: clinical and genetic traits of the FXS, an explanation of the test, meaning of the different result categories (Normal, IM, PM and FM) and recommendations for the participant in each case (Figure S1).
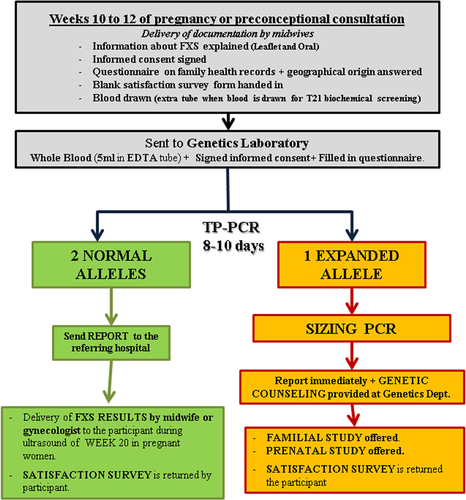
Workflow for the pilot screening for the Fragile X syndrome (FXS)
After receiving information on FXS, those who chose to participate in the study filled in a consent form and provided a written answer of a survey of the family health history and the geographic origin of the maternal grandparents of the fetus (Figure S2). Those that declined participation were asked to answer a short questionnaire on the reasons for their refusal. The informed consent had to be filled in and signed; otherwise the FXS test was not performed.
As an exception, blood was drawn specifically for the study, after informed consent, from women who were attended by midwives in a preconception consultation. Preconception participants were provided with the same information and forms as pregnant participants. Of note, not all gynecologists and midwives of the public health system in the Balearic Islands (IB-Salut) participated in the study, and thus, not all pregnant women had access to the study during the recruitment period.
Seven to 10 days post recruitment, written negative screening reports were returned to participants by the gynecologist or midwife and genetic counseling was offered. If a PM or IM was detected, the participant was immediately contacted by phone and given an appointment at the Genetics Department with a Genetics MD. During consultation, the written report with the detailed findings (repeat size of PM or IM) and risks for the fetus and family were explained, and prenatal testing options were explained and offered. In addition, if agreed to by the participant, a cascade family study was initiated to identify additional members of the family carrying a PM or IM alleles. The participant was told which members of the family were suitable for cascade screening using the information from the family pedigree and she was the person responsible for communicating this option to her family members. In the case of IM alleles, the same procedure was followed, and allele stability within the family was evaluated to inform the patients in case there was evidence for allelic instability (members of the same family with different repeat sizes for the IM allele).
Instrumentation
Awareness of the disease was measured by asking participants at enrollment, after reading the information sheet, to indicate if they had any previous knowledge of FXS. The question was: “Before today, have you ever heard about FXS?” (Figure S2).
In addition, each patient received a blank satisfaction survey form to be completed and returned after the patient received the written report with the results of the FXS test (Figure S3). This survey sheet contained seven questions. Questions 1 to 4 were intended to measure satisfaction of participant with: information provided, care provided before and after results of test, and time passed from blood collection to results (Scale: 0 = totally unsatisfied to 10 = totally satisfied). Question 5 was intended to measure anxiety generated by the test (Scale: 0 = no anxiety to 10 = great anxiety). Question 6 asked if the anxiety generated by the test was offset by the usefulness of the results (Scale: 0 = not at all to 10 = completely). And finally, question 7 asked if anxiety produced by the test increased anxiety already present and caused by pregnancy itself (Scale: 0 = no increase to 10 = great increase).
Sample Processing Procedures
All blood samples were referred to the Genetics department of Son Espases University Hospital from hospitals and health centers in the Balearic Islands (see additional Table S1 for center list and number of patients) from November 2012 to March 2014. A total of 3,731 blood samples from women of childbearing age were tested, including 3,413 pregnant and 318 non-pregnant women. Genomic DNA was extracted from EDTA blood samples using standard procedures (Promega, Wizard Genomic). FMR1 CGG repeat allele size determination, AGG interruptions, and carrier status were determined by a triplet primed PCR (TP-PCR) approach as described in the literature (Basehore et al. 2012; Lyon et al. 2010; Nolin et al. 2013; Yrigollen et al. 2012). The genomic DNA samples were analyzed using lab-validated FMR1 assay utilizing commercially available FMR1 TP-PCR reagents (Abbott Molecular Inc., Illinois, USA). Samples showing a number of repeats higher than normal limits (≥45 repeats) were reanalyzed using lab-validated FMR1 assay utilizing commercially available FMR1 sizing reagents (Abbott Molecular Inc., Illinois, U.S.A), to determine sizes of expanded alleles with precision.
Data Analysis
The PM or IM carrier frequency was defined as the number of heterozygotes with the PM or IM allele, respectively, divided by the total number of individuals, and 95 % confidence intervals (CI) were calculated as previously described (Newcombe 1998).
Results
Summary of Results
We tested blood samples from 3, 371 women, of which 3,413 were pregnant (91.5 %) and 318 were non-pregnant (8.5 %). In addition, we received only 0.8 % (n = 29) declines to participate in the study (Table 1). There were 10,530 recorded births in the Balearic Islands in the year 2013, and thus, our study was offered to and covered about 35 % of annual pregnancies. Geographic origins of the parents of participants were obtained and are summarized in Fig. 2. We found that 60 % of parents of participants were born outside of the Balearic Islands. Furthermore, 68 % of participants had at least one parent born outside of the Balearic Islands. Molecular tests identified a total of 35 PM and 108 IM alleles. The resulting frequency of PM carriers from the observed results was 1 in 106 [95 % confidence interval (CI), 1/77-1/155] and the mean CGG length was 61 repeats (Range: 55–83). The estimated frequency of IM carriers was 1 in 35 (95 % CI, 1/29-1/42) and the mean CGG length was 48 repeats (Range: 45–54).
| Total # women tested | 3,731 |
| # women declined to participate | 29 |
| # pregnant women tests | 3,413 |
| # preconception women tests | 318 |
| # FM detected (>200 reps.) | 0 |
| # PM detected (55–200 reps.) | 35 |
| # IM detected (45–54 reps.) | 108 |
| # IM cascade family studies initiated | 14 |
| # PM cascade family studies initiated | 16 |
| # of additional IM individuals detected through cascade family testing | 19 |
| # of additional PM individuals detected through cascade family testing | 16 |
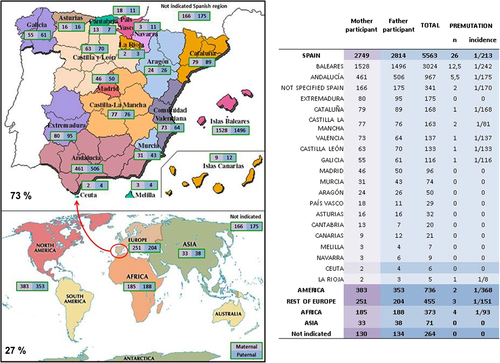
Geographic origins of parents of participants. a Maps with numbers of parents (fathers and mothers) for each Spanish region or continent. b Table with numbers and incidence of PM calculated with the total number of parents for each geographic region
As a result of the detection of IM or PM alleles, a total of 30 participants agreed to initiate a family cascade screening study. Cascade screening was performed in 14 families with an IM allele and 16 families with a PM allele. Through these family studies we ascertained a total of 19 IM carriers and 16 PM carriers outside of the women initially recruited (Table 1). All pregnant women decided to continue their pregnancy normally. Detailed molecular results obtained from this FXS screening study and the prenatal and family studies are summarized in another paper (Alfaro Arenas et al. 2016).
Awareness of the Disease
At enrollment, after reading the information sheet, participants were asked in a preliminary questionnaire to indicate if they had any previous awareness of FXS. The question was: “Before today, have you ever heard about FXS?” (Figure S2). In total, 25 % (n = 950) answered that they had heard before about the FXS. This suggests that 75 % of our female population is unaware of the existence of FXS.
Self-Reported FXS-Associated Health Factors
The preliminary questionnaire asked if the participant had records of intellectual disability, tremor/ataxia or premature ovarian insufficiency in their family history, and the geographical origin of the parents of the participant (Figure S2). Three-hundred ninety-five of 3,731 participants self-reported a positive familial history of intellectual disability (~10 %), including 7 self-reported Down syndrome affected members; 244 women reported a premature ovarian insufficiency history within their family (6.5 %); and 174 reported tremor/ataxia (4.6 %). Table 2 summarizes the data obtained from the participants’ self-reported family health history and Table 3 contains a list of the self-reported diseases. Among the 395 women with a positive familiar history of intellectual disability, 4 IM carriers and 5 PM carriers were identified, although in one of these positive cases the self-reported cause of intellectual disability was Down syndrome. Among the 244 participants who reported a premature ovarian insufficiency history within their family, 3 IM carriers and 3 PM carriers were identified. Tremor/ataxia was reported in 174 cases, including 2 IM carriers and 1 PM carrier. In total, the frequency of PM in participants who reported a family history of FXS-related health problems is similar to that of those that did not report any family history (0.9 vs 1 %).
| CGG repeat size | Self-reported family health history | |||
|---|---|---|---|---|
| ID and/or POI and/or Ataxia/Tremor | No reported History | |||
| n | %* | n | %a | |
| Normal | 699 | 97.8 | 2927 | 97.0 |
| Intermediate | 9 | 1.3 | 61 | 2.0 |
| Premutation | 7 | 1.0 | 28 | 0.9 |
| Full mutation | 0 | 0 | 0 | 0 |
| TOTAL | 715 | 100 | 3016 | 100 |
- ID intellectual disability, POI premature ovarian insufficiency aPercentages do not add to 100 due to rounding of numbers to a decimal place
| Self-reported family history | Self-reported diagnostic | n°. Of cases |
|---|---|---|
| ID | Down syndrome | 7 |
| Autism | 2 | |
| ID + omphalocele | 1 | |
| Cerebral paralysis | 1 | |
| problems at birth | 1 | |
| Asperger | 1 | |
| Not reported diagnostic | 382 | |
| Total | 395 | |
| POI | Breast cancer | 1 |
| Surgical removal | 1 | |
| Not reported diagnostic | 242 | |
| Total | 244 | |
| TREMOR/ATAXIA | Vertigo | 1 |
| Parkinson | 10 | |
| Multiple sclerosis | 2 | |
| Epilepsy | 1 | |
| Dementia | 1 | |
| Alzheimer | 1 | |
| Not reported diagnostic | 158 | |
| Total | 174 |
Acceptability of the FXS Test
To evaluate the acceptability of FXS screening, we included a question in the preliminary survey for women who declined to participate in the study, to indicate the reason. We received a very small number of declines (0.8 %; N = 29). These women indicated causes of non-acceptance that were mainly lack of interest (38 %), followed by fear (17 %), and religious reasons (4 %) (Figure S4).
Anxiety and Satisfaction
Measures that we implemented to minimize anxiety included: 1) test information was provided by midwives and gynecologists together with that of the routine trisomy biochemical screening; 2) Participants were given a telephone number to which they could call in and speak with the project coordinator/genetics counsellor in case they had questions on the offered test. If the telephone answers were not satisfactory they were given an in person appointment at the Genetics department to answer all their questions and concerns; and 3) Patients with a PM or IM result were immediately given an appointment with a genetics MD/counsellor to discuss all the options, and to try to address any created stress. All women in whom a PM was detected accepted the offered genetic consultation, while a small proportion of women with a normal result called in to request more information and very few of these came in for a consultation (numbers not recorded).
Once the results of the FXS test had been received, participants completed a satisfaction survey consisting of seven questions (Figure S3). A total of 607 women (16.3 %). completed and returned the survey. Results are presented in Table 4. In summary, satisfaction with information, care provided, and waiting time for the results was high, averaging 8.4. The anxiety generated by the study was low, averaging 2.7 and the usefulness of the test was rated with an average of 6.
| Questions of the satisfaction survey | Mean |
|---|---|
| Satisfaction with the information provided on the FXS | 8.4 |
| Satisfaction with the care provided during the process (from the proposal of screening to delivering of results) | 8.6 |
| Satisfaction with care received (genetic counseling, etc.) after knowing the results of the tests | 8.6 |
| Satisfaction with the time between blood collection and receipt of the results | 8.2 |
| Anxiety generated by the FXS screening | 2.9 |
| The anxiety generated by the test has been offset by the usefulness of the results obtained | 6.0 |
| The anxiety generated by this study has increased the anxiety caused by the pregnancy itself | 2.6 |
- Scale: 0 to 10, where a higher score indicates greater satisfaction, perceived utility, and anxiety
Geographic Origins of Participants
The participants were asked to specifically indicate geographic origin of their mother and father in order to be able to distinguish potential ethnic or geographical differences in PM and IM incidences (Fig. 2). We found that only approximately 40 % of the mothers or fathers of participants had been born in the Balearic Islands. Of the remaining 60 %, 34 % were born in other geographical regions of Spain, and 26 % in other countries. In total, 68 % of participants had alleles (either paternal, maternal or both) originating outside of the Balearic Islands in the previous generation. In addition, when possible we traced the allelic origin (paternal and maternal) of participants with a PM or IM allele using the information of the cascade familial studies. When we calculated the incidence of PM across the global population of parents, we found that those of Balearic origin showed one of the lowest incidences of PM compared to parents with other Spanish or foreign origins. For example, Balearic-born parents (male and female together) showed an incidence of PM of 1/242, while the second most numerous Spanish-origin group from Andalucía, showed an incidence of 1/175. However, because of low numbers within each group, none of these differences in PM frequencies among regions and continents was statistically significant (p > 0.05).
Screening Costs
We calculated the direct costs per test based on this pilot study. This calculation takes into account general laboratory reagents and personnel (a full time technologist that performs tests, analysis and reports). The amount comes up to 5 € per sample to which we have to add the price of reagents for the triplet-primed PCR (TP-PCR). Market prices for commercial kits of FXS triplet-primed PCR currently range around 60 € per sample, although this amount could be considerably lowered if bought in bulk. If we consider that the price of an FXS reagents kit could be lowered to between 30 and 45 €/ patient by buying in bulk 10,000 samples, and estimate indirect costs (genetic counseling, use of hospital facilities, etc.) in 25 % of direct costs, we could come up with a range of prices per test of between 43.75 and 57.50 €. If we assume 10,000 pregnancies per year in the Balearic Islands and a 100 % uptake, an estimation of the yearly cost for screening in pregnant women would be between 437,500 and 575,000 € for a population of around 1 million inhabitants. These figures would decrease over time as pregnant women get tested and cascade family studies are performed. This is because the total numbers of tests should decrease year to year as the proportion of women and families in the population who know their FXS status increases.
Discussion
Currently, FXS can be easily identified with accurate and effective molecular screening methods (e.g., TP-PCR which was used in this study) (Alfaro Arenas et al. 2016). We present here findings of the first FXS population-based study in Spain on the prevalence of premutation and intermediate CGG repeat alleles in women of reproductive age and the first European pilot study on an unselected population of pregnant women or women who are planning pregnancy. In our study population, we observed a premutation prevalence rate of 1 in every 106 females, which to our knowledge is the highest prevalence rate reported for females in the literature (Berkenstadt et al. 2007; Hantash et al. 2011; Jang et al. 2014; Seltzer et al. 2012; Tassone et al. 2012). We also found an intermediate allele (45–54 repeats) prevalence rate of 1 in 35 females which is similar to that reported in the United States (Seltzer et al. 2012). The results obtained from the FXS screening study and the resulting prenatal and family studies (cascade screening) are summarized in Table 1 and in a separate molecular report (Alfaro Arenas et al. 2016).
Awareness of the Disease, Geographic and Health Factors
The FXS is the most common cause of inherited intellectual disability, and thus is a relatively frequent disease. However, participants when asked in a preliminary questionnaire to indicate if they had previous knowledge of the FXS, only 25 % had heard of FXS and its consequences before this study. These results show a need for more education in order to increase awareness of the disease within our population. Studies like the present one in which information was provided orally by midwives, obstetricians and geneticists and in written form by a leaflet (figure S1) should certainly contribute to such awareness. The observed low levels of awareness of FXS argue for the need to implement genetic counseling only by knowledgeable professionals in any institutionalized screening. In fact, none of the PM and IM carriers identified in our study opted for pregnancy termination, which in our view reflects that a pregnant PM carrier when confronted with the outcomes and available options by prepared genetic counsellors, understands them and rarely adopts drastic and potentially non-proportionate measures. Furthermore, most participants identified as PM or IM allele carriers reported transmitting the information to their relatives and families and expressed high interest in having cascade screening among their members.
The observation that a majority of participants had parents originating outside the Balearic Islands is a reflection of the great influx of migrant population over the last 60 years in the Balearic Islands. In fact, the region has increased from 422,000 inhabitants in 1950 to 1,111,000 in 2013, mainly because of immigration from other parts of Spain and the World. In this context, we have tried to indirectly find global PM frequencies (male + female) for each of the Spanish regions by considering the self-reported geographic origin of the parents of participants. The same was done for parents born in other countries. We found important differences in PM frequencies between these populations (see Fig. 2). The lowest PM frequency among Spanish parents (only groups larger than 100 considered) is that of those indigenous to Extremadura, 0 PM (N = 175), followed by those from the Balearic Islands, 1/242 (N = 3024). The highest frequency was among those from Castilla-La Mancha 1/81 (N = 163), followed by Galicia 1/116 (N = 116).
Internationally we find the highest PM frequency among African (mostly North African) parents (1/93; N = 373) and the lowest among American (mostly South and Central American) (1/368; N = 736). However, when the parental population is divided, the statistical potency is lost and none of these differences are significant. In any case, the observation of a relatively low PM frequency among the Balearic Islands native population would argue against any differentiating trait of Balearic Islands’ natives compared to other populations. Furthermore, it makes it very unlikely that the high prevalence observed in this study is originated by founder effects and isolation of the Balearic population through history.
Participants were also asked for family records of intellectual disability, tremor/ataxia or premature ovarian insufficiency, and 10 % reported a positive familial history of intellectual disability, 6.5 % of premature ovarian insufficiency and 4.6 % of tremor/ataxia. Participants with a self-reported family history of intellectual disability, ovarian insufficiency and/or ataxia/tremor show similar PM incidences as those with no family history (1.0 % vs 0.9 %) (Table 1). This result suggests that voluntarily forwarded self-reported history of clinical conditions associated with FXS does not increase significantly the probability of detecting expanded alleles in comparison to participants who do not report such clinical conditions in their families. These findings suggest that obtaining a family history does not effectively screen for FXS, or enhance the effectiveness of a population-level screen for FXS.
Anxiety and Reasons for Refusal
We received a very small number of declines to participate in the study. The recorded reasons for non-acceptance argue against religious or ethical beliefs as a cause of frequent declines within our population. In addition, we found high levels of satisfaction and low self-reported anxiety. These results indicate a high voluntary acceptance of the study by pregnant and non-pregnant women and that the screening program resulted with a high level of satisfaction and low levels of stress.
Study Limitations
Not all gynecologists and midwives participated in the study, and thus, not all pregnant women had access to the study during the recruitment period. In total, 3,731 blood samples from women of childbearing age were tested, including 3,413 pregnant (91.5 %) and 318 non-pregnant women (8.5 %). In 2013, there were a total of 10,530 recorded births in the Balearic Islands, and thus our study covered roughly 35 % of annual pregnancies. To our knowledge, only 0.8 % of the women refused to participate in the study, and thus we have no reason to believe that the pregnant female population that was screened is biased in any way. There are some concerns, however, regarding the extent to which the present sample is representative of the total Balearic Islands female population. Participants came from the public health system, which is used by most of the immigrants and working class people but less used by the wealthier part of society. Thus, possible biases may be overrepresentation of immigrants, under representation of affluent members of society, and other unknown reasons for bias that could not be assessed. In addition, only 16 % of participants returned the satisfaction survey, and thus the satisfaction results may not be representative for all the women of the study.
Practice Implications
There is ongoing debate regarding the utility of population screening for FXS, and whether it should be done neonatally or by testing women of reproductive age (preconception or in pregnant women). Testing of women of reproductive age, in our view, has advantages over neonatal screening because: 1) Female carriers of premutations are 2–3 times more frequent than male ones (~1/150 ♀ vs. ~1/400♂) (Hill et al. 2010); 2) Expansion from premutation to full mutation only occurs in mother to child transmissions; 3) During a woman's reproductive life, premutations do not produce an obvious phenotype that makes them easily detectable and 4) Once a premutation is detected, prenatal diagnosis can be offered and different reproductive options are available for future pregnancies.
In 2005, Musci and colleagues, calculated a cost effective screening if the cost per sample was under 95$ assuming that the calculated health care and social cost of a person suffering from the FXS is 14,858$/ year (Musci et al. 2005). In our pilot study no pregnancies with a FM were detected, but in such case it is likely that many women would choose to terminate their pregnancy. Pregnancy termination is not the only option and early detection of FXS has advantages because affected offspring can benefit from early intervention and, even early therapy, if ongoing clinical trials for a pharmacological treatment are successful (Berry-Kravis 2014). Furthermore, these calculations do not include other diseases associated with the FXS PM, like POI and FXTAS which would also benefit from screening. Knowledge of being a carrier of a PM allows for early treatment and avoids a diagnostic odyssey to find the cause of illness after the initial symptoms. Thus, if we take these calculations as applicable to our population, expenses within a screening setting as ours should be highly cost-effective.
Ideally, it would be best to screen women before pregnancy because it gives women more options in case they are PM carriers. Women could opt in these cases for in vitro fertilization techniques together with preimplantation diagnostics or donor oocytes. However, implementation of public screening on all fertile women preconceptionally also carries implicit ethical considerations, such as deciding the best age to do it (e.g., should it be done in adolescents or only in consenting adults?), and in what context (How do you access women? Gynecologists, pediatricians caring for females between 16 and 18, specific consultation at any age?; Added to existing screening programs or implemented as a new population screen?). In our view, for practical reasons, it could be more difficult and costly to have all women undergo the FXS test before pregnancy. It would mean a considerably larger portion of women to be screened per year and a totally new screening program to be implemented in the public health system. Practical arguments in favor of screening for FXS during pregnancy in our community are that we can easily take advantage of the biochemical screening for aneuploidies that are in place, as we have done in this study, at very little additional cost or stress for participants.
Conclusion
In conclusion, the results of this study present evidence of low added stress, perceived usefulness, and efficacy of pregnancy and/or preconception screening of women for FXS in a public health system. Thus, this study suggests that FMR1 screening for FXS in women who are pregnant is a useful tool that should be considered for implementation.
Future Research Recommendations
Further studies in other populations and health systems may be useful to demonstrate and corroborate the study findings. Future research and implementation of non-invasive prenatal screening may also provide totally new outlooks and ways in which FXS screening can be performed.
Below is the link to the electronic supplementary material.
Acknowledgments
We thank participants and professionals who contributed to this study. We also thank Natalia Marlowe, John Norton, Birgit Reinhardt, Koldo Aurrekoetxea and Judy H Yu from Abbott Molecular for their help.
Compliance with Ethical Standards
Conflict of Interest
Authors Ramona Alfaro Arenas, Jordi Rosell Andreo and Damián Heine Suñer have been funded by an Abbott Laboratories Inc. grant.
Human Studies and Informed Consent
‘All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.’
Animal Studies
No animal studies were carried out by the authors for this article.




