Differential effects of dietary protein on early life-history and morphological traits in natterjack toad (Epidalea calamita) tadpoles reared in captivity
Abstract
The production of high quality amphibian larvae through optimal diets is a critical component of amphibian conservation breeding programs. Larval period, survival, body weight and total length are frequently used as metrics of adequate nutrition. However, the effects of nutrition on tadpole and metamorph morphology are rarely tested in detail. In the present study, we analyzed the most common metrics and six other larval and post-metamorphic morphological traits in natterjack toads (Epidalea calamita) fed with three different commercial fish diets, varying in protein content (32.0%, 38.3%, and 46.2%). Our results suggest that early life-history (tadpole growth, development, and survival) and morphological traits of E. calamita tadpoles are differentially affected by the percentage of dietary protein. As protein content increased, tadpoles exhibited larger bodies along with shorter tail fins; however, with no significant differences in total length. Larval period was similar across treatments but mortality was lower in high-protein diet. At high-protein diets the metamorphs revealed significantly longer bodies, and wider heads and hind legs, but there was no significant difference in the average weight across all dietary treatments. Based on our results, feed containing 46.2% protein promotes growth, development and survival of E. calamita tadpoles better than either of the other two feeds tested. The use of other body measures beyond weight, tadpole total length, and snout-vent length in studies of amphibian nutrition in captivity may assist the selection of appropriate diets to optimize tadpole survival and metamorph fitness. Zoo Biol. 32:457–462, 2013. © 2013 Wiley Periodicals Inc.
INTRODUCTION
Nutrition is one of the main husbandry issues when rearing amphibian larvae, particularly within conservation breeding programs [Browne and Zippel, 2007]. Despite its crucial importance, the knowledge of amphibian nutritional requirements among researchers and zoo staff does not reflect the increasing number of species that require captive breeding programs (see Altig et al. [2007] for details regarding feeding ecology of amphibian larvae) and only comprises a minor fraction of the current literature on amphibian captive rearing [Browne et al., 2011].
Dietary protein plays an important role in the growth and development of amphibian larvae [Kupferberg, 1997] and, therefore, a knowledge of the dietary protein requirements of any species is essential to optimize its health and development. Most of the studies published on amphibian nutrition are directed to those few species used in frog farming [e.g., Martínez et al., 1993, 1994; Carmona-Osalde et al., 1996; Sretarugsa et al., 1997]. However, in conservation breeding programs, larval husbandry aims to improve long-term fitness and viability of populations of a wide range of amphibian taxa [Gascon et al., 2007], and few advances in larval nutrition research have been made [e.g., McCallum and Trauth, 2002; Banks et al., 2008; Matson et al., 2010]. Short larval developmental periods and large body sizes at metamorphosis are desirable, as these traits enhance individual fitness: (1) younger age and higher survivorship to maturity, (2) larger size at first reproduction, and (3) higher fecundity [Werner, 1986; Smith, 1987; Semlitsch et al., 1988].
The optimal dietary protein level for a species is frequently assessed by one to five larval traits—survival, growth, larval period, body weight, and tadpole total length—and one post-metamophic trait, the snout-vent length [e.g., Martínez et al., 1994; Banks et al., 2008; Matson et al., 2010]. However, there are other morphological traits besides body total length, such as tadpole tail shape and metamorph's leg and head sizes, that are possibly affected by diet and have major consequences on larval [Van Buskirk and McCollum, 2000; Dayton et al., 2005] and metamorph locomotion [Álvarez and Nicieza, 2002; Tejedo et al., 2010] and juvenile feeding [Tejedo et al., 2010], respectively.
In this study, we evaluated the effects of dietary protein content on early life history traits (tadpole growth, development, and survival) and tadpole and metamorph morphometrics in a bufonid, the natterjack toad (Epidalea calamita). E. calamita breeds in temporary ponds [Tejedo and Reques, 1992], taking 2 weeks to 3 months to metamorphose [Tejedo, 1993; Reques and Tejedo, 1995]. Tadpoles browse mostly on detritus and periphyton [Diaz-Paniagua, 1985]. Bufonids are of particular interest for captive breeding since this family includes several species facing enigmatic declines [Stuart et al., 2004]. In particular, E. calamita populations have declined in recent decades at the species' range margins, mainly due to loss and degradation of suitable habitat, and might be threatened by the chytrid fungus in some areas [Beja et al., 2009].
No study so far has evaluated both early life history and morphological responses to different diets in anuran larvae. Such knowledge is a critical component of husbandry practice for the rearing of amphibian larvae, and vital in conservation breeding programs to provide metamorphs with the morphological characteristics that optimize fitness and survivability.
MATERIALS AND METHODS
Experimental Design
A single egg string of E. calamita was collected from a temporary pond near Grândola, Portugal in February 2009 (38°10′N; 8°57′W; 180 m elevation). A subsample of 75 tadpoles was selected when the first larvae reached Gosner [1960] stage 25. Tadpoles were individually placed in hemispheric polyethylene containers with 250 ml aged tap water and then randomly allocated to three experimental groups of 25 each, corresponding to different dietary treatments. Dietary treatments were selected from the most commonly used commercial granulated fish foods (Sera® GmbH, Heinsburg, Germany) to provide a wide range of dietary protein while otherwise providing complete nutrition (see Table 1).
| P32 | P38 | P46 | |
|---|---|---|---|
| Nutrient content (%) | |||
| Protein | 32.0 | 38.3 | 46.2 |
| Fat | 7.2 | 6.1 | 6.2 |
| Fiber | 5.6 | 3.9 | 3.7 |
| Ash | 9.3 | 9.5 | 11.2 |
| Moisture | 6.4 | a | a |
| Composition (%) | |||
| Animal source | 37.5 | 25.0 | 62.5 |
| Vegetable source | 50.0 | 62.5 | 12.5 |
| Other | 12.5 | 12.5 | 25.0 |
- a No information provided.
A randomized block design was used with one replicate of each dietary treatment within every block, and blocks were randomly displaced in the shelves every week. Water changes were performed and tadpoles were fed with the same amount of food (≈10% of mean body weight), according to their respective treatment, three times per week until metamorphosis was reached.
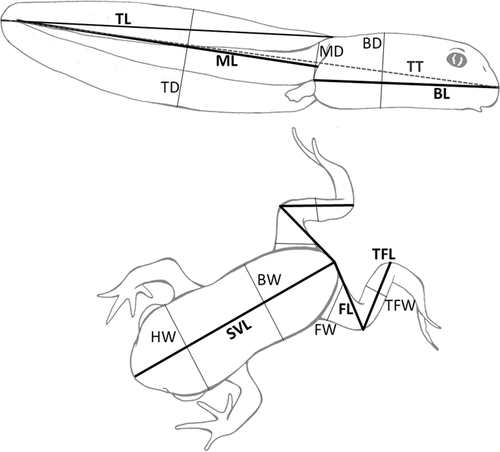
Each tadpole was weighed individually (±0.01 g) and photographed against a measuring scale, at four moments: (i) the start of the experiment, (ii) 1 month later, (iii) the onset of metamorphosis (Gosner stage 42), and (iv) the end of metamorphosis (Gosner stage 46). Mean body weight and morphology of tadpoles (Fig. 1) did not significantly differ among treatments at the start of the experiment (P > 0.05; see Statistical Analysis below). Larval period (in days) or date of death were also recorded.
Powder-free vinyl gloves were used to minimize the risk of contamination and possible mortality [Cashins et al., 2008]. Rearing containers were kept under natural conditions of air temperature and photoperiod (from 12L:12D in March to 14L:10D in early June). Water temperature fluctuated naturally between 12°C and 22°C. The first individual completed metamorphosis on day 58, and the experiment was completed on day 96 when the last individual was measured.
Statistical Analysis
Overall proportions of survival to metamorphosis within dietary treatments were computed. Since data did not meet the assumptions of normality and/or homogeneity of variances, non-parametric Kruskal–Wallis (K–W) tests were used to analyze the effects of diet on body weight (W), larval period, and relative growth rate (RGR = 100 × (Wf − Wi)/Wi), and to compare the day of and stage at the moment of tadpole death among treatments.
Morphological traits (Fig. 1) were measured from images using SigmaScan Pro 5.0.0 software (Aspire Software International, Ashburn, VA). Intra-observer reliability in image measurement was assessed using the Standard Error of Measurement (SEM) calculation [Weir, 2005]. Since average SEM was negligible (0.31 mm), we performed analyses on measurement data without error.
To compare body measurements among treatments, we employed a method frequently used on studies of induced phenotypic variation in tadpoles [e.g., Van Buskirk and McCollum, 2000; Relyea, 2001]. Firstly, a principal components analysis performed on log transformed measurements was conducted for size correction [McCoy et al., 2006]; as all traits loaded strongly and positively on the first axis, which is assumed to represent “body size,” their measurements were regressed against the PC1 scores, and the residuals compared using one-way ANOVAs. Measures of right and left sides of metamorphs' traits were averaged to eliminate asymmetry effects [Van Buskirk and Saxer, 2001]. Tuckey HSD post hoc tests were performed when treatment effects were significant (P < 0.05 for all tests).
RESULTS
Body Weight and Life History Traits
There were no significant effects of diet on body weight 1 month after the beginning of the experiment (Table 2). At the onset of metamorphosis, tadpoles fed with the highest protein content exhibited significantly higher growth rates (K–W χ2 = 7.16, df = 2, P < 0.05) and body weights (K–W χ2 = 6.51, df = 2, P < 0.05) compared to those fed low-protein diets. Nevertheless, there were no significant differences on larval period or on metamorphs' body weight among treatments. Survival to metamorphosis was higher in the group subjected to the highest protein content but the lowest protein content did not induce the highest mortality (Table 2). There were no differences in the mean day or stage of death among treatments.
| Kruskal–Wallis | P32 | P38 | P46 | ||
|---|---|---|---|---|---|
| df | χ2 | ||||
| Initial body weight (g) | 2 | 3.84 | 0.017 ± 0.001 | 0.015 ± 0.001 | 0.015 ± 0.001 |
| Body weight at day 30 (g) | 2 | 1.42 | 0.073 ± 0.003 | 0.077 ± 0.003 | 0.076 ± 0.002 |
| Body weight at onset of metamorphosis (g) | 2 | 6.51* | 0.089 ± 0.005a | 0.093 ± 0.003a | 0.103 ± 0.002b |
| Body weight of metamorphs (g) | 2 | 4.28 | 0.067 ± 0.004 | 0.073 ± 0.005 | 0.076 ± 0.003 |
| Relative growth rate (%) | 2 | 7.16* | 461.4 ± 66.2a | 563.9 ± 66.9a | 676.9 ± 56.1b |
| Larval period (days) | 2 | 1.37 | 57.1 ± 1.4 | 58.9 ± 1.5 | 57.2 ± 0.9 |
| Survival to metamorphosis (%) | 60 | 40 | 76 | ||
| Stage of death | 2 | 0.749 | 33.70 ± 1.74 | 35.20 ± 1.60 | 38.17 ± 3.00 |
| Day at death | 2 | 0.176 | 38.40 ± 8.12 | 41.80 ± 6.22 | 46.17 ± 11.28 |
- Values given are mean ± SE. Asterisk (*) denotes significance of difference among treatments (P < 0.05, Kruskal–Wallis non-parametric test) and means that are significantly different are shown with a different letter.
Morphology
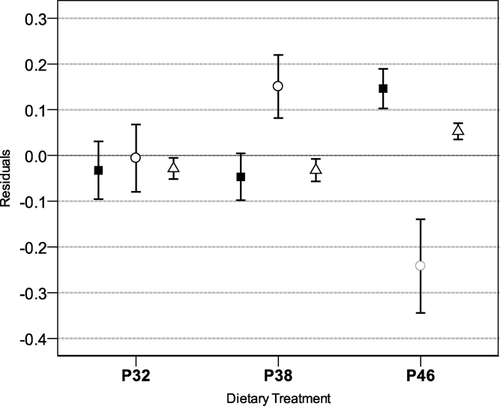
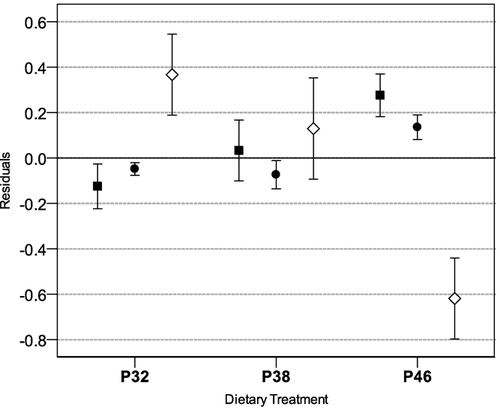
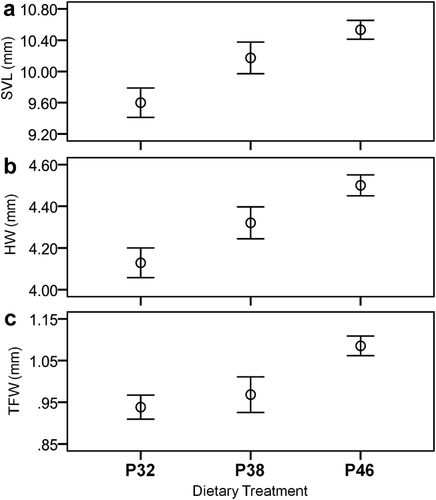
After 1 month of experiment, it was already possible to find significant differences in body and tail shapes (Fig. 2). Tadpoles fed the highest dietary protein content exhibited significantly longer bodies and more muscular tails with thinner fins (ANOVA: BL, F = 4.37, df = 2.57, P < 0.05; MD, F = 5.29, df = 2.57, P < 0.01; and TD, F = 5.16, df = 2.57, P < 0.01). At the onset of metamorphosis, tadpoles fed the highest dietary protein content exhibited larger bodies and shorter tail fins than those of remaining treatments (ANOVA: BL, F = 3.94, df = 2.50, P < 0.05; BD, F = 5.30, df = 2.50, P < 0.01; TL, F = 7.93, df = 2.50, P < 0.01; Fig. 3). There were significant differences among diet treatments in snout-vent length, head, and tibio-fibula width of the newly metamorphosed tadpoles (ANOVA: SVL, F = 9.32, df = 2,41, P < 0.01; HW, F = 9.65, df = 2,41, P < 0.01; TFW, F = 7.84, df = 2,41, P < 0.01; Fig. 4). Individuals fed the highest dietary protein content achieved significantly longer bodies, and wider heads and tibias than those fed lower dietary protein contents. No skeletal malformations were detected in our study.
DISCUSSION
Our results suggest that percentage dietary protein can differentially influence multiple larval and post-metamorphic traits in anurans. No significant effects of percentage dietary protein were detected on metamorphs' weight or larval period, whereas there were significant effects on multiple morphological traits and growth rates, in both tadpoles and resulting metamorphs. Tadpoles grew faster and had higher survival at the highest dietary protein level tested, exhibiting larger larval bodies and shorter tails, and producing larger metamorphs. Furthermore, metamorphs had also wider heads and thicker hind legs. Wide heads may increase feeding capability [Tejedo et al., 2010] and thus increase survival of newly metamorphosed toads, whereas thicker hind legs are probably the result of an increased muscular mass, enhancing dispersal, and/or fleeing ability. Similarly to other amphibian species, large size at metamorphosis may also promote reproductive success in E. calamita as size is positively related with fecundity (both clutch and egg size) in E. calamita females [Tejedo, 1992a], and with growth rates and mating success in males [Tejedo, 1992b]. Nevertheless, advantages of a large body at metamorphosis may not be permanent under poor habitat/captivity conditions during the juvenile phase [Sinsch et al., 2010; Verschooren et al., 2011].
Tadpole rearing represents a demanding task for zoological institutions but few nutrition studies are available to support their conservation breeding programs. Although poor in proteins and other nutrients, lettuce-based diets are still being used in conservation breeding programs [e.g., Banks et al., 2008; McFadden et al., 2008]. The use of nutrient-poor diets in these programs might represent serious consequences to larval growth and development [Matson et al., 2010]. Alternatively, commercial diets formulated for fish can be provided but there is little information about how their quality influences larval growth and post-metamorphic health in anurans. A recent study revealed that Lithobates sphenocephalus tadpoles fed with low levels of dietary protein (13.8%) become more susceptible to the infection by Batrachochytrium dendrobatidis than those fed with a high-protein diet (47.6%) [Venesky et al., 2012]. In general, high-protein diets (>35%) improve tadpole growth and health, but optimal dietary protein level may vary between species. For example, Lithobates catesbeianus tadpoles fed diets with 39–45% protein achieved better results regarding feed conversion, growth, and survival than those fed diets with 30–35% protein [Carmona-Osalde et al., 1996; Sretarugsa et al., 1997]. Also, Hoplobatrachus tigerinus and Pelophylax perezi tadpoles grew faster and survived better when fed diets containing 39% [Sretarugsa et al., 1997] and both 39% and 45.5% dietary protein [Martínez et al., 1993], respectively. Although a bufonid with fast-growing tadpoles, E. calamita showed similar responses to different dietary protein levels as those of ranids, considering larval growth and survival, and metamorph dimensions. On the other hand, high dietary protein levels can negatively affect tadpole growth due to the additional energy that may be required for protein metabolism [Martínez et al., 1993] but this was not observed in our study. Additionally, there are nutritional components other than protein that need to be investigated and correctly dosed, such as lipids [e.g., Scott et al., 2007], vitamins [e.g., Niazi and Saxena, 1968], and carotenoids [e.g., Ogilvy et al., 2012].
Larval traits used to evaluate individual fitness in breeding programs have been poorly researched. Our results suggest that if only body weight is considered, the effects of diet on overall tadpole dimensions will not be accurately interpreted. Also, diet-induced differences on tadpole body and tail lengths go unnoticed when only tadpole total length is measured. Some traits that may provide long-term benefits to larvae and metamorphs that are seldom researched, such as hind leg and head width [Tejedo et al., 2010], were shown to be significantly affected by percentage dietary protein. More studies are needed on amphibian nutrition in order to enhance our understanding of larval growth and to lead to more successful conservation breeding programs. We recommend the measurement of other morphological traits beyond those already in use.
ACKNOWLEDGMENTS
We would like to give special thanks to the Reptile House's staff of Lisbon Zoo, Susana Silva, Paulo, and Lena, for the support and assistance during the whole study period, and to the curator Telma Araújo for helping with the logistics. We also thank our anonymous reviewers for their extensive and helpful comments on the manuscript. We are grateful for financial support from FCT (Portugal) through its Pluriannual Funding Program (to R. Rebelo); amphibian capture and handling was licensed by ICNB, Portugal (nos. 321/08/CAPT and 23/09/CAPT).




