The Sequence Makes the Difference: On the Reaction of Phosphorus-centered Biradical [⋅P(μ-N-tBuPh3C)2P⋅] with Tolan
Abstract
The biradical [⋅P(μ-N-tBuPh3C)2P⋅] (6T) can be generated by reduction of a cyclic dichlorophosphane, [ClP(μ-N-tBuPh3C)2PCl], utilizing magnesium. If this biradical is treated with alkynes, e. g. tolan, Ph−C≡C−Ph, the addition product, a [2.1.1.] bicyclic species, [P(μ-N-tBuPh3C)2P(Ph−C=C−Ph)] (7), is obtained. However, if the reduction process with magnesium is carried out in the presence of tolan the reaction mixture finds a different reaction channel, ultimately yielding a magnesium(II) double salt, including a [Mg(THF)2]2+ cation with a [tBuPh3C−N−P−N−CtBuPh3]− and a novel tolan-stabilized [(PhC=CPh)P−N−P(PhC=CPh)]− anion as counterions. This novel anion can also be conceived as anionic [2.2.1] bicycle with a [N]− ion trans-annular bridging a 1,4-diphosphabenzene.
That main group-centered biradicals can be used to activate small molecules has been shown several times.1-7 In particular, formal cyclo-butadienyl derivatives of the Niecke biradical, [⋅CCl(μ-Mes*P)2C(Cl)⋅] (1,3-diphospha-cyclo-butane-2,4-diyle, Mes*=2,4,6-tri-tert-butylphenyl=“super-mesityl”),5, 8-14 such as [⋅P(μ-N−R)2P⋅],15 are particularly well suited, and so it is not surprising that they can activate molecules with single and multiple bonds, often leading to [2.1.1] bicycles (Scheme 1).6, 16 In recent years, we have focused primarily on four-membered pnictogen-centered biradicals of the type [⋅P(μ-N−R)2P⋅] (6R) with R=Ter (=2,6-Mes2C6H3, Mes=2,4,6-Me3C6H2), Hyp (=tris(trimethylsilyl)-silyl),17 as well as Bbp (=2,6-bis[bis(trimethyl-silyl)methyl]phenyl)18 and have shown that these phosphorus-centered biradicals are particularly suitable for molecular activation applications.19 These biradicals (6R) can be prepared easily starting from the 1,3-dichloro-cyclo-diphosphadiazanes (5, Scheme 2) by reduction with magnesium turnings in almost quantitative reactions. However, to prevent oligomerization (especially dimerization), the substituent on the nitrogen must be sterically very demanding.6, 20 For this purpose, the Ter substituent on nitrogen has been mainly used, but also the Hyp or Bbp substituents. Since we are also interested in getting rid of these sterically demanding substituents for follow-up chemistry, we looked at trityl-based substituents, hoping to split them off by strong nucleophiles. In this context, we have worked out a new synthetic route for the preparation of a trityl-substituted biradical, [⋅P(μ-N-tBuPh3C)2P⋅] (6T), bearing the tris(4-tert-butyl-phenyl)methyl-substituent (tBuPh3C=T, Scheme 2). The synthesis as well as the use of the biradical 6T in the reaction with tolan, which led to a very unusual result, is reported here.
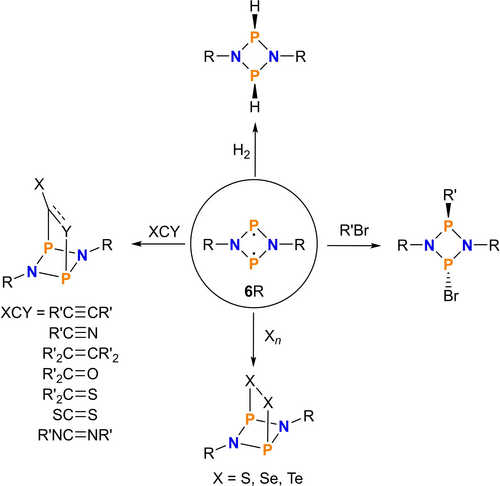
Activation of small molecules bearing single and multiple bonds using biradicals of the type [⋅P(μ-N−R)2P⋅] (6R, R=Ter, Hyp, and Bbp).16
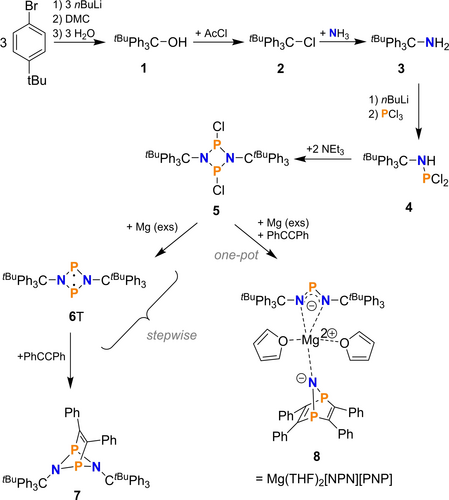
Multi-step synthesis of biradical 6T and its reaction with tolan. Depending on how the reaction is carried out, different products were isolated for the reaction with tolan (stepwise→7 vs. one-pot procedure→8).
We started this project by developing a synthesis route for the unknown biradical [⋅P(μ-N-tBuPh3C)2P⋅] (6T). Starting from 1-bromo-4-tert-butylbenzene, we succeeded in isolating 6T in a six-steps synthesis route as displayed in Scheme 2. The first three steps represented high-yielding processes (yields: 99 (1), 83 (2), and 97 % for 3), while the synthesis of tris(4-tert-butylphenyl)amino(dichloro)phosphane (4) gave only a yield of 46 % due to frequent washing of the reaction mixture to remove LiCl (see ESI). Addition of a strong base such as Et3N to aminodichlorophosphane 4 yields 1,3-dichloro-2,4-tris(4-tert-butylphenyl)methyl-cyclo-1,3-diphospha-2,4-diazane (5) in a straight forward reaction (yield: 79 %). The final step, the reduction with magnesium turnings afforded the formation of biradical 6T in an almost quantitative reaction. Unfortunately, we faced a separation problem, as biradical 6T displayed a low solubility like MgCl2 in organic solvents such as THF, benzene, fluorobenzene, CH2Cl2, DME or toluene. To prove the existence of 6T, we reacted the mixture biradical 6T / MgCl2 with tolan because it is known that biradicals of type 6R (R=Ter, Hyp) react to give [2.1.1.] bicyclic compounds such as 7. Indeed, after completion of the reduction with magnesium as indicated by 31P NMR reaction control, addition of tolan led to a soluble product which was identified by its characteristic chemical shift in the NMR spectrum (δ[31P{1H}]=231, cf. 246 ppm 6Ter⋅tolan).19 To generate more tolan addition product 7, we performed the reaction a second time, but this time we added tolan along with the Mg turnings in a one-pot synthesis to save time (Scheme 2). To our surprise, this resulted in the formation of a completely different reaction product, namely a magnesium double salt with the formal composition [Mg(THF)2]2+[(tBuPh3C−N)2P]−[N(P2C4Ph4)]−. Single crystals suitable for X-ray structure analysis could be obtained from a saturated THF solution at ambient temperatures, so that the molecular structure of the magnesium salt could be unequivocally determined (Figure 1). Besides single crystal structure elucidation, magnesium salt 8 was fully characterized by means of elemental analysis, NMR and vibrational spectroscope (see analytical data). For example, the 31P NMR data in C6D6 solution clearly show the presence of a separated NPN (389.9) and PNP moiety (158 ppm); both signals appeared as singlets. Compound 8 can be prepared in bulk, is stable under argon atmosphere and decomposes above 130 °C.
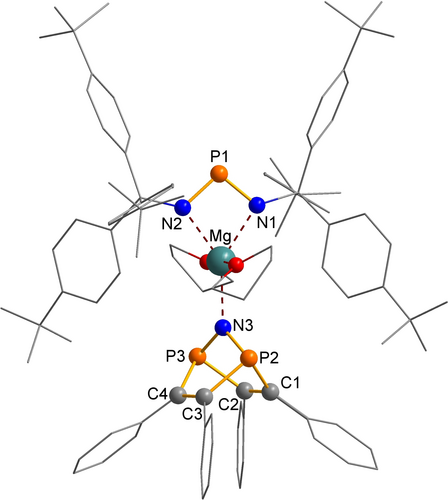
Molecular structure of magnesium salt 8 in the crystal. Color code: P orange, N blue, O red, C grey, Mg turquoise. Selected bond lengths [Å] and dihedral angles [°]: P1−N1 1.606(3), P1−N2 1.610(3), P2−N3 1.638(3), P3−N3 1.653(2), Mg−N1 2.134(3), Mg−N2 2.133(3), Mg−N3 2.129(3), P2−C1 1.903(4), P2−C3 1.901(4), P3−C2 1.900(4), P3−C4 1.897(5), C1−C2 1.344(5), C3−C4 1.356(5); N1−P1−N2 99.2(1), N1−Mg−N2 70.1(1), N1−Mg−N3 145.0(1), N2−Mg−N3 144.9(1), P2−N3−P3 109.2(2).
Simple stoichiometric consideration led to the assumption that four equivalents of magnesium and tolan reacted with three equivalents of dichloro-cyclo-diazadiphosphane 5 to afford two equivalents of magnesium salt 8 along with two equivalents of MgCl2 and tBuPh3C−Cl (Scheme 3). The formation of tBuPh3C-Cl was proven by NMR spectroscopy (see ESI). It should be noted that this formal stoichiometric reaction does not represent the reaction mechanism. It can be assumed that this heterogenous reaction includes donor-stabilized magnesium(I) species21 as well as oligomers of the type [P2N2RxCly ⋅ (tolan)z]n− stabilized by complexation at the magnesium centre.
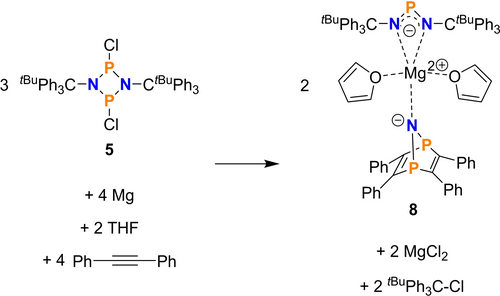
Stoichiometric reaction of Mg, tolan and dichloro-cyclo-diazadiphosphane 5 yielding double salt 8.
Due to complex reaction, we are not able to propose a reaction mechanism. However, the formal [R−N−P−N−R]− anion has been observed and isolated in the reaction of 6Ter with NHCs (N-Heterocyclic Carbene) yielding [P(NHC)2]+[Ter−N−P−N−Ter]−.22 The latter reaction means that a formal P+ ion was excised from the biradical 6Ter and stabilized by two NHCs, which, however, is impossible in the course of the formation of 8. Hence, we assume that both anions in 8 are formed from a formal “N3P3” trimeric precursor stabilized as ligand at the magnesium cation to split into [NPN]− (=[(tBuPh3C−N)2P]−) as well as a [PNP]− (=[N(P2C4Ph4)]−) ions upon reaction with the tolan. Especially the latter anion, [N(P2C2Ph4)]−, is very unusual as it represents a formal 1,4-diphospha-benzene (also known as 1,4-diphosphinines)23-27 that is bridged by a N− ion. Furthermore, we would like to point out that the reaction can also proceed without in-situ biradical formation (6Ter), for example, by first reducing the tolan, which can then react with the dichlorophosphane 7.
Orange block-shaped crystals of magnesium salt 8 crystallize in the triclinic space group P with two formula units per unit cell (Figure 1). In the crystal, magnesium salt 8 forms a molecular complex of the type [Mg(THF)2]2+[NPN]−[PNP]− (with [NPN]−=[(tBuPh3C−N)2P]−) and [PNP]−=[N(P2C4Ph4)]−, Scheme 2). Between these molecular complexes in the solid no significant short intermolecular distances, which are indicative for significant interactions, were found as the inorganic core Mg[NPN][PNP] sits in a pocket formed by two tBuPh3C substituents and two C2Ph2 substituents. The coordination sphere around the Mg2+ ion is best described as distorted trigonal bipyramidal with the two oxygen of the two coordinating THF molecules in the axial position (∢(O1−Mg−O2)=175.7(1)°, d(Mg−O1)=2.172(2) Å, d(Mg−O2)=2.177(2) Å; Σrcov(Mg−O)=2.02 Å).28 The formal trigonal plane is formed by the two N atoms of the [NPN]− ligand (∢(N1−Mg−N2)=70.1(1)°; d(Mg−N1)=2.134(3) Å, d(Mg−N2)=2.133(3) Å; Σrcov(Mg−N)=2.10 Å)28 and the apical N atom of the [PNP]− ligand (d(Mg−N1)=2.129(3) Å). As expected, compared to the rather acute N1−Mg−N2 angle, the N3−Mg−N1 and N3−Mg−N2 angles are strongly increased (145.0(1)°, 144.9(1)°). The N1−P1−N2 angle is considerably smaller than the P2−N3−P3 angle with 109.2(2)°. Both N−P bonds of the [NPN]− ion are significantly shorter with 1.606(3) and 1.610(3) Å compared to the N−P bonds of the [PNP]− ion (1.653(2) and 1.638(3) Å). Therefore, partial double bonding can be discussed for the P−N bonds in [NPN]−, while the P−N bonds in [PNP]− represent highly polarized single bonds with a significantly smaller degree of π bonding (cf. Σrcov(P−N)=1.82 Å, Σrcov(P=N)=1.62 Å,29 including polarization effects according to Schomaker and Stevenson: Σrcov(P−N)=1.76 Å, Σrcov(P=N)=1.52 Å).30 It should be noted that the small degree of π bonding within the [PNP]− ion (with tri-coordinated P atoms) can be attributed to hyperconjugative effects31, 32 between the lone pair (LP) of the N atom (p-type atomic orbital=p-AO) and the four antibonding P−C bonds (p-AO-LP(N)→σ(P−C) bonds, E2=40.6 kcal mol−1) according to natural bond analysis (NBO, see below).33, 34 The P−C bonds between 1.89 and 1.90 Å are in the range of a typical single bond (Σrcov(P−C)=1.86 Å).28 As expected, the C−C bonds within the six-membered P2C4 ring are in the range of a classic double bond (1.344(5) and 1.356(5) Å; Σrcov(C=C)=1.34 Å).28 The C1−P2−P3−C4 torsion angle is 98.8(2)°, thus featuring a puckered six-membered P2C4 heterocycle, while both P2C2 moieties are almost planar with a deviation from planarity smaller than 2°.
with two formula units per unit cell (Figure 1). In the crystal, magnesium salt 8 forms a molecular complex of the type [Mg(THF)2]2+[NPN]−[PNP]− (with [NPN]−=[(tBuPh3C−N)2P]−) and [PNP]−=[N(P2C4Ph4)]−, Scheme 2). Between these molecular complexes in the solid no significant short intermolecular distances, which are indicative for significant interactions, were found as the inorganic core Mg[NPN][PNP] sits in a pocket formed by two tBuPh3C substituents and two C2Ph2 substituents. The coordination sphere around the Mg2+ ion is best described as distorted trigonal bipyramidal with the two oxygen of the two coordinating THF molecules in the axial position (∢(O1−Mg−O2)=175.7(1)°, d(Mg−O1)=2.172(2) Å, d(Mg−O2)=2.177(2) Å; Σrcov(Mg−O)=2.02 Å).28 The formal trigonal plane is formed by the two N atoms of the [NPN]− ligand (∢(N1−Mg−N2)=70.1(1)°; d(Mg−N1)=2.134(3) Å, d(Mg−N2)=2.133(3) Å; Σrcov(Mg−N)=2.10 Å)28 and the apical N atom of the [PNP]− ligand (d(Mg−N1)=2.129(3) Å). As expected, compared to the rather acute N1−Mg−N2 angle, the N3−Mg−N1 and N3−Mg−N2 angles are strongly increased (145.0(1)°, 144.9(1)°). The N1−P1−N2 angle is considerably smaller than the P2−N3−P3 angle with 109.2(2)°. Both N−P bonds of the [NPN]− ion are significantly shorter with 1.606(3) and 1.610(3) Å compared to the N−P bonds of the [PNP]− ion (1.653(2) and 1.638(3) Å). Therefore, partial double bonding can be discussed for the P−N bonds in [NPN]−, while the P−N bonds in [PNP]− represent highly polarized single bonds with a significantly smaller degree of π bonding (cf. Σrcov(P−N)=1.82 Å, Σrcov(P=N)=1.62 Å,29 including polarization effects according to Schomaker and Stevenson: Σrcov(P−N)=1.76 Å, Σrcov(P=N)=1.52 Å).30 It should be noted that the small degree of π bonding within the [PNP]− ion (with tri-coordinated P atoms) can be attributed to hyperconjugative effects31, 32 between the lone pair (LP) of the N atom (p-type atomic orbital=p-AO) and the four antibonding P−C bonds (p-AO-LP(N)→σ(P−C) bonds, E2=40.6 kcal mol−1) according to natural bond analysis (NBO, see below).33, 34 The P−C bonds between 1.89 and 1.90 Å are in the range of a typical single bond (Σrcov(P−C)=1.86 Å).28 As expected, the C−C bonds within the six-membered P2C4 ring are in the range of a classic double bond (1.344(5) and 1.356(5) Å; Σrcov(C=C)=1.34 Å).28 The C1−P2−P3−C4 torsion angle is 98.8(2)°, thus featuring a puckered six-membered P2C4 heterocycle, while both P2C2 moieties are almost planar with a deviation from planarity smaller than 2°.
NBO and MO calculations were performed (NBO=Natural Bond Orbital, MO=Molecular Orbital, PBE0/def2svp+gd3bj) to get insight into the charge transfer and bonding within coordination compound 8. The energetically most favoured Lewis formula for 8 according to NBO analyses is Lewis formula A in Scheme 4, which features formally separated Mg2+, [NPN]−, [PNP]− ions and two neutral THF molecules. However, as indicated by the donor-acceptor energies as well computed NPA charges (Natural population analysis), there are donor-acceptor interactions between the ligands and the central Mg2+ ion, which is also illustrated by the partial charge of magnesium with +1.77e corresponding to a charge transfer of ca. 0.23e (Figure 2). Interestingly, amongst the donors are also all P atoms of the two ligands, which interact via their lone pair with the empty s-orbital localized at the Mg2+ ion. The donor-acceptor energies involved in these interactions are in the range between 5–15 kcal mol−1. A closer look at the [NPN]− ion reveals the delocalization of the π bond as depicted in the resonance B ↔ C (Scheme 4). Delocalization of one of the two lone pairs (which sits in a p-AO) at the formally negatively charged nitrogen atom in the [PNP]− ion into the four σ*(P−C) bonds also introduces some π character along the P−N−P unit, while weakening the P−C bonds (resonance D ↔ H). Delocalization of the p-type LP at the apical N is also evident in the MO picture as illustrated by the HOMO (Figure 3). The P−N single as well as double bonds in both anions are also strongly polarized towards the N atom ([NPN]−: σ(P−N) 26/74 %, π(P−N) 23/77 %; [PNP]−: σ(P−N) 27/73 %).
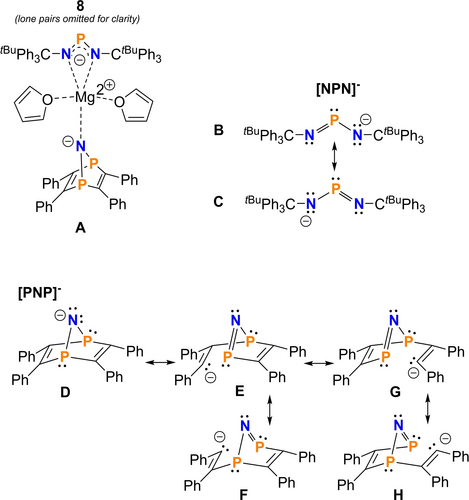
Lewis representations according to NBO analysis for 8 and each of the anions [NPN]− and [PNP]−.
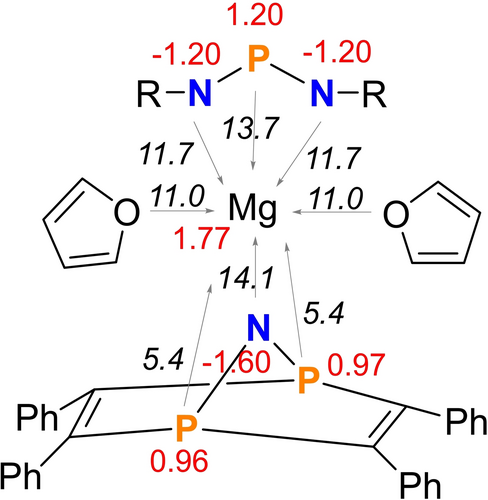
NBO charges (in e, red color) and donor-acceptor energies (E2) approximated by second order perturbation theory) of the LP(ligand atom)→s-orbital(Mg2+) interaction (E2 in italics and kcal mol−1) for magnesium salt 8 (PBE0/def2svp+gd3bj).
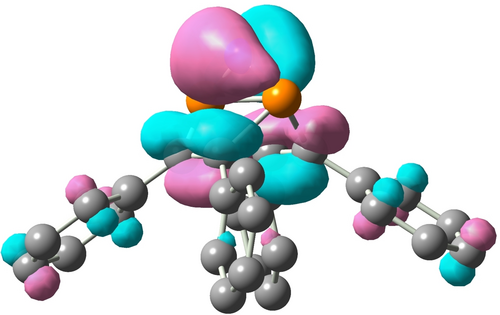
HOMO of the [PNP]− ion.
In conclusion, we have been able to show that depending on the reaction sequence biradical 6T, generated from Mg and [Cl−P(μ-N-tBuPh3C)2P−Cl] (5), can either form a [2.1.1] bicyclic compound in the reaction with tolan, or an ion complex of the type [Mg(THF)2]2+[NPN]−[PNP]− featuring a novel [NPN]=[N(P2C2Ph4]− ion, when the reduction process is carried out in the presence of tolan. This novel anion can also be regarded as an anionic [2.2.1] bicycle with a [N]− ion trans-annular bridging a 1.4-diphosphabenzene.
Experimental Section
General information along with all experimental spectra can be found in the supporting information.
Synthesis of 8. 1,3-dichloro-2,4-tris(4-t-butylphenyl)methyl-cyclo-1,3-diphospha-2,4-diazane (1.25 g, 1.27 mmol) and diphenylacetylene (0.22 g, 1.24 mmol) were combined and dissolved in THF (20 mL). The colorless solution was quickly added to a flask with activated magnesium turnings and the reaction mixture was stirred with a magnetic glass stir bar overnight. The obtained dark red solution was separated from unreacted magnesium by syringe and all volatile components were removed in vacuo (1 ⋅ 10−3 mbar). The red residue was dissolved in n-hexane (5 mL) and the suspension was filtered. The solvent was removed from the filtrate in vacuo (1 ⋅ 10−3 mbar) and the red solid residue was re-dissolved in THF (5 mL). After concentration, the solution was stored at −30 °C, resulting in the deposition of orange block-shaped crystals. The supernatant was removed by syringe. The supernatant was concentrated, resulting in a second crop of orange crystals, which were treated in the same manner. The crystals of the first and second crop were combined and dried in vacuo (1 ⋅ 10−3 mbar) for two hours at 40 °C (water bath). Yield: 0.35 g (0.24 mmol, 38.7 %).
Single crystals suitable for X-ray diffraction can be grown from saturated THF solution at ambient temperature.
Mp. 130 °C (decom.). CHN calcd. (found) in %: C 80.73 (80.59), H 7.38 (8.07), N 3.14 (2.59). 1H NMR (300 K, C6D6, 300.13 MHz): δ=1.18 (s, 27H, C(CH3)3), 1.22 (s, 27H, C(CH3)3), 1.35 (br. s, 8H, thf-CH2), 3.52 (br s, 8H, thf-CH2), 6.90-7.08 (m, 12H, Artolan−CH), 7.21 (m, 16H, ArPh−CH), 7.36 (d, 4H, 3J(1H,1H)=7.8 Hz, ArPh−CH), 7.48 (d, 4H, 3J(1H,1H)=7.8 Hz, ArPh−CH), 7.50–7.55 (m, 8H, Artolan−CH). 13C{1H} NMR (298.2 K, C6D6, 125.76 MHz): δ=25.57 (s, thf-CH2), 31.45 (s, C(CH3)3), 34.33 (s, C(CH3)3), 68.23 (s, thf-CH2), 90.13 (s, CCtolan), 124.94 (s, ArPh−CH); 125.44 (s, ArPh−CH), 126.63 (s, Artolan−C), 128.29 (s, Artolan−CH), 129.66 (s, Artolan−CH), 131.92 (s, Artolan-CH), 141.93 (s; ArPh−CH); 148.90 (s, Artolan−C). 31P{1H} NMR (298.6 K, C6D6, 121.51 MHz): δ=158.1 ([PNP]−), 389.9 (s, [NPN]−). IR (ATR, 32 scans, cm−1):  =3027.85 (w), 2958.81 (s), 2903.15 (m), 2866.05 (m), 1600.50 (w), 1506.71 (m), 1474.77 (m), 1460.34 (m), 1441.79 (w), 1393.35 (m), 1361.40 (m), 1311.93 (w), 1267.62 (m), 1241.85 (w), 1200.63 (w), 1160.44 (w), 1108.91 (m), 1070.78 (w), 1016.16 (m), 961.53 (w), 939.89 (w), 914.13 (w), 886.30 (w), 839.93 (m), 820.34 (s), 791.49 (w), 755.42 (m), 691.52 (s), 605.98 (w), 577.13 (m), 535.90 (w). Raman (633 nm, 20 s, 20 scans, cm−1):
=3027.85 (w), 2958.81 (s), 2903.15 (m), 2866.05 (m), 1600.50 (w), 1506.71 (m), 1474.77 (m), 1460.34 (m), 1441.79 (w), 1393.35 (m), 1361.40 (m), 1311.93 (w), 1267.62 (m), 1241.85 (w), 1200.63 (w), 1160.44 (w), 1108.91 (m), 1070.78 (w), 1016.16 (m), 961.53 (w), 939.89 (w), 914.13 (w), 886.30 (w), 839.93 (m), 820.34 (s), 791.49 (w), 755.42 (m), 691.52 (s), 605.98 (w), 577.13 (m), 535.90 (w). Raman (633 nm, 20 s, 20 scans, cm−1):  =3063.05 (3), 2959.85 (2), 2922.86 (2), 2902.41 (2), 2863.47 (1), 2218.03 (8), 2146.96 (1), 1593.03 (10), 1481.07 (1), 1442.13 (1), 1268.85 (1), 1199.73 (2), 1153.97 (1), 1138.40 (9), 1104.32 (4), 1024.49 (1), 995.29 (9), 742.17 (7), 702.26 (1), 638.01 (2), 614.64 (1), 574.73 (1), 536.76 (1). MS (CI, pos, thf, m/z): 411 [tBuPh3C]+.
=3063.05 (3), 2959.85 (2), 2922.86 (2), 2902.41 (2), 2863.47 (1), 2218.03 (8), 2146.96 (1), 1593.03 (10), 1481.07 (1), 1442.13 (1), 1268.85 (1), 1199.73 (2), 1153.97 (1), 1138.40 (9), 1104.32 (4), 1024.49 (1), 995.29 (9), 742.17 (7), 702.26 (1), 638.01 (2), 614.64 (1), 574.73 (1), 536.76 (1). MS (CI, pos, thf, m/z): 411 [tBuPh3C]+.
Acknowledgments
We wish to thank the University of Rostock and the LIKAT Rostock for financial support. This research was supported by the Deutsche Forschungsgemeinschaft (DFG, SCHU 1170/12-2). We thank Verena Looks for her enthusiastic synthetic work at the beginning of this project. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




