Predicting the outcome of psychotherapy for chronic depression by person-specific symptom networks
Abstract
Psychotherapies are efficacious in the treatment of depression, albeit only with a moderate effect size. It is hoped that personalization of treatment can lead to better outcomes. The network theory of psychopathology offers a novel approach suggesting that symptom interactions as displayed in person-specific symptom networks could guide treatment planning for an individual patient. In a sample of 254 patients with chronic depression treated with either disorder-specific or non-specific psychotherapy for 48 weeks, we investigated if person-specific symptom networks predicted observer-rated depression severity at the end of treatment and one and two years after treatment termination. Person-specific symptom networks were constructed based on a time-varying multilevel vector autoregressive model of patient-rated symptom data. We used statistical parameters that describe the structure of these person-specific networks to predict therapy outcome. First, we used symptom centrality measures as predictors. Second, we used a machine learning approach to select parameters that describe the strength of pairwise symptom associations. We found that information on person-specific symptom networks strongly improved the accuracy of the prediction of observer-rated depression severity at treatment termination compared to common covariates recorded at baseline. This was also shown for predicting observer-rated depression severity at one- and two-year follow-up. Pairwise symptom associations were better predictors than symptom centrality parameters for depression severity at the end of therapy and one year later. Replication and external validation of our findings, methodological developments, and work on possible ways of implementation are needed before person-specific networks can be reliably used in clinical practice. Nevertheless, our results indicate that the structure of person-specific symptom networks can provide valuable information for the personalization of treatment for chronic depression.
Several treatment options are available for depression, including a variety of pharmacotherapies and psychotherapies. These treatments are usually evaluated with respect to their overall efficacy in patient samples, and their average effect size has been reported to be moderate1-3. Still, a considerable number of people do not respond to current therapies4-8.
While the available evidence suggests that treatment for depression reduces the average symptom severity of a whole group, such group-level effects cannot be directly applied to the individual patient8. Individual differences in treatment efficacy and their sources are commonly disregarded in group comparison designs such as those of randomized controlled trials6. This limits the information base for clinicians aiming to make evidence-based treatment choices for their individual patients1, 6, 9, 10. Therefore, research on personalized mental health treatment, also called “precision mental health care”, has gained considerable popularity10-12.
A novel approach to the personalization of treatments is offered by the network theory of psychopathology. This theory assumes that mental disorders arise and are sustained because symptoms causally interact with each other13. The occurrence of one symptom is thought to cause the occurrence of further symptoms, and mutual interactions among symptoms cause the persistence of mental health problems. From this perspective, treatment should target factors causing specific symptoms, or specific symptoms, or associations among symptoms14.
Person-specific symptom networks which show associations among symptoms for an individual have been proposed for individualized treatment planning13, 15, 16. More precisely, based on the network theory, it has been suggested that a person-specific symptom network could indicate which specific symptoms or symptom associations should be primarily targeted by treatment for a given individual13, 14, 17. The centrality hypothesis suggests that symptoms which are most central in the network, i.e. which have the most or strongest associations with other symptoms, should be targeted, because an improvement of these symptoms would lead to the improvement of many other symptoms14, 18, 19. Additionally, the theory suggests that treatment should reduce (strong) associations among symptoms, so that the occurrence of one symptom ceases to induce other symptoms14.
So far, there is little empirical evidence to support the centrality hypothesis and the prioritization of strong symptom associations. Previous research showed that centrality indices based on cross-sectional between-person networks could not predict symptom change accurately, especially when compared to traditionally used non-network parameters, such as baseline symptom severity18, 19. Similarly, the connectedness of baseline cross-sectional networks did not predict treatment response when accounting for baseline symptom severity variance20.
However, studies of cross-sectional symptom networks are based on variation between individuals, i.e., on how different persons of a certain population differ in their symptom occurrence at a specific time point. They inform on whether individuals in a population showing a symptom at a specific time point are also more likely to show other symptoms at the same time point21. Thus, cross-sectional networks show symptom associations in a specific group. This contrasts to longitudinal symptom networks which can be constructed with repeated observations of each individual22. These networks are based on variation within each individual person over time, i.e. on how symptom occurrence differs over time for a given individual21. Consequently, longitudinal networks can show for a given person whether the occurrence of a symptom is related to the occurrence of another symptom at a neighbouring time point and, therefore, allow the investigation of person-specific symptom associations15, 21, 23.
In sum, longitudinal networks indicate symptom associations specific to an individual, while cross-sectional networks show symptom associations for a certain group. It has been argued that findings obtained in cross-sectional analyses of group effects cannot be directly applied to an individual person without strong and frequently unrealistic assumptions5, 9. Further, treatment is thought to introduce changes in symptom dynamics within each individual person12. Therefore, longitudinal person-specific networks seem more likely to entail relevant information for treatment response than cross-sectional networks24, 25.
Initial research on longitudinal symptom networks showed that treatment-related hypotheses of the network theory could be partially confirmed with empirical data26. Further, person-specific network parameters together with some covariates – i.e., initial functional impairment, sex, education level, personality style, and difficulty to attend treatment – predicted dropout during treatment for mood and anxiety disorders with notable accuracy27.
Still, longitudinal person-specific symptom networks derived from repeated measurements diverged from the patients’ own perception of their symptom networks28, and methodological challenges for estimating longitudinal person-specific networks remain15, 25, 29. Furthermore, there is no consensus yet on how to select treatment targets based on person-specific symptom networks29. A major obstacle is that there is uncertainty about which of the various parameters describing the structure of the person-specific network should be selected for treatment planning. Two of the most frequently considered options are information on the centrality of symptoms and information on the pairwise associations among symptoms.
If parameters that describe the structure of person-specific symptom networks before treatment or their change during treatment are predictive of treatment outcome, this could provide further support to the value of symptom networks for planning and monitoring treatment. In this study, we aimed to assess how the structure of longitudinal person-specific symptom networks before treatment, and their change during psychotherapy (either disorder-specific or non-specific), relate to treatment outcome in patients with chronic depression.
METHODS
Procedure
This study used data from a large multisite randomized controlled trial of psychotherapy for chronic depression30-32. Patients from eight clinical sites in Germany were randomized to receive either disorder-specific (cognitive behavioral analysis system of psychotherapy, CBASP) or non-specific (supportive) psychotherapy over 48 weeks. Depression severity was measured at baseline, at treatment termination, and one and two years after treatment ended, using the 24-item version of the Hamilton Rating Scale for Depression (HRSD-24)33. The assessment was done by blinded, experienced and trained raters31. Additionally, patients rated their depression severity at each session using the 28-item Inventory of Depressive Symptomatology (IDS)34. We included only IDS items that align with the nine depression symptoms of the DSM-5, and dichotomized them before analysis.
The ethics committees of the University of Freiburg and of all participating centres approved the study, and all participants provided written consent. The original study was preregistered at clinicaltrials.gov (NCT00970437). All analyses performed in this study were preregistered on the Open Science Framework (see supplementary information).
Statistical analysis
Person-specific symptom networks
An overview of data analysis is provided in Figure 1. All person-specific symptom networks are based on the estimation of a univariate multilevel model for each symptom. This model estimates whether the presence of a symptom at each treatment session is associated with the presence of this symptom and all other symptoms at the previous session. In this model, temporal symptom associations can change linearly throughout treatment, i.e. get weaker or stronger, and these changes across time can differ between treatment groups.
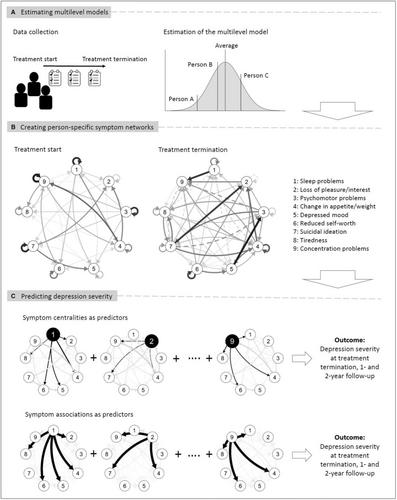
The multilevel model includes random effects for each individual for the temporal symptom associations and their change. This means that, in addition to modelling the temporal symptom associations and their change during treatment at the group level, i.e. for the “average person”, the model also provides so-called random effect estimates. These estimates indicate how strongly each individual person differs from the average, and show for each individual how strongly the symptoms are associated with each other at baseline and how these associations change during treatment.
We used the random effect estimates for each individual to construct person-specific symptom networks. We calculated for each individual how strongly all symptoms were associated with each other at each session and combined this information in person-specific symptom networks for that session. These networks show how each symptom at a given time point is related to itself and to all other symptoms at the next time point.
Panel B in Figure 1 shows an exemplary symptom network of an individual patient. In this example, at treatment start, the presence of sleep problems predicted the presence of this symptom at the next session, as indicated by the arrow pointing from this symptom to itself. Sleep problems also predicted loss of pleasure/interest, change in appetite/weight, depressed mood, reduced self-worth, and tiredness, as indicated by the respective arrows. The session-to-session associations of this symptom changed throughout treatment, which can be seen in the network at treatment termination.
To describe the person-specific symptom networks, we used symptom centrality and pairwise symptom association measures.
As symptom centrality measure, we used outstrength centrality, which shows how connected a symptom is in a given network. We calculated the outstrength of each symptom by summing the absolute strength of all “outgoing” associations of that symptom with all other symptoms in the respective person-specific network. In the example from an individual patient in Figure 1, the centrality of sleep problems at treatment start is calculated by summing up the strength of its outgoing associations with itself, loss of pleasure/interest, change in appetite/weight, depressed mood, reduced self-worth, and tiredness (see Figure 1, Panel C). We then computed the change in outstrength of each symptom during treatment.
As pairwise symptom association measure, we used the magnitude of each symptom association present in the network (i.e., the strength of each arrow in the network). The pairwise symptom associations were calculated for each possible pair of symptoms, ranging from the sleep problems → loss of pleasure/interest to the concentration problems → tiredness association in the exemplary patient depicted in Figure 1, Panel C. To evaluate the change of the pairwise symptom associations, we used the corresponding change estimates from the previously described multilevel model.
Prediction of observer-rated depression severity
The network symptom centralities and pairwise symptom associations were used to predict treatment outcomes (Figure 1, Panel C). Five different models with different predictors and different statistical approaches were evaluated. Table 1 provides an overview (see supplementary information for a more detailed description).
| Potential predictors | Covariate model | Baseline symptom-centrality model | Change symptom- centrality model | Baseline symptom- association model | Change symptom- association model |
|---|---|---|---|---|---|
| Common covariates | x | x | x | x | x |
| Symptom centrality of all symptoms before treatment | x | x | x | x | |
| Change of the symptom centrality of all symptoms during treatment | x | x | |||
| All pairwise symptom associations before treatment | x | x | |||
| Change in all pairwise symptom associations during treatment | x | ||||
| Number of (selected) predictors | 10 | 19 | 28 | 24 (TT) | 37 (TT) |
| 30 (FU1) | 27 (FU1) | ||||
| 16 (FU2) | 14 (FU2) |
- Common covariates included observer-rated depression severity at baseline, treatment group, and trial site. TT – treatment termination, FU1 – one year after treatment termination, FU2 – two years after treatment termination.
All predictive models were evaluated with respect to the outcome variables represented by observer-rated depression severity measured with the HRSD-24 at the end of treatment, and one year and two years after treatment termination. Missing data for observer-rated depression severity were imputed using the data augmentation algorithm35.
The covariate model only included variables commonly used as predictors in randomized controlled trials, i.e. observer-rated baseline depression severity measured by the HRSD-24, treatment group, and trial site. The baseline symptom-centrality model and the change symptom-centrality model added symptom-wise parameters as predictor variables. The former included the centrality measure outstrength of all symptoms at baseline in addition to the common covariates as predictors. The latter also included the change of the outstrength of each symptom during treatment. These three models were evaluated using Bayesian hierarchical linear regression. We used Markov Chain Monte Carlo sampling methods with weakly informative priors to estimate the models.
Additionally, we used machine learning to investigate two models that incorporated also information on pairwise symptom associations. The baseline symptom-association model included observer-rated depression severity at baseline, treatment group, trial site, outstrength of all symptoms at baseline, and the strength of all symptom associations at baseline as potential predictors. The change symptom-association model also added, to the pool of potential predictors, the change of the outstrength of all symptoms during treatment, and the rate of change of all symptom associations during treatment. We used an Empirical Bayes Elastic Net (EBEN) algorithm36 to select the most important from the large number of potential predictors for each model. The penalization parameters for the EBEN were determined using leave-one-out cross-validation. Based on the selection of non-zero predictors by the EBEN algorithm, we ran linear regression analyses using Markov Chain Monte Carlo sampling methods with weakly informative priors for the selected predictors and all outcomes (see also supplementary information).
As a measure of predictive accuracy, we calculated Bayesian R2 and adjusted it by accounting for the number of predictors and sample size37. Further, for each model and each outcome, we investigated calibration plots contrasting observed and predicted values. To formally compare all models, we used the posterior distributions of the adjusted R2 to calculate the probability that one model was superior to the other.
Several sensitivity analyses were conducted to evaluate the robustness of the results. First, we investigated if including the baseline severity of each specific symptom rather than the sum-score of all symptoms in the covariate model improved its predictive accuracy. Second, we assessed the effect of adding a two-way interaction term between the (change in) symptom centralities and the treatment group to the symptom-centrality models. Third, we calculated the symptom-association models including only symptom associations without symptom centralities in the pool of potential predictors. Fourth, we explored if using expected influence instead of outstrength as measure of symptom centrality influenced the predictive performance of the models. Expected influence is the sum of the raw strengths of each symptom's associations with other symptoms. Finally, we repeated all main analyses with complete cases only, to assess the impact of data imputation.
All analyses were done in R version 4.2.0 using the packages brms38 and EBglmnet36.
RESULTS
The current study included 254 patients (65.4% women, mean age: 44.9 years), who, on average, rated their depressive symptoms 23 times during treatment (5,842 data points). Data on the outcome measure (observer-rated depression severity measured by the HRSD-2433) were available for 254 persons at baseline, for 228 persons at the end of treatment, for 193 persons one year after treatment termination, and for 184 persons two years after treatment termination.
The explained variance for each model, as indicated by Bayesian R2 adjusted for the number of predictors, is displayed for all models in Table 2 and Figure 2. This measure shows how much interindividual difference in depression severity after treatment can be explained by the predictor variables of each model. We also calculated the probability that a given model explains more variance than another model.
| Covariate model | Baseline symptom- centrality model | Change symptom- centrality model | Baseline symptom- association model | Change symptom- association model | |
|---|---|---|---|---|---|
| Treatment termination | 0.11 (0.05, 0.19) | 0.25 (0.17, 0.33) | 0.36 (0.29, 0.43) | 0.42 (0.34, 0.48) | 0.56 (0.50, 0.61) |
| 1-year follow-up | 0.12 (0.06, 0.20) | 0.24 (0.16, 0.32) | 0.29 (0.21, 0.37) | 0.45 (0.38, 0.51) | 0.45 (0.38, 0.51) |
| 2-year follow-up | 0.10 (0.03, 0.17) | 0.18 (0.09, 0.25) | 0.20 (0.12, 0.28) | 0.24 (0.16, 0.32) | 0.22 (0.14, 0.29) |
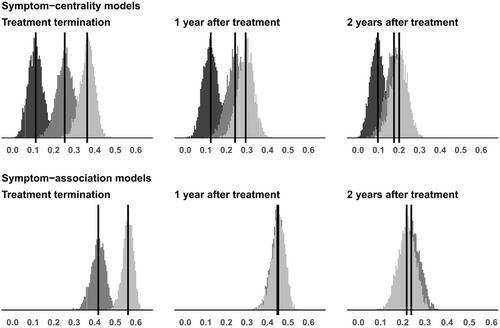
While common covariates (i.e., baseline depression severity, treatment group, and trial site) explained only 11% of the variance in post-treatment depression severity, the model also including the centrality of all symptoms at baseline accounted for 25% of the variance (see Table 2 and Figure 2). This baseline symptom-centrality model explained more variance than the covariate model with a probability of 0.99.
Adding the change of each symptom's centrality as predictor to the model increased the explained variance in depression severity at the end of treatment to 36% (see Table 2 and Figure 2). This change symptom-centrality model had a higher explained variance than the covariate and the baseline symptom-centrality model with a probability of 1 and 0.98, respectively.
Both models that used symptom associations as input had a higher predictive accuracy. Symptom associations and common covariates at baseline explained 42% of depression severity at treatment termination (see Table 2 and Figure 2). This symptom-association model outperformed the baseline symptom-centrality model with a probability of 1. Including the change in pairwise symptom associations during treatment increased the explained variance to 56% (see Table 2 and Figure 2). This change symptom-association model had a very high probability of outperforming any of the other models in predicting depression severity at treatment end (from 0.999 to 1).
The superiority of the prediction with symptom associations in contrast to common covariates was also visible for depression severity measured one and two years after treatment termination, while the superiority of the prediction with symptom associations compared to symptom centralities was evident one year but not two years after treatment ended (see Table 2 and Figure 2). The superiority of models that also included change in symptom centralities and symptom associations with respect to those that only included symptom centralities and symptom associations at baseline largely disappeared for depression severity one and two years after treatment termination (see Table 2 and Figure 2). For explaining the variance of depression severity two years after treatment, all models outperformed the common covariate model, without highly probable differences between them (see supplementary information). Analyses with non-adjusted R2 instead of adjusted R2 showed a similar pattern of findings, with slightly larger R2 for all models (see supplementary information).
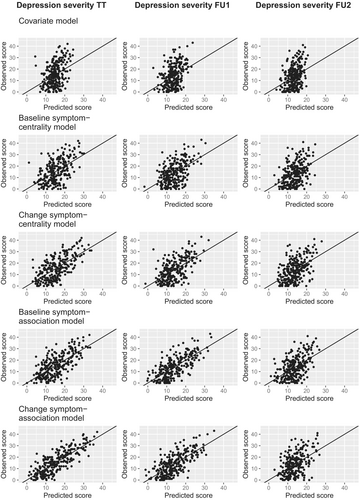
The predictive accuracy and the superiority of the symptom-association models are also reflected in the calibration plots contrasting observed and predicted values for depression severity at each time point and for each model (see Figure 3). The calibration plots show the weakest correspondence between predicted and observed values of depression severity at the end of treatment for the common covariates model, and the strongest match between predicted and observed values for the symptom-association models. This pattern is replicated for depression severity one year after the end of treatment, whereas the models show roughly equal performance for predicting depression severity two years after the end of treatment (see Figure 3).
All sensitivity analyses suggested robustness of our results (see Table 3). First, including the severity of each individual symptom at the beginning of treatment instead of the summed baseline symptom severity improved the performance of the common covariate model only marginally. Second, adding an interaction term between treatment group and the network parameters to the symptom-centrality models did not markedly improve their predictive performance. Third, the symptom-association models that included only symptom associations as potential predictors showed comparable performance to the symptom-association models that also included the symptoms’ outstrength. Fourth, the findings did not change substantially when we used the expected influence (i.e., the sum of the raw strengths of each symptom's associations with other symptoms) instead of outstrength for each symptom for measuring centrality. Finally, the sensitivity analyses with complete cases showed very similar findings (see Table 3 and supplementary information).
| Analyses | Adjusted R2 with 95% credible intervals | ||
|---|---|---|---|
| Treatment termination | 1-year follow-up | 2-year follow-up | |
| 1. Covariate model including the severity of each symptom at baseline | 0.10 (0.02, 0.17) | 0.10 (0.03, 0.18) | 0.10 (0.03, 0.17) |
| 2. Symptom-centrality models including the interaction between each symptom centrality and treatment group | |||
| Baseline symptom-centrality model | 0.24 (0.16, 0.31) | 0.23 (0.15, 0.31) | 0.19 (0.11, 0.26) |
| Change symptom-centrality model | 0.36 (0.28, 0.42) | 0.28 (0.20, 0.35) | 0.21 (0.13, 0.28) |
| 3. Symptom-association models including only symptom associations as possible predictors | |||
| Baseline symptom-association model | 0.41 (0.34, 0.48) | 0.53 (0.47, 0.58) | 0.24 (0.15, 0.31) |
| Change symptom-association model | 0.53 (0.47, 0.58) | 0.46 (0.39, 0.52) | 0.25 (0.17, 0.32) |
| 4. All models with expected influence as symptom centrality measure | |||
| Baseline symptom-centrality model | 0.23 (0.14, 0.30) | 0.21 (0.13, 0.29) | 0.18 (0.10, 0.26) |
| Change symptom-centrality model | 0.40 (0.32, 0.46) | 0.27 (0.19, 0.35) | 0.23 (0.14, 0.30) |
| Baseline symptom-association model | 0.39 (0.31, 0.46) | 0.45 (0.38, 0.51) | 0.24 (0.16, 0.32) |
| Change symptom-association model | 0.53 (0.47, 0.58) | 0.46 (0.39, 0.51) | 0.25 (0.17, 0.33) |
| 5. All models estimated with complete cases | |||
| Covariate model | 0.11 (0.04, 0.18) | 0.13 (0.05, 0.22) | 0.08 (0.01, 0.16) |
| Baseline symptom-centrality model | 0.26 (0.17, 0.34) | 0.28 (0.19, 0.37) | 0.21 (0.12, 0.30) |
| Change symptom-centrality model | 0.40 (0.33, 0.47) | 0.34 (0.25, 0.42) | 0.24 (0.14, 0.32) |
| Baseline symptom-association model | 0.44 (0.37, 0.50) | 0.47 (0.38, 0.53) | 0.26 (0.17, 0.35) |
| Change symptom-association model | 0.61 (0.55, 0.65) | 0.43 (0.35, 0.50) | 0.34 (0.24, 0.42) |
DISCUSSION
In this study, we assessed if and how the structure of person-specific symptom networks based on self-rated depression symptoms relates to treatment outcome, operationalized as observer-rated depression severity at three time points after treatment. We investigated models including symptom centrality information, i.e. the outstrength of each symptom, and models based on symptom association information, i.e., the magnitude of each pairwise symptom association.
We found that information on the person-specific symptom network structure strongly improved the accuracy of the prediction of depression severity after treatment compared to common covariates recorded at baseline. This improvement was also notable for predicting depression severity one and two years after treatment termination. Models including changes in symptom centralities or associations outperformed models including only baseline symptom centralities and/or associations for prediction of depression severity at treatment termination.
Furthermore, we found that models including pairwise symptom associations predicted depression severity at the end of treatment and one year after treatment termination substantially better than models including each symptom's centrality. However, this difference was found to be negligible two years after treatment termination. Additional analyses further supported the superior prediction of treatment outcome based on symptom associations as opposed to centrality parameters.
In addition to the common covariates considered in this study, several previous investigations tested other possible predictive variables such as the number and duration of depressive episodes, the subtypes of depression, personality traits, age, and employment status39-43. In general, the proportion of explained variance ranged between 5 and 20% for variables collected before treatment, sometimes reaching up to 40% when data collected during treatment were used44-49. In this study, we did not use any input beyond the repeated measurement of individual symptoms, but still obtained a similar or higher predictive performance than models based on those variables.
When using person-specific symptom networks for treatment planning and monitoring, it is important to determine the specific network information that should be prioritized. Clinicians could focus on specific symptoms or specific symptom associations14, 17, 50. The current study suggests that a focus on specific pairwise symptom associations could be more useful than focusing on symptom-wise centrality measures, i.e. the outstrength of each symptom.
So far, treatment recommendations and evaluations mostly rely on the presence or the severity of a specific disorder. However, previous research showed that individuals with the same depression severity markedly differed in how symptoms related to each other51. Together with our findings, this suggests that an assessment and monitoring of symptom associations, in addition to the assessment of symptom severity, provides additional information relevant for individualized treatment planning.
If the current results are replicated and more information is gained on which specific symptom associations are important for a given population or individual, person-specific networks may open new paths for clinical practice. Clinicians could gather data on symptoms of the patient at the outset of treatment and subject these to network analyses. Subsequently, the network could be examined to determine which of the pairwise symptom associations that are known to predict outcome are present in this individual patient. If, for example, it emerges that in a given patient there is a strong association between sleep disturbance and loss of pleasure, the treatment for this patient could focus on weakening this association by training strategies to engage in pleasurable activities even in the presence of sleep disturbance. In a sense, this would be a data-enhanced version of classic functional analysis52.
Additionally, person-specific networks might reveal patterns of interactions between behaviors, emotions, cognitions and somatic experiences that sustain the mental disorder. For example, a person-specific symptom network of an individual patient could show that his/her low self-worth leads to avoidance behaviors, which lead to loss of pleasure and depressed mood, and subsequently to even more strongly reduced self-worth. Then, treatment could be personalized to break such vicious circles of self-sustaining symptoms. In sum, person-specific symptom networks could help identify maladaptive symptom associations and potentially provide insight into the self-sustaining mechanisms of the mental disorder for a specific person and, therefore, guide individualized treatment.
A major challenge for the use of person-specific networks in clinical practice is their reliable estimation15, 53. These networks can be estimated reliably only with a high number of repeated measures for each individual54-56. Alternatively, Bayesian network modelling might enable a robust estimation of person-specific networks with less data points per person, because prior information could be incorporated57. This information could be based on how symptom interactions are perceived by the clinician or by the patient, or stem from large group-level studies on within-person symptom interactions58.
For the implementation of network analyses in clinical practice, a desirable solution would be a comprehensive decision support tool consisting of: a) largely automated data collection before and during treatment by easy-to-use and privacy-preserving apps; b) automated and continuously updated data analysis formally combining the collected data with data from similar patients and settings; c) a clear presentation of the results visually (e.g., network diagrams) and numerically (e.g., probabilities) with accompanying interpretations and recommendations for treatment. Such data-informed treatment planning and monitoring tools are being developed54, 59, 60.
The presented findings need to be considered under the light of some limitations. First of all, this study is exploratory. Therefore, our results need to be subjected to replication efforts. This is particularly relevant because the limited sample size prevented us from cross-validating our model. We attempted to address this problem by quantifying uncertainty with Bayesian analysis and adjusting R2 for the number of predictors, but the risk of overfitting remains. Second, the person-specific networks were estimated with data from all time points during treatment. Therefore, we used a different measure of depression severity as outcome (the observer-rated HRSD-24) than the depression measure used for creating the underlying model for the symptom networks (the self-rated IDS) and also investigated outcomes one and two years after treatment termination. Finally, the current results were obtained in a sample of people with chronic depression undergoing psychotherapy, so future research needs to show whether and to what extent the findings are relevant to other samples or settings.
Overall, we could show that person-specific networks offer valuable information that could be used for personalized treatment planning, monitoring and evaluation in persons with chronic depression. There are still various open questions, such as how person-specific networks should be estimated or constructed, or which specific network parameters should be chosen. Still, our findings suggest that individualizing treatment for depression on the basis of symptom networks might be a valuable path to follow and that machine learning approaches could be powerful tools in this respect.
Our results emphasize the importance of symptom associations and support the notion of depression as a dynamic system of interacting symptoms. Understanding what works for whom has been an essential question for decades, and approaching it from a network perspective might offer new opportunities for improving treatment effects for the individual.
ACKNOWLEDGEMENTS
Supplementary information on this study is available at https://osf.io/eqbzt/?view_only=146fb7b7665c486da895654017e65deb.




