A scoping review of randomized clinical trials for pain management in pediatric tonsillectomy and adenotonsillectomy
Abstract
Objectives
To examine the volume, topics, and reporting trends in the published literature of randomized clinical trials for pharmacologic pain management of pediatric tonsillectomy and adenotonsillectomy and to identify areas requiring further research.
Data Sources
PubMed (National Library of Medicine and National Institutes of Health), Scopus (Elsevier), CINAHL (EBSCO), and Cochrane Library (Wiley).
Methods
A systematic search of four databases was conducted. Only randomized controlled or comparison trials examining pain improvement with a pharmacologic intervention in pediatric tonsillectomy or adenotonsillectomy were included. Data collected included demographics, pain-related outcomes, sedation scores, nausea/vomiting, postoperative bleeding, types of drug comparisons, modes of administration, timing of administration, and identities of the investigated drugs.
Results
One hundred and eighty-nine studies were included for analysis. Most studies included validated pain scales, with the majority using visual-assisted scales (49.21%). Fewer studies examined pain beyond 24 h postoperation (24.87%), and few studies included a validated sedation scale (12.17%). Studies have compared several different dimensions of pharmacologic treatment, including different drugs, timing of administration, modes of administration, and dosages. Only 23 (12.17%) studies examined medications administered postoperatively, and only 29 (15.34%) studies examined oral medications. Acetaminophen only had four self-comparisons.
Conclusion
Our work provides the first scoping review of pain and pediatric tonsillectomy. With drug safety profiles considered, the literature does not have enough data to determine which treatment regimen provides superior pain control in pediatric tonsillectomy. Even common drugs like acetaminophen and ibuprofen require further research for optimizing the treatment of posttonsillectomy pain. The heterogeneity in study design and comparisons weakens the conclusions of potential systematic reviews and meta-analyses. Future directions include more noninferiority studies of unique comparisons and more studies examining oral medications given postoperatively.
Key points
-
This novel scoping review highlights the heterogeneity of research in pediatric posttonsillectomy pain management.
-
Even common drugs like acetaminophen and ibuprofen require further research for optimizing the treatment of pediatric posttonsillectomy pain.
INTRODUCTION
Tonsillectomy is a common pediatric ambulatory surgical procedure, second in incidence only to myringotomy with tube insertion in the United States.1 In 2010, 289,000 ambulatory tonsillectomy procedures were performed in children younger than 15 years of age.2 Over the past 35 years, the incidence rates of tonsillectomy have increased significantly, with obstructive sleep-disordered breathing and recurrent throat infections cited as the most common indications for surgery.3-5
The management of posttonsillectomy pain is oftentimes inadequate and studies have identified tonsillectomies as surgical procedures that induce high levels of pain.6-8 Although too little pain medication may result in significant patient discomfort and suffering, too much pain medication may result in serious adverse events. Postoperative hemorrhage is a well-described risk for tonsillectomy.9 Nonsteroidal anti-inflammatory drugs (NSAIDs) have the theoretical potential to further increase the risk for postoperative bleeding.10 While opioid analgesics do not increase hemorrhage risk, the risks for respiratory depression and death have been well documented with the use of opioids, as emphasized by the 2013 and 2017 US Food and Drug Administration black box warning and contraindication statements.11, 12
In 2019, The American Academy of Otolaryngology (AAO) released an updated Clinical Practice Guideline for pediatric tonsillectomy. The authors strongly recommended the use of intraoperative intravenous (iv) steroids and postoperative ibuprofen and acetaminophen for pain. Furthermore, the authors strongly recommended against the use of postoperative codeine.13 Otherwise, there is no clear consensus or standardization for pediatric tonsillectomy pain regimens. The Clinical Practice Guidelines also highlighted the need for increased research comparing pharmacologic postoperative pain medications, as well as a standardized set of outcomes.13
There exists a large amount of published high-level evidence for pharmacologic pain management in pediatric tonsillectomy/adenotonsillectomy. The clinical applicability of these studies varies significantly due to the extensive heterogeneity in pain treatment regimens, such as differences in the timing of administration, dosing, and modes of administration. These differences create difficulties in conducting high-quality systematic reviews and meta-analyses of randomized-controlled trials, which summarize the best available research on a specific question. In contrast, a scoping review, which aims to broadly examine what has been published rather than answer a clinical question, would provide the needed mapping of the overwhelming amount of literature.14 Furthermore, a scoping review would provide guidance for a clinical trial design that may allow for future high-level systematic reviews and meta-analyses. The purpose of this scoping review is to examine the volume, topics, and reporting trends in the published literature of randomized controlled trials for pain management of pediatric tonsillectomy and adenotonsillectomy and to identify areas requiring further research.
METHODS
Systematic literature search
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines.15 A research librarian (E. B.) with expertise in conducting systematic reviews developed detailed search strategies in the following four databases: PubMed (National Library of Medicine, National Institutes of Health), Scopus (Elsevier), CINAHL (EBSCO), and Cochrane Library (Wiley). The search strategies used a combination of subject headings (e.g., Medical Subject Headings [MeSH] in PubMed) and keywords for the following three concepts: tonsillectomy/adenotonsillectomy, pain, and pediatrics. The PubMed search strategy was modified for the other three databases, replacing MeSH terms with appropriate subject headings, when available, and maintaining similar keywords. The search strategies for each database are detailed in Supporting Information Appendix 1. The databases were searched from inception through May 7, 2021, and results were limited to the English language only. To identify additional articles, the reference lists of relevant articles were hand searched, as well as citing articles. References were uploaded to Covidence systematic review software (Veritas Health Innovation) and screened for relevance.
Selection criteria
Only randomized controlled trials with the primary or secondary objective of examining pain improvement with specific pharmacologic intervention in pediatric tonsillectomy patients were included. Abstracts were first independently reviewed by two reviewers (M. S. and B. D. L.) to first identify all randomized controlled studies pertaining to the treatment of tonsillectomies in patients aged 0–18 years. Non-English studies, nonhuman studies, and nonjournal articles were excluded. Studies were considered for inclusion in the final analyses if they were: (1) randomized controlled or comparison trials, (2) involving only patients aged 0–18 years, (3) examining patients that underwent tonsillectomy, tonsillotomy, or adenotonsillectomy, and (4) examining the effects of pharmacologic interventions on postsurgical pain. Quality improvement studies or studies that involved nonpharmacologic interventions (e.g., hot and cold meals, acupressure, or glossopharyngeal block) were excluded. Additionally, studies that included patients that underwent other concomitant procedures (e.g., myringotomy with tympanostomy tube placement) were excluded.
Data collection and analysis
Measures that are relevant to the health care providers and the general public were extracted by two reviewers (M. S. and B. D. L.) and included in the analysis and discussion. Disagreements were resolved by way of discussion. Collected measures included demographics (age, weight, body mass index [BMI], sex, American Society of Anesthesiologists [ASA] physical status, and country where the study was conducted), pain-related outcomes (pain scale used, time points examined, and postoperative analgesic requirements), sedation scores, nausea/vomiting, postoperative bleeding, type of drug comparisons, mode of administration, and timing of administration. These measures were all reported as counts and percentages of publications mentioning the item. Drug comparisons, irrespective of mode and timing of administration, were also counted. In instances of incomplete data, an attempt was made to contact the primary author via email for clarification or sharing of primary data.
The results of this scoping review are presented according to the recommendations of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).16 All graphs and tables were performed with Microsoft Excel version 2008 (Microsoft), Prism version 8.0.0 (GraphPad Software), and Cochrane Review Manager (RevMan) version 5.4 (Nordic Cochrane Center, Cochrane Collaboration, 2011). Pooled analysis was performed with each measure was weighted according to the number of publications and were summarized by frequency (N) and percentage (%).
Quality of evidence assessment
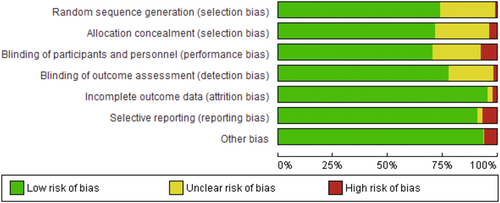
The level of evidence for each selected article was evaluated by the Oxford Center for Evidence-Based Medicine.17 The risk of bias was assessed according to the Cochrane Handbook for Systematic Reviews of Interventions version 6.2, 2021. Two authors (M. S. and B. D. L.) performed a pilot assessment on three studies to check for consistency of assessment. Both then performed independent risk assessments on the remaining studies. All disagreements were resolved by way of discussion. The risk of bias items included the following: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. The risk of bias for each aspect was graded as low, unclear, or high.
RESULTS
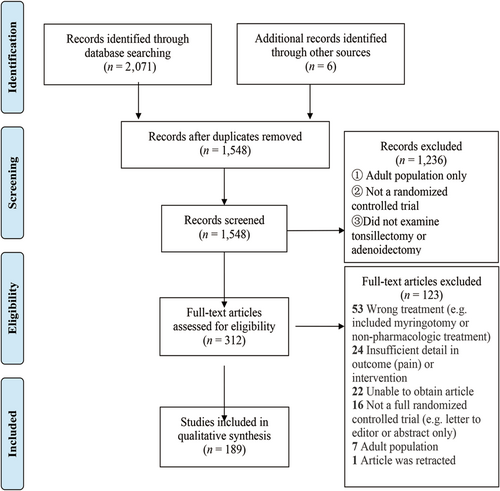
A total of 189 randomized controlled and comparison trials met the inclusion criteria for analysis.18-206 A PRISMA diagram outlining the literature search is shown in Figure 1, and the risk of bias graph is shown in Figure 2. Risk of bias was “unclear” for 16.78% of items, and 4.01% were “high risk.” All studies met Level 2 criteria for the Oxford level of evidence. The included studies were published between 1985 and 2021 (188 articles in total, Turkey 48, US 32, Iran 30, others 78).
The demographics examined by the literature are shown in Table 1. No studies included only children under 12 years of age (maximum age, 11 years); therefore, we examined studies that included only children under 13 years of age. Most studies focused on children with a maximum age of 7–12 (58.20%), but several studies included patients up to 13–18 years (38.62%) without age subgroup analysis. Most studies reported body weight (71.43%), whereas only 10 (5.29%) studies reported BMI. Most studies indicated ASA 1 (65.61%) and 2 (60.32%) as inclusion criteria.
| Variable | Number of publications | Publications (%) |
|---|---|---|
| Maximum patient age | ||
| 7–12 | 110 | 58.20 |
| 13–18 | 73 | 38.62 |
| Unclear | 6 | 3.17 |
| Weight | 135 | 71.43 |
| Body mass index | 10 | 5.29 |
| Sex | 158 | 83.60 |
| American Society of Anesthesiologists (ASA) status | ||
| ASA 1 | 124 | 65.61 |
| ASA 2 | 114 | 60.32 |
| ASA 3 | 7 | 3.70 |
| No info | 65 | 34.39 |
The reported pain-related outcomes are shown in Table 2. Only two studies (1.06%) did not include a pain scale, and nearly half of the studies used a visual-assisted scale (49.21%). Sixteen studies used multiple pain scales. Approximately one in four studies examined time points at and beyond 24 h, whereas a greater majority (84.66%) included time points in the immediate postoperative period. Postoperative analgesic requirements were reported in 76.19% of studies, whereas only approximately 23.28% of studies reported sedation scores.
| Variable | Number of publications | Publications (%) |
|---|---|---|
| Pain scale | 186 | 98.41 |
| Observational scales | 69 | 36.51 |
| CHEOPS | 44 | 23.28 |
| FLACC | 14 | 7.41 |
| Hanallah | 7 | 3.70 |
| TPPPS/PPPM | 2 | 1.06 |
| CHIPPS | 1 | 0.53 |
| CPSC | 1 | 0.53 |
| Visual-assisted scales | 93 | 49.21 |
| VAS | 45 | 23.81 |
| Faces (unspecified) | 19 | 10.05 |
| Wong-Baker | 10 | 5.29 |
| McGrath | 9 | 4.76 |
| Oucher | 9 | 4.76 |
| Maunuksela | 1 | 0.53 |
| Numeric pain scale | ||
| Numeric rating scale | 3 | 1.59 |
| Objective pain score | 19 | 10.05 |
| Unvalidated | 15 | 7.29 |
| No pain scale used | 2 | 1.06 |
| Time points (h) examined | ||
| ≤24 | 160 | 84.66 |
| ≥24 | 47 | 24.87 |
| Postoperative analgesia requirements | 144 | 76.19 |
| Number of patients | 76 | 40.21 |
| Time to first request | 51 | 26.98 |
| Doses (frequency, amount, or number) | 81 | 42.86 |
| Sedation scores | 44 | 23.28 |
| Aldrete | 2 | 1.06 |
| MOAA/S | 1 | 0.53 |
| Ramsay | 12 | 6.35 |
| Riker SAS | 1 | 0.53 |
| Vancouver | 1 | 0.53 |
| Wilson | 6 | 3.17 |
| Unvalidated | 21 | 11.11 |
| Nausea and vomiting | 140 | 74.07 |
| Postoperative bleeding | 90 | 47.62 |
- Abbreviations: CHEOPS, Children's Hospital of Eastern Ontario Pain Scale; CHIPPS, Children and Infants Postoperative Pain Scale; CPSC, Conscious Presence and Self Control Scale; FLACC, Face, Legs, Activity, Cry, Consolability Scale; MOAA/S, Modified Observer's Assessment of Alertness/Sedation Scale; PPPM, Parents' Postoperative Pain Measure; PPPS, Toddler-Preschool Postoperative Pain Scale; Riker SAS, Riker Sedation-Agitation Scale; VAS, Visual Analog Scale.
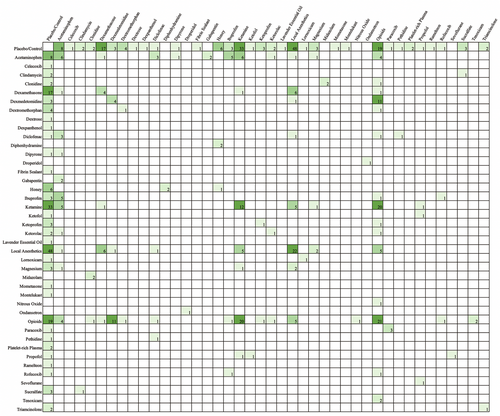
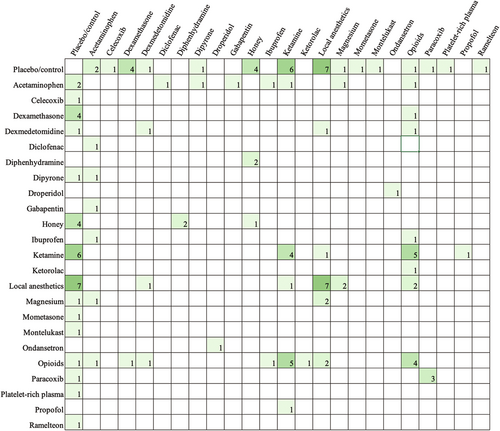
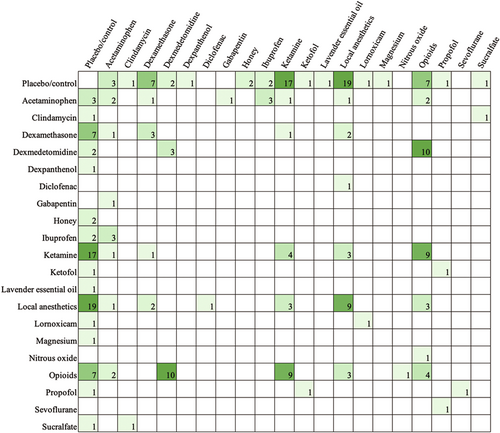
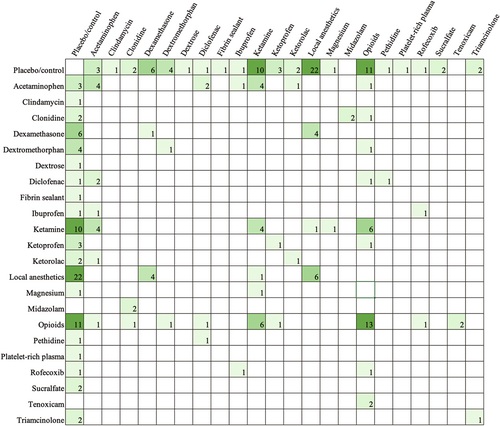
Table 3 shows the types of comparisons made in the literature. The majority of papers included a placebo or control comparison (62.43%). Most papers aimed to compare different drugs (47.09%), whereas fewer studies sought to compare the timing of administration (3.17%), different modes of administration for the same (9.52%) or different drugs (5.82%), and dose differences (7.41%) (Tables 4 and 5). Across all studies that examined the effects of acetaminophen, nine unique doses between 10 mg/kg (postoperatively every 4 h as needed) and 100 mg/kg (perioperative single dose) were used. Figure 3 exhibits all drug comparisons. Figures 4-6 show drug comparisons split by year of publication. There were fewer comparisons involving opioids after 2015 compared to prior years, whereas studies on local anesthetics and ketamine persisted through all years of publication.
| Variable | Number of publications | Publications (%) |
|---|---|---|
| Drug to placebo/control comparison | 118 | 62.43 |
| Only examines placebo/control comparison | 55 | 29.10 |
| Same drug, different dose | 14 | 7.41 |
| Different drugs, not combined | 89 | 47.09 |
| Drug a compared to drug A + drug B | 22 | 11.64 |
| Same drug, different modes of administration | 18 | 9.52 |
| Different drugs, different modes of administration | 11 | 5.82 |
| Timing of administration | 6 | 3.17 |
| Different drugs, different modes of administration, and different timing of administration | 2 | 1.06 |
- Note: The included studies examined 12 different modes of administration (Table 4). The most common were intravenous injection and peritonsillar injection. Regarding the timing of administration (Table 5), most studies compared presurgical or intrasurgical pharmacologic interventions. In contrast, only 23 (12.17%) studies examined postsurgical drug administrations. The frequency of comparisons made for oral drugs given postoperatively is shown in Figure 7.
| Mode of administration | Number of publications | Publications (%) |
|---|---|---|
| Intramuscular injection | 12 | 6.35 |
| Intranasal spray | 2 | 1.06 |
| Inhalation | 3 | 1.59 |
| Intravenous injection | 75 | 39.68 |
| Intravenous—patient-controlled administration | 2 | 1.06 |
| Mouthwash, oral rinse, or oral spray | 6 | 3.17 |
| Oral | 29 | 15.34 |
| Peritonsillar infiltration | 56 | 29.63 |
| Rectal | 15 | 7.94 |
| Subcutaneous injection | 1 | 0.53 |
| Sublingual | 1 | 0.53 |
| Topical to operation site | 18 | 9.52 |
| Unclear | 3 | 1.59 |
| Timing of administration | Number of publications | Publications (%) |
|---|---|---|
| Before surgery | 22 | 11.64 |
| Night before surgery | 1 | 0.53 |
| Within 2 h of surgery | 21 | 11.11 |
| At induction | 15 | 7.94 |
| After induction, before the end of surgery | 102 | 53.97 |
| After intubation | 3 | 1.59 |
| Preincision | 77 | 40.74 |
| During operation | 4 | 2.12 |
| After tonsils removed | 10 | 5.29 |
| At end of surgery | 14 | 7.41 |
| Postoperation | 23 | 12.17 |
| Mixed | 13 | 6.88 |
| Unclear | 2 | 1.06 |
DISCUSSION
Our work provides the first scoping review of pain and pediatric tonsillectomy. All randomized controlled and comparison trials regarding pharmacologic interventions for pain management in pediatric tonsillectomy and adenotonsillectomy were evaluated. This study provides future researchers guidance by showing which areas require additional randomized controlled trials.
Although this study contains elements similar to a systematic review (i.e., inclusion of a PRISMA diagram, focuses only on randomized controlled trials, assessing the risk of bias), the aim of the study fits within the category of scoping reviews. Specifically examining randomized controlled trials was warranted because pediatric tonsillectomy is a common procedure with high accessibility for randomized controlled trials. Furthermore, the large number of published randomized controlled trials creates expectations for the ability to conduct powerful systematic reviews and meta-analyses. However, our study highlights the heterogeneity in methodology and pain treatment comparisons in the existing literature, thereby elucidating the difficulties in conducting high-level systematic reviews and meta-analyses of randomized controlled trials.
Quality of included studies
The moderate amounts of unclear bias (16.78%) highlight the need for clear reporting of methodology regarding randomization and blinding. In particular, for surgeries like tonsillectomies, the ideal protocol would minimize the number of different operating surgeons and surgical methods involved in these clinical trials. Nonetheless, the overall critical appraisal of studies indicated an acceptably low risk of bias for most studies.
Demographics and patient characteristics
Specific to children, developmental changes in physiology (e.g., puberty) may affect the efficacy, toxicity, and dosing regimens for various medications.207 In our study, we sought to investigate pre- and postpubescent time points. Due to factors of geography, culture, and genetics, the onset of puberty and adolescence varies for each individual, and determining a universally accepted age cutoff becomes challenging. There were many studies that included patients up to 13–18 years of age (39.15%) and these studies did not provide relevant subgroup analyses. In such cases, assigning accurate conclusions to children at different stages of development becomes difficult. It is preferable that future studies develop inclusion criteria for patient age that reflect subgroups of interest. If multiple subgroups are of interest, then subgroup analyses sharpen the ability to develop recommendations specific to an individual patient.
Studies often reported weight but very seldom reported BMI. This may be due to the fact that the definitions of obesity differ between children and adults. For children, obesity is defined as BMI at or above the 95th percentile when compared to children of the same age and sex.208 Weight varies significantly across pediatric patients; the utility of comparing the weight between a 5-year-old child and a 17-year-old adolescent is questionable. Even with height reported alongside weight as lumped means and standard deviations, the pertinent pharmacologic characteristics of individual patients still become lost. Overweight and obese patients generally have larger volumes of distribution of lipophilic medications. For children of different age groups and body sizes, lean body mass, blood volume, and changes in the percentage of total body water influence the volume of distribution for hydrophilic medications.209 Although BMI alone does not convey all the bodily characteristics of a patient, the metric is much more informative than weight alone when examining groups of patients. Future studies should consider reporting the number of pediatric patients who meet the criteria for overweightness (85th–95th percentile for BMI) and obesity (95th percentile or greater).208
ASA status was focused on those related to healthy patients (ASA 1) and mild to moderate systemic disease that is well-controlled (ASA 2). This is appropriate for examining drug effects as a patient will have fewer confounders related to pain and reporting of pain. Furthermore, most studies properly characterized the number of male and female patients per group.
Outcomes
Pain scales were used in 98.41% of studies, with the majority preferring visual-assisted scales over observational scales. Because pain is subjective in nature and pain perception varies across cultures and individuals, there is no clear preferred method of collecting and reporting pain data. However, the usage of both an objective and observational scale in addition to a subjective visual-assisted scale may improve the generalizability and validity of findings. Furthermore, the person who obtains the data (i.e., study staff vs. patient vs. parent) must be described and consistent across the study. Analgesia requirements in the immediate postoperative period may acceptably act as surrogates for pain perception; however, the requirements for administering rescue analgesia must be well-delineated, including the pain scale used. After a patient is discharged from the hospital, confounders for analgesia requirements increase, and specific guidance should be given to caretakers to improve the accuracy and validity of data.
Reports on sedation were of interest as both a complication of medications as well as a confounder for pain. Patients that received pre- and intraoperative interventions may have pain ratings affected by increased or decreased sedation related to the intervention. Furthermore, opioids have been noted to increase the risk for excessive sedation, respiratory depression, and death in children.210-213 Twenty-three studies utilized six different validated sedation scores. This finding highlights the need to encourage the use of sedation scores and also to standardize methods for quantifying and reporting sedation.
Approximately half of the studies reported postoperative bleeding rates. This complication is specifically relevant to tonsillectomy due to the increased morbidity and mortality associated with hemorrhage.9 The risk for postoperative hemorrhage is theoretically increased further by the use of NSAIDs.10 Previous studies have indicated no increased bleeding risk with the use of ibuprofen, but recent studies suggest the possibility of increased bleeding severity with ibuprofen.214-217 Furthermore, studies have reported increased bleeding risk with ketorolac, but a meta-analysis suggested that ketorolac may not truly increase the risk for postoperative hemorrhage in pediatric tonsillectomy.218 However, our current work has shown that ketorolac only has five comparisons for pain published in the literature. Even if ketorolac does not induce a risk of bleeding, the intervention should first be determined to be a suitable analgesic for this patient population. More clinical trials on ketorolac and ibuprofen, specifically compared with acetaminophen and placebo/controls should, therefore, be conducted.
Areas requiring further investigation
Despite a large number of studies and drug comparisons for pediatric tonsillectomy, there is still no clear consensus on a superior pain treatment regimen. A large barrier is the heterogeneity of comparisons across different dimensions, which include different drugs, modes of administration, timing of administration, duration of treatment, and dosages. A study should ideally examine differences in only one dimension.
Initial efforts should focus on fully understanding drugs with clear safety profiles, which currently include ibuprofen and acetaminophen.13 Acetaminophen/paracetamol/propacetamol, medications strongly recommended for pediatric tonsillectomy, has only six self-comparisons published in the literature for pediatric tonsillectomies. These studies compared oral versus rectal, oral versus intramuscular injection, intravenously before induction versus after surgery, oral perioperative 40 versus 100 mg/kg (single dose), acetaminophen versus acetaminophen with ibuprofen, and oral versus rectal acetaminophen with codeine.31, 87, 92, 126, 133, 146 Even for this common drug, the literature has not adequately determined the best mode of administration (i.e., intravenous vs. rectal vs. oral), timing of administration, dosages, or duration of treatment postoperatively. These issues become magnified for other less used and less investigated drugs. Furthermore, the wide variation in dosing for acetaminophen highlights the need to determine a standardized dose for comparison studies. After fully characterizing common and relatively safe drugs, researchers can better conduct noninferiority trials for other medications of interest.
The most recent Pediatric Tonsillectomy Clinical Practice Guidelines had expressed the need for increased research comparing oral pharmacologic postoperative pain medications.13 Our findings show that only approximately one in four studies examined time points beyond 24 h after surgery, demonstrating the referenced paucity in the literature for home pain control regimens. Further emphasizing this point, only 29 (15.34%) studies examined oral medications. As exhibited in Figure 7, even the most common postsurgical oral pain medications have not been adequately investigated. For such a common and long-established procedure, this finding likely stems from the difficulties in obtaining data from patients after discharge. Without clear standards for follow-up and with follow-ups oftentimes conducted by phone calls, obtaining reliable survey data can be challenging for researchers. In contrast, measures in the immediate postoperative period are more readily accessible.

Only 22 comparisons could be made for add-on regimens (i.e., drug A compared to drug A with drug B). These add-on studies emphasize the ongoing support for multimodal regimens.219 However, because the efficacy of individual drugs has not yet been determined, it would be premature to target multimodal regimens.
Limitations
This study is limited by the scope of the project. Because the authors decided to only include patients in randomized controlled and comparison trials, data from retrospective and observational prospective studies are not considered. Also, the nature of pain deriving from tonsillectomy and adenoidectomy are likely different, and therefore the authors excluded any articles only examining adenoidectomy. However, most publications used tonsillectomy with or without adenoidectomy as inclusion criteria. Ideally, our work would have examined only adenoidectomy, tonsillectomy, or adenotonsillectomy, rather than a combination of the latter two surgeries. There are additional factors that may influence post-tonsillectomy pain, such as surgical instrumentation and technique.220 However, these separate factors should be directly compared in randomized-controlled trials, rather than analyzed through studies aiming to compare pharmacologic interventions. Furthermore, our work does not examine differences across time of publication. Because we included articles from 1985 to 2021, changes in reporting patterns may have already occurred that are not visible from our summative information. Finally, scoping reviews are not designed to assess the quality of the empirical evidence. More targeted systematic reviews would inform the field of the quality of relevant research in these areas.
CONCLUSIONS
Our work elucidates the reporting trends and areas requiring further research for pharmacologic pain management in pediatric tonsillectomy and adenotonsillectomy. Researchers should analyze outcomes for age groups with consideration for varying stages of development, and more studies should provide BMI information in addition to descriptive statistics of weight. Studies should utilize and report validated sedation scores, and complications such as postoperative hemorrhage and nausea/vomiting should be addressed.
With drug safety profiles considered, the literature does not have enough data to determine which treatment regimen provides superior pain control in pediatric tonsillectomy. There is currently inadequate data on oral medications given postoperatively, including acetaminophen and ibuprofen. Timing of drug administration, modes of administration, and duration of treatment require more research. Furthermore, the strength of any systematic reviews and meta-analyses would be diminished by the significant heterogeneity in reporting trends and comparisons made in the existing literature. Future studies should first focus on understanding drugs with adequate safety profiles, which currently include ibuprofen and acetaminophen. Thereafter, noninferiority trials with other drugs of interest should be considered. We hope this scoping review provides a compelling framework for the future reporting and conduct of randomized clinical trials for posttonsillectomy pain management.
AUTHOR CONTRIBUTIONS
Michael Shih, Barry D. Long, and Clarice S. Clemmens: design, conduct, analysis, and manuscript conception. Phayvanh P. Pecha, David R. White, and Yi-Chun Carol Liu: design, analysis, and manuscript conception. Emily Brennan: design, conduct, and manuscript conception. Mariam I. Nguyen: conduct, analysis, and manuscript conception.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
This review contains only data derived from published articles. No new data was generated.
This study was a review and thus no ethics statement or IRB approval was needed.




