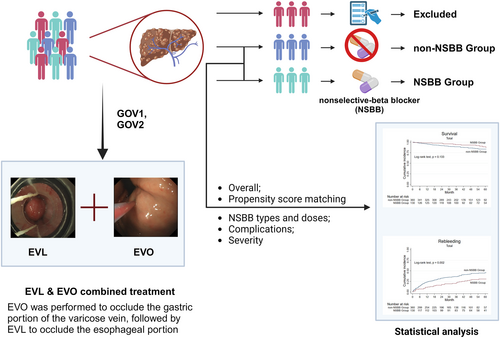
Secondary Prophylaxis of Cirrhotic Gastric Variceal Bleeding: Addition of Non-Selective Beta-Blockers to Endoscopic Combined Treatment
Ling Wu and Xiaoquan Huang share first, and Shiyao Chen and Detlef Schuppan share senior authorship.
Funding: This study was supported by the National Key R&D Program of China (2023YFC2507500) and the Shanghai Committee of Science and Technology (22Y11907400), Prof. Dr. Dr. Detelf Schuppan has received project-related support from the German Research Foundation (DFG) Collaborative Research Center (CRC) grants SFB 1066 project B3 and CRC 1292 project B8, and grant support from EU Horizon 2020 projects under grant agreements nr. 634413 (EPoS, European Project on Steatohepatitis), and nr. 777377 (LITMUS, Liver Investigation on Marker Utility in Steatohepatitis).
ABSTRACT
Background
It remains unclear whether the addition of non-selective beta-blockers (NSBB) provides further benefit after combined use of tissue adhesive and endoscopic variceal ligation for bleeding gastroesophageal varices.
Objective
This is the first cohort study comparing the secondary prophylactic efficacy of adding NSBB to combined endoscopic treatment in cirrhotic patients with gastric varices (without inclusion of isolated gastric varices [IGVs], which are rare in patients with cirrhosis without splanchnic thrombosis).
Methods
We retrospectively analyzed two matched large cohorts of cirrhotic patients with gastric varices who received combined endoscopic treatment and were assigned to receive NSBB treatment or not as secondary prophylaxis. Further sub-analyses were performed. The 5-year overall survival and rebleeding rates were compared.
Results
A total of 490 patients were enrolled, 130 of whom took NSBB. After propensity score matching (PSM), both groups comprised 130 patients. No significant difference between the NSBB and non-NSBB groups was observed regarding 5-year survival (87.40% vs. 83.64%, HR 0.62, 95% CI 0.33–1.17), while NSBB decreased the 5-year rebleeding rate from 45.7% to 30.64% (HR 0.57, 95% CI 0.39–0.82). The same was true when comparing the 2 groups after PSM. NSBB (HR 0.54, 95% CI 0.37–0.79) and splenectomy (HR 0.65, 95% CI 0.44–0.96) independently reduced rebleeding but did not affect survival. In patients without splenectomy, NSBB treatment improved survival (HR 0.43, 95% CI 0.21–0.89) and reduced rebleeding (HR 0.52, 95% CI 0.34–0.78).
Conclusion
We show for the first time that adding NSBB to combined endoscopic therapy significantly reduced recurrent variceal bleeding in cirrhotic patients but did not improve overall survival, except for patients without splenectomy. The addition of NSBB for patients receiving combined endoscopic treatment of gastroesophageal varices provides benefits but does not prolong overall survival.
Graphical Abstract
Abbreviations
-
- CI
-
- confidence intervals
-
- CT
-
- computed tomography
-
- CTA
-
- computed tomography angiography
-
- EVL
-
- esophageal variceal ligation
-
- EVO
-
- endoscopic variceal obturation
-
- GOV1
-
- gastroesophageal varices type 1
-
- GOV2
-
- gastroesophageal varices type 2
-
- HR
-
- hazard ratios
-
- HVPG
-
- hepatic venous pressure gradient
-
- IQR
-
- interquartile range
-
- NSBB
-
- non-selective beta-blocker
-
- PSM
-
- propensity score matching
-
- PVT
-
- portal vein thrombosis
Summary
-
Summarize the established knowledge on this subject:
- ◦
Gastroesophageal variceal bleeding is a medical emergency, and it remains controversial whether to add nonselective-beta blockers (NSBBs) after combined endoscopic application of tissue adhesive for gastric varices followed by esophageal variceal ligation.
- ◦
-
What are the significant and/or new findings of this study?
- ◦
We evaluated for the first time the secondary prophylactic efficacy of adding NSBBs to endoscopic combined variceal treatment (obliteration and ligation) of gastroesophageal varices (GOV1 and GOV2) in cirrhotic patients, which significantly reduced recurrent variceal bleeding in cirrhotic patients but did not improve overall survival, except for patients without splenectomy.
- ◦
1 Introduction
Gastroesophageal varices are present in approximately 50% of cirrhotic patients [1]. The cirrhosis-related age-standardized mortality is high [2]. Gastroesophageal variceal bleeding is a medical emergency with a high mortality and rebleeding rate, which is even higher in patients receiving no antiviral therapy for hepatitis B or C [3]. Endoscopic variceal ligation (EVL) and endoscopic variceal obliteration (EVO) with cyanoacrylate are recommended for patients with acute variceal bleeding [4], but both treatments have no impact on portal pressure.
Portal hypertension is the primary pathophysiological mechanism underlying gastroesophageal varices. Lowering portal pressure improves the prognosis [5] and is a key prophylaxis for variceal (re)bleeding according to the Baveno VII consensus [4]. Typically, a hepatic venous pressure gradient (HVPG), reflecting portal pressure, at or above 12 mmHg, predicts a high likelihood of variceal bleeding [6]. Non-selective beta-blockers (NSBBs) reduce portal blood flow and associated portal pressure by targeting beta-receptors on blood vessels and the heart [7]. NSBBs have also been shown to reduce the risk of other cirrhosis-related complications such as hepatic functional decompensation [7, 8]. However, NSBBs should be carefully and comprehensively considered in cirrhotic patients, especially those with ascites and unstable hemodynamics [9]. Thus, in patients with portal hypertension maintaining heart rate above 55 beats per minute is necessary to improve prognosis, when visceral and peripheral vasodilation, water and sodium retention occur and where NSBBs can reduce heart rate and cardiac output, further exacerbating ascites and neurohumoral metabolic disorders, finally promoting the onset of hepatorenal syndrome and compromising survival.
If there are no contraindications to beta-blockers, the combination of NSBB and EVL is the first-line treatment for the prevention of recurrent esophageal variceal hemorrhage [4], while no data on NSBB therapy in patients with gastric varices have been published. Although gastric varices are less frequent, they have a higher rebleeding rate and are more difficult to treat than esophageal varices. Gastroesophageal varices type 1 (GOV1) and type 2 (GOV2) are varices in direct continuity from the esophagus to the stomach (lesser and greater curvatures, respectively) [10]. We chose EVL after EVO for treatment of GOV1 and GOV2, which tended to have a better secondary prophylactic effect according to our clinical experience. Here we evaluated, for the first time, the efficacy of the addition of NSBB compared with standard endoscopic follow-up after combined endoscopic treatment of gastroesophageal variceal bleeding in a retrospective analysis of a large cohort of well-defined patients. Moreover, we performed subgroup analysis to identify the characteristics of patients who may have benefited from the addition of NSBB after combined endoscopic treatment.
2 Patients and Methods
2.1 Study Cohorts
This retrospective cohort study was conducted at a single tertiary academic hospital in China, specializing in liver diseases (Zhongshan Hospital, Shanghai, China). Patients with cirrhotic varices who received nonemergent combined EVL and EVO treatment to prevent rebleeding between January 2010 and December 2017 were reviewed. Patients without an episode of variceal bleeding, with a history of malignancy, or immediately lost to follow-up after discharge were excluded. Patients were divided into two groups according to whether they received additional NSBB (propranolol or carvedilol) after endoscopic combined treatment. The study protocol was approved by the Medical Ethics Committee of the Zhongshan Hospital, Fudan University (B2015-007).
Cirrhosis was diagnosed based on a previous liver biopsy, laboratory tests, or imaging findings, and the severity of cirrhosis was graded according to the Child-Pugh Score. Cirrhosis-related portal hypertension was assessed using computed tomography angiography (CTA), and the presence of gastroesophageal varices was confirmed by endoscopy (GOV1 and GOV2). The HVPG was measured as the wedged hepatic venous pressure measured by inflation of the balloon catheter in the right hepatic vein minus the free hepatic venous pressure measured by the sensor in the right hepatic vein inserted from the right jugular vein [11]. The presence of portal vein thrombosis (PVT) was determined using contrast-enhanced computed tomography (CT) to detect non-enhancing low-density segments in the portal vein. The first episode of gastroesophageal variceal bleeding was confirmed by endoscopy with bleeding directly from a gastric or esophageal varix or by blood in the stomach in the presence of gastroesophageal varices and no other recognizable cause of bleeding [12].
2.2 Patient Characteristics and Follow-Up
All patients were re-evaluated every 8 weeks until both gastric and esophageal varices were eradicated, with annual follow-up for EGV for 5 years to receive endoscopic treatment if needed. The therapeutic regimen for each patient followed the guidelines at that time. (i.e., the Baveno V consensus [13] and Baveno VI consensus [4].)
2.3 Interventions
Initial combined endoscopy was performed using an electronic endoscope GIF-XQ260 (Olympus, Tokyo, Japan) to determine the grade of varices and decide the treatment options. Firstly, gastric varices were treated with EVO by injecting N-butyl-cyanoacrylate (COMPONT, Beijing, China) using the sandwich method (lauromacrogol–cyanoacrylate–lauromacrogol) described by us [14], followed by EVL, with six multi-band ligators (Cook Endoscopy, Winston-Salem, NC, USA) was performed in a spiral ascending fashion from 1 cm above the Z-line [15]. Multiple-site injections were administered to completely obturate the gastric varices in one session. Patients were followed up every 2 months after the combined endoscopic treatment, with re-ligation or re-sclerotherapy if necessary, and then every six to 12 months after the eradication of varices.
Patients were divided into NSBB and non-NSBB groups according to the endoscopist's decision following the first combined endoscopic treatment (EVL and EVO). Proceeding this way was justified since there was no consensus on additional NSBB prescription. However, if patients lived nearby and were more easily available for outpatient assessment of liver function and determination of their quality-of-life status, they were more likely to be assigned additional NSBB. Patients in the NSBB group were prescribed different oral doses of propranolol or carvedilol to achieve a resting heart rate of 55–60 beats per minute while maintaining a systolic blood pressure of at least 90 mmHg. Either being prescribed and taking propranolol up to 30 mg/day or carvedilol up to 10 mg/day, or achieving an approximate 25% reduction in heart rate [16] was defined as a standard NSBB intake, otherwise, low NSBB intake.
Considering that splenectomy used to be the main surgical treatment for complicated variceal bleeding in China, patients usually received splenectomy 10–20 years ago to control massive variceal bleeding or to improve thrombocytopenia. Therefore, some patients included in our study had undergone splenectomy, but all splenectomies were performed more than 10 years ago, and patients who underwent splenectomy after combined endoscopic treatment were not included.
2.4 Assessment of Clinical Outcome
The primary outcome was overall survival, defined as the time from the first combined endoscopic treatment until death from any cause. The secondary endpoint was rebleeding, defined as the time to recurrent variceal bleeding due to portal hypertension at least 120 h after the first variceal hemorrhage and endoscopic treatment, manifesting as recurrent hematemesis or hematochezia in any of the following settings: (1) hospital admission; (2) a drop of 30 g/L hemoglobin or requirement for blood transfusion; and (3) death within 6 weeks, according to international guidelines [12].
2.5 Statistical Analysis
Continuous variables are expressed as mean ± standard error if the values were distributed normally; otherwise, as median with interquartile range (IQR). Each categorical variable is presented as the total number and the corresponding percentage n (percentage). Statistically significant differences in variables between the two groups were tested using Student's unpaired t-test for continuous variables that met both normal distribution and homogeneity of variance hypotheses, χ2 test for categorical variables and Mann–Whitney U test for all other variables. Levene's test was used to validate the equality of variances. Propensity score matching (PSM) with a 0.02 match tolerance was used to reduce bias from confounding variables. Time-to-event endpoints, including overall survival and rebleeding, were analyzed using the Kaplan-Meier method, and the differences were assessed by the log-rank test. The effects of clinically important prognostic factors on endpoints were assessed using univariate and multivariate Cox proportional hazards models, as reflected by hazard ratios (HRs) and 95% confidence intervals (CIs). The effects of different factors on rebleeding were assessed using univariate and multivariate logistic regression, as reflected by odd ratios (HRs) and 95% confidence intervals (CIs). All reported p values were two-sided and considered statistically significant if they were 0.05 or less. Data were processed using SPSS (version 24.0; SPSS Inc., Chicago, IL, USA) and STATA 13.0 (StataCorp LLC., Texas, USA).
3 Results
3.1 Baseline Characteristics of the Included Patients
A total of 573 patients with cirrhotic varices received combined EVL and EVO treatment to prevent rebleeding. 83 patients were excluded for the following reasons: no history of variceal bleeding (n = 21), a history of malignancy (n = 57), and immediate loss to follow-up after discharge (n = 5). Finally, 490 patients with gastroesophageal varices (GOV1 and GOV2) were included in the study with complete data sets: 130 patients were treated with NSBB continuously following the first combined endoscopic treatment, while 360 patients did not receive NSBB (Figure 1). As shown in Table 1, baseline characteristics were comparable between the two groups, except for male gender, presence of type 2 diabetes, and mild encephalopathy, which were more prevalent in the non-NSBB group than in the NSBB group. Notably, (other) signs of hepatic decompensation, including HVPG and Child-Pugh class, did not differ between the groups.
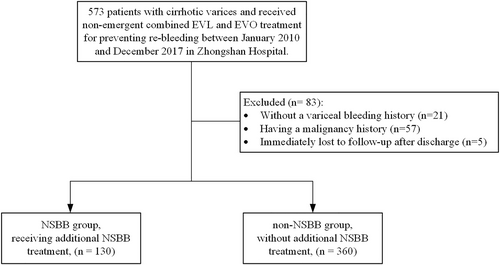
The flow chart of this retrospective study cohort. EVL, esophageal variceal ligation; EVO, endoscopic variceal obturation.
| Entire group | p value | Propensity score matched group | p value | |||||
|---|---|---|---|---|---|---|---|---|
| All (n = 490) | Non-NSBBs (n = 360) | NSBBs (n = 130) | All (n = 260) | Non-NSBBs (n = 130) | NSBBs (n = 130) | |||
| Age (years) | 56 (18) | 56 (17) | 52.8 ± 12.3 | 0.136 | 54 (19) | 54 (19) | 52.8 ± 12.3 | 0.953 |
| Male (%) | 319 (65.1%) | 244 (67.8%) | 75 (57.7%) | 0.039 | 152 (58.5%) | 77 (59.2%) | 75 (57.5%) | 0.801 |
| Etiology of cirrhosis | 0.301 | 0.339 | ||||||
| HBV/HCV | 292 (59.6%) | 223 (62.0%) | 69 (53.1%) | 154 (59.23%) | 85 (65.4%) | 69 (53.1%) | ||
| AILD | 29 (5.9%) | 21 (5.8%) | 8 (6.2%) | 15 (5.8%) | 7 (5.4%) | 8 (6.2%) | ||
| Alcohol | 48 (9.8%) | 35 (9.7%) | 13 (10.0%) | 21 (8.06%) | 5 (3.8%) | 13 (10.0%) | ||
| Others | 132 (26.9%) | 88 (24.4%) | 44 (33.9%) | 77 (29.6%) | 33 (25.4%) | 44 (33.9%) | ||
| HVPG (mmHg) | 14.0 ± 6.2 | 14.2 ± 6.3 | 13.7 ± 5.9 | 0.539 | 13.8 ± 6.2 | 13.9 ± 6.5 | 13.7 ± 5.9 | 0.815 |
| Diabetes | 108 (22.0%) | 88 (24.4%) | 20 (15.4%) | 0.033 | 37 (14.2%) | 17 (13.1%) | 20 (15.4%) | 0.594 |
| Hypertension | 89 (18.2%) | 64 (18.8%) | 25 (19.2%) | 0.713 | 46 (17.7%) | 21 (16.2%) | 25 (19.2%) | 0.516 |
| Splenectomy | 107 (21.8%) | 83 (23.1%) | 24 (18.5%) | 0.277 | 50 (19.2%) | 26 (20%) | 24 (18.5%) | 0.753 |
| Hemoglobin (g/L) | 87 (33) | 89 (32.5) | 87.5 ± 22.2 | 0.444 | 86 (32) | 87 (34) | 87.5 ± 22.2 | 0.829 |
| Platelet (*109/L) | 71 (64) | 69.5 (61.5) | 73.5 (67) | 0.732 | 67 (57) | 64 (56) | 73.5 (67) | 0.210 |
| Albumin (g/L) | 35.0 ± 4.8 | 34.8 ± 4.7 | 35.5 ± 5.1 | 0.114 | 35.4 ± 0.3 | 35.3 ± 4.4 | 35.5 ± 5.1 | 0.668 |
| Total bilirubin (μmol/L) | 13.15(8.2) | 13 (8.2) | 13.5 ± 7.15 | 0.993 | 13.4 (7.8) | 12.9 (7.7) | 13.5 (8.155) | 0.876 |
| Direct bilirubin (μmol/L) | 6 (4.6) | 6 (4.6) | 6.15 (4.6) | 0.763 | 6.2 (4.3) | 6.25 (4.1) | 6.15 (4.6) | 0.875 |
| Prothrombin time (s) | 13.8 (2) | 13.9 (1.9) | 13.7 (2) | 0.099 | 13.8 (2) | 14.1 ± 1.5 | 13.7 (2) | 0.077 |
| Creatinine (μmol/L) | 65.3 (22.1) | 65.4 (22.4) | 64.05 (18.95) | 0.624 | 65 (18.9) | 66 (18.3) | 64.045 (18.945) | 0.580 |
| Ascites | 242 (49.4%) | 184 (51.1%) | 58 (44.6%) | 0.204 | 118 (45.4%) | 60 (46.2%) | 58 (44.6%) | 0.803 |
| Encephalopathy | 14 (2.9%) | 11 (3.1%) | 3 (2.3%) | 0.018 | 6 (2.3%) | 3 (2.3%) | 3 (2.3%) | 1.000 |
| Child-Pugh score | 7 (3) | 7 (3) | 6 (1) | 0.484 | 6 (1) | 6 (3) | 6 (1) | 0.921 |
| Child-Pugh grade | 0.343 | 0.207 | ||||||
| A | 240 (49.0%) | 170 (47.2%) | 70 (53.8%) | 136 (52.3%) | 66 (50.8%) | 70 (53.8%) | ||
| B | 234 (47.8%) | 179 (49.7%) | 55 (42.3%) | 118 (45.4%) | 63 (48.5%) | 55 (42.3%) | ||
| C | 16 (3.3%) | 11 (3.1%) | 5 (3.8%) | 6 (2.3%) | 1 (0.8%) | 5 (3.8%) | ||
| Portal vein thrombosis | 191 (39.0%) | 139 (38.6%) | 52 (40.0%) | 0.781 | 100 (38.5%) | 48 (36.9%) | 52 (40.0%) | 0.610 |
| Splenectomy (among PVT) | 66(34.6%) | 49(64.7%) | 17(32.7%) | 0.741 | 13(27.1%) | 17(32.7%) | 0.541 | |
- Note: Data are presented as mean ± standard deviation, median (interquartile range), and n (proportion).
- Abbreviations: AILD, autoimmune liver disease; HBV, hepatitis B virus; HCV, hepatitis C virus; HVPG, hepatic venous pressure gradient, PVT, portal vein thrombosis.
The variables entered in the Logistic Regression model for propensity score estimation included age, gender, Child-Pugh class, and other concomitant conditions (diabetes, hypertension, coronary heart disease, splenectomy, portal vein thrombosis), which were considered to be clinically important for patient prognosis. After PSM, both groups comprised 130 patients, and none of the baseline characteristics showed significant differences between the two groups. The baseline characteristics of patients included in and excluded from PSM were comparable, except for gender and diabetes (Supporting Information S3: Table S1).
3.2 Cumulative Incidences of Overall Survival and Rebleeding
3.2.1 Overall Cohort
The 1-, 3-, and 5-year cumulative overall survival rates before PSM were 95.41%, 89.20%, and 83.64% in the non-NSBB group and 98.44%, 94.15%, and 87.40% in the NSBB group, respectively, with no significant difference between the two groups (p = 0.133) (Figure 2A). However, rebleeding rates were significantly different between the two groups favoring NSBB treatment, with 1-, 3-, and 5-year cumulative incidence rates of 23.57%, 36.33%, and 45.65% in the non-NSBB group and 11.79%, 21.16%, and 30.64% in the NSBB group (p = 0.002) (Figure 2B). After PSM, the overall survival was not significantly different (p = 0.119), and rebleeding was significantly lower in the NSBB group (p = 0.007, log-rank test) (Figure 2C,D).
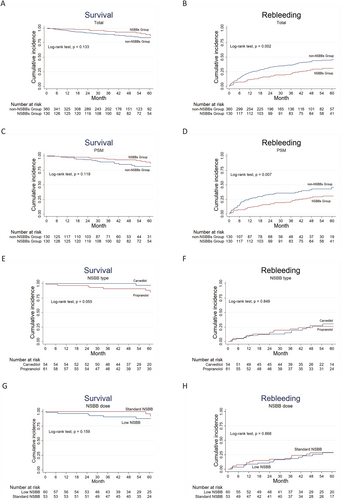
Cumulative survival and incidence of rebleeding in different groups. (A) Overall survival, (B) cumulative incidence of rebleeding. (C) Overall survival, (D) cumulative incidence of rebleeding after PSM. The blue lines represent the non-NSBB group and the red lines represent the NSBB group. (E) Overall survival and (F) cumulative incidence of rebleeding in patients taking different NSBBs. Blue lines represent carvedilol and red lines represent propranolol. (G) Overall survival and (H) cumulative incidence of rebleeding in patients taking different doses of NSBB. Blue lines represent standard NSBB, and the red lines represent low NSBB. The numbers of patients at risk at different time points are presented below the graph. NSBB, non-selective beta-blocker.
3.2.2 Effect of Different NSBB Treatments
Furthermore, the different NSBB prescriptions (carvedilol vs. propranolol; standard NSBB vs. low NSBB) did not show significant differences in rebleeding (p = 0.849) or survival (with a trend in favor of carvedilol over propanolol, p = 0.055) (Figure 2E,F), nor was there a difference between patients receiving standard and low NSBB (p = 0.868 and 0.159, respectively) (Figure 2G,H).
3.2.3 Complications of Hepatic Decompensation
Since complications related to liver failure negatively impact prognosis, overall survival rates and rebleeding rates were compared between the NSBB and non-NSBB groups (post-PSM) according to baseline Child-Pugh grade and the presence or absence of ascites, diabetes, hypertension, portal vein thrombosis (PVT), or splenectomy status (Figure 3 and Figure S1). In contrast to the results based on all patients (n = 490), where overall survival was comparable between the NSBB and non-NSBB groups, the addition of NSBB had a statistically significant effect on overall survival in patients without ascites and those without splenectomy (p = 0.0461 and 0.019, respectively) (Figure 3B,F); Regarding rebleeding, patients without ascites, patients with PVT, or patients with splenectomy had no statistically significant difference after additional NSBB treatment (p > 0.05 in all 3 classifications) (Figure 4B,C and E), different from the comparison based on all patients (with or without NSBB treatment).
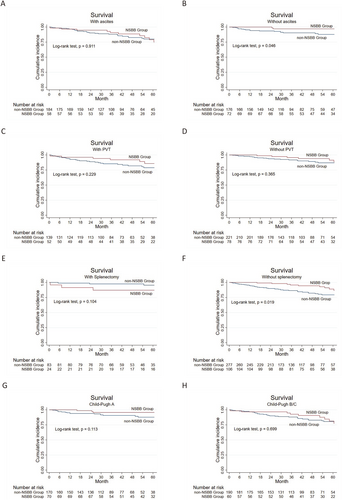
Cumulative overall survival in the non-NSBBs versus the NSBBs group with comparable baseline or complication. Patients (A and B) with or without ascites, (C and D) with or without PVT, (E and F) with or without splenectomy, (G and H) with different Child-Pugh grades. The blue lines represent the non-NSBB, and the red lines represent the NSBB group. The number of patients at risk at different points of time is presented below the graph. NSBB, non-selective beta-blocker.
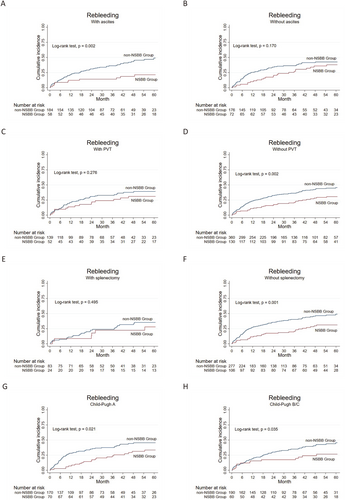
Cumulative incidences of rebleeding in the non-NSBBs group and the NSBBs group with the same specific baseline or complication. Patients (A and B) with or without ascites, (C and D) with or without PVT, (E and F) with or without splenectomy, (G and H) with different Child-Pugh grades. The blue lines represent the non-NSBB, and the red lines represent the NSBB group. The numbers of patients at risk at different points of time are presented below the graph. NSBB, non-selective beta-blocker.
3.2.4 Splenectomy for Patients With PVT
Patients with splenectomy tend to have a high risk of developing portal vein thrombosis. Comparing patients with PVT and with or without splenectomy, those with splenectomy displayed significantly higher overall survival and lower rebleeding rates (p = 0.016 and 0.025, respectively) (Figure 5A,B). After stratification based on the use of NSBB, no significant differences were observed between patients with PVT and with or without splenectomy treated with NSBB (p = 0.339 and 0.810, respectively) (Figure 5C,D). However, survival was significantly improved and rebleeding was reduced in splenectomized patients versus patients without splenectomy who did not receive NSBB (p = 0.001 and 0.005, respectively) (Figure 5E,F).
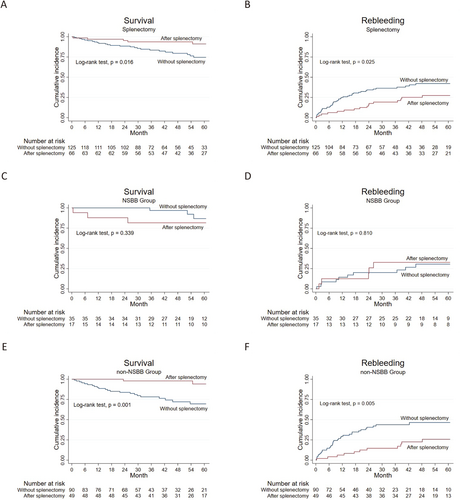
Cumulative survival and incidence of rebleeding in patients with PVT with or without splenectomy. Cumulative survival and incidence of rebleeding in (A and B) all patients with PVT, (C and D) PVT patients with NSBBs treatment, and (E and F) PVT patients without NSBBs treatment. The blue lines represent patients without splenectomy, and the red lines represent patients with splenectomy. The numbers of patients at risk at different points of time are presented below the graph. NSBB, non-selective beta-blocker; PVT, portal vein thrombosis.
3.3 Risk Factors Analysis
Independent predictors including age, use of NSBB, concomitant hypertension, diabetes, splenectomy, portal vein thrombosis, and Child-Pugh score were included in the multivariate Cox regression analysis for all-cause mortality. Additional NSBB did not significantly affect overall mortality, whereas old age, splenectomy, and portal vein thrombosis were independent predictors of all-cause mortality (HR 1.05, 95% CI 1.02–1.09; HR 0.33, 95% CI 0.14–0.80; HR 1.73, 95% CI 1.00–2.99, respectively). Additional NSBB, concomitant diabetes, splenectomy, and Child-Pugh score might have a mild influence on rebleeding, all with p-values of less than 0.2. Further multivariate Cox regression analysis showed that both NSBB use (HR 0.54, 95%CI 0.37–0.79) and splenectomy (HR 0.65, 95%CI 0.44–0.96) independently reduced the risk of rebleeding (Table 2). In addition, after controlling other potential predictors, the use of NSBBs (OR 0.59, 95%CI 0.37–0.92) was associated with a 41% reduction in the odds of rebleeding according to logistic regression (Supporting Information S3: Table S2).
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | ||
| All-cause mortality | Age | 1.06 | 1.04–1.09 | < 0.001 | 1.05 | 1.02–1.08 | < 0.001 |
| Gender | 1.00 | 0.58–1.71 | 0.991 | ||||
| (Male vs. female) | |||||||
| Group | 0.62 | 0.33–1.17 | 0.137 | 0.61 | 0.32–1.17 | 0.137 | |
| (NSBB vs. non-NSBB) | |||||||
| Hypertension | 1.74 | 0.98–3.10 | 0.061 | 1.00 | 0.54–1.85 | 0.991 | |
| (Yes vs. No) | |||||||
| Diabetes | 1.84 | 1.06–3.20 | 0.029 | 1.15 | 0.65–2.03 | 0.637 | |
| (Yes vs. No) | |||||||
| Splenectomy | 0.35 | 0.15–0.81 | 0.015 | 0.35 | 0.14–0.83 | 0.018 | |
| (Yes vs. No) | |||||||
| Portal vein thrombosis | 1.68 | 1.00–2.82 | 0.052 | 1.72 | 1.00–2.96 | 0.049 | |
| (Yes vs. No) | |||||||
| Child-Pugh score | 1.31 | 1.10–1.56 | 0.002 | 1.16 | 0.97–1.39 | 0.094 | |
| Rebleeding | Age | 1.00 | 0.99–1.02 | 0.598 | |||
| Gender | 0.99 | 0.73–1.35 | 0.957 | ||||
| (Male vs. female) | |||||||
| Group | 0.57 | 0.39–0.82 | 0.003 | 0.54 | 0.37–0.79 | 0.001 | |
| (NSBB vs. non-NSBB) | |||||||
| Hypertension | 0.96 | 0.65–1.43 | 0.847 | ||||
| (Yes vs. No) | |||||||
| Diabetes | 1.29 | 0.92–1.83 | 0.144 | 1.17 | 0.82–1.67 | 0.377 | |
| (Yes vs. No) | |||||||
| Splenectomy | 0.68 | 0.47–1.00 | 0.054 | 0.65 | 0.44–0.96 | 0.03 | |
| (Yes vs. No) | |||||||
| Portal vein thrombosis | 0.86 | 0.63–1.18 | 0.352 | ||||
| (Yes vs. No) | |||||||
| Child-Pugh score | 0.92 | 0.82–1.03 | 0.156 | 0.90 | 0.81–1.01 | 0.078 | |
- Abbreviations: CI, confidence interval; HR, hazard ratio; NSBB, non-selective beta-blocker.
4 Discussion
The effect of NSBB in patients with cirrhotic gastric varices is controversial [10, 16, 17], and clinical evidence for the addition of NSBB after combined endoscopic therapy is lacking. Unlike previous research that focused on only one endoscopic prophylactic treatment (EVL or EVO alone), our study included cirrhotic patients with gastroesophageal varices who received combined endoscopic treatment, that is, EVO for gastric varices, followed by EVL for esophageal varices, as secondary prophylaxis.
Currently, clinical evidence on NSBB as secondary prophylaxis for EGV is mixed, and the association between NSBB and mortality differs, especially NSBB treatment after EVO, which still needs more evidence to be clarified. In end-stage cirrhosis, there were still discrepancies as to the effect of propranolol on mortality [18, 19], perhaps because the heterogeneity of varices (the types of varices in both retrospective studies are not clear) and treatment regimens led to divergent effects (the study that analyzed patients receiving octreotide showed no effect on mortality [18], while the other showed an increase in mortality [19]). To avoid potential confounding effects, we restricted the study to patients with both GOV1 and GOV2 who received EVL after EVO. Our study showed that the addition of NSBB did not have a significant effect on overall survival, although a trend towards lower mortality was found (HR 0.62, 95% CI 0.33–1.17), in line with prior studies on (mainly) esophageal varices [20, 21]. Another study showed that the same combined EVL and NSBB therapy, compared to EVL alone, was effective in reducing mortality in patients with Child B/C rather than Child A cirrhosis, suggesting that the severity of cirrhosis may also influence the outcome of secondary prophylactic therapies [22]. We stratified Child-Pugh grades to evaluate the effect of additional NSBB; the results were the same as those of the whole group that had comparable Child-Pugh grades. On the contrary, Chen [17] et al. found that additional carvedilol to EVO did not decrease rebleeding of gastric varices yet generated more adverse effects; a randomized controlled trial showed that the addition of propranolol to EVO did not benefit in reducing rebleeding or mortality in gastric varices [23]. However, in this study, patients only received EVO, and isolated gastric varices were included, which can bleed with a lower HVPG and are associated with a higher morbidity and mortality rate. In addition, the effects of carvedilol and traditional NSBB propranolol on secondary prophylaxis are still controversial, so we also compared carvedilol and propranolol. Although, we did not find statistically significant differences between propranolol and carvedilol in overall survival and rebleeding, we observed a trend of improved survival with carvedilol (Figure 2B). In this vein, our results are in line with prior studies reporting that carvedilol positively affected survival and more efficiently reduced rebleeding than propranolol through a better HVPG response [24, 25].
It is crucial to identify the common characteristics of patients who may benefit from additional NSBB treatment. A retrospective study showed that additional NSBB did not reduce rebleeding of esophageal varices compared to EVL alone but reduced mortality in patients with compensated cirrhosis [26], contrary to the conclusion of a meta-analysis that the combination of NSBB and EVL significantly reduced mortality in patients with Child-Pugh B/C but not with Child-Pugh A, while the combination was superior to EVL alone in preventing variceal rebleeding in patients with Child-Pugh A[22]. And our study found that the addition of NSBB to the combined endoscopic treatment decreased the incidence of rebleeding in patients with ascites or Child-Pugh B/C, while there was no benefit on overall survival. We speculate that the side effects of NSBBs counteracted the benefit of reduced rebleeding, as they may have exacerbated the unstable hemodynamics; therefore, NSBB should be carefully considered in patients with severe complications [27, 28]. Moreover, the presence of gastric varices usually results in a more complicated collateral circulation than when only esophageal varices are present, and the treatment of gastric collaterals is essential to the prognosis of GOV [29]. Therefore, in the first endoscopic session to treat GOV1 and GOV2 varices, we performed EVO to occlude the gastric portion of the varicose veins, followed by EVL to occlude the esophageal portion, assuming that successive and combined endoscopic treatment should have a synergistic beneficial effect in preventing recurrent bleeding. These varices are mainly supplied by the left and posterior gastric veins, draining into the systemic circulation through esophageal and paraesophageal varices and/or the inferior phrenic vein [11]. Thus, we observed in our clinical practice that obturation of gastric varices ameliorated the extent of connecting esophageal varices.
Notably, splenectomy was found to be an independent predictor of overall survival in our study, in line with its beneficial role in survival in our previous study of patients with schistosomiasis-related portal hypertension [30]. It is therefore explainable that additional NSBB treatment played a beneficial role in improving overall survival and reducing rebleeding in patients without splenectomy. Splenectomy has been the main surgical treatment for complicated variceal bleeding in China previously [31]. In our study, 107 (21.8%) patients underwent splenectomy 10–20 years ago to control massive variceal bleeding or ameliorate thrombocytopenia. When subdividing patients on NSBBs into splenectomized and non-splenectomized groups, rebleeding was equally low and survival remained unaffected, irrespective of whether NSBB treatment was comparable after splenectomy. In contrast, in patients without NSBB treatment, splenectomy both reduced rebleeding and improved overall survival, and we conclude that NSBB treatment may benefit patients without splenectomy. As PVT is an important post-splenectomy complication, we further stratified PVT patients according to splenectomy. In patients with PVT, splenectomy tended to both improve overall survival and reduce the rate of rebleeding, while the further comparison based on NSBBs treatment showed that splenectomy still played a beneficial role in improving overall survival and reducing the rate of rebleeding in the group that did not receive NSBBs treatment, while the addition of NSBBs did not further improve outcomes. Thus, NSBB treatment may benefit non-splenectomized patients with PVT.
Giannitrapani [32] et al. proposed that NSBB might promote PVT, and were mainly concerned with the hemodynamic changes induced by NSBB. Splenectomy not only blocks excessive blood flow from the splenic artery but also reduces the amount of blood flowing from the splenic vein into the portal vein, which contributes to the reduction of HVPG; therefore, patients with portal hypertension benefit from splenectomy. In our study, NSBB showed a beneficial role in non-splenectomized patients, even with PVT, which can be explained by their effect of reducing HVPG. We speculate that lowering HVPG could be beneficial in secondary prophylaxis of cirrhotic gastric variceal bleeding, while the correlation between HVPG and the secondary prophylaxis in patients with cirrhotic gastric varices receiving combined endoscopic treatment needs further exploration.
It should be noticed that the doses of NSBBs in our study were lower than those in most other studies. Apart from the used NSBBs and the types of varicose veins, we also paid attention to the doses of NSBBs, as there are no strict criteria for the doses of NSBB treatment, which are usually titrated until heart rate and blood pressure reach defined thresholds of heart rate and blood pressure [9]. A real-world, multicenter, retrospective study showed that low-dose NSBBs (≤ 80 mg/day) performed better than high-dose NSBBs (> 80 mg) in reducing mortality in both primary and secondary prophylaxis [33]; and the crude comparison based on alcoholic cirrhosis with spontaneous bacterial peritonitis also showed that a high dose of NSBB (160 mg of propranolol) was associated with an increased risk of death compared with a lower dose (80 mg) [34]. The recommended doses of NSBBs for secondary prophylaxis in our center were: carvedilol, starting at 6.25–12.5 mg per day and propranolol starting at 10 mg twice a day up to the tolerated dose [35], which is much lower than that in the reports from other countries [1]. During our clinical practice, we found that patients tended to achieve the target heart rate with a relatively low dose of NSBBs, so we defined the intake of propranolol up to 30 mg/day or carvedilol up to 10 mg/day as quantitatively acceptable NSBB intake (sufficient NSBB intake), otherwise as insufficient NSBB intake. These doses were adjusted to a reduction of the heart rate of approximately 25%, in line with international criteria. With these doses, there were statistically significant differences between sufficient and insufficient NSBB intake groups for both mortality and rebleeding rates in our cohort of Chinese patients.
Our study had limitations. The lack of follow-up portal-hepatic vein pressure gradient measurements hampered further exploration of the correlations between clinical outcomes and additional NSBB treatment. While intrahepatic shunts such as intrahepatic venous-venous shunts, arterial-venous shunts and arterial-portal shunts are common in decompensated cirrhosis, and about 39% of patients in our study had PVT, both intrahepatic shunts and PVT may affect the accuracy of pressure measurement; therefore, it would have been informative to perform additional HVPG and vascular imaging analysis. Moreover, this was a retrospective cohort study in a single center, and patients were assigned according to the preference of the treating physician.
In conclusion, the addition of NSBB to combined endoscopic therapy in patients with complex esophagogastric varices could significantly reduce the rate of recurrent bleeding but improved overall survival only in patients who did not undergo splenectomy. The addition of NSBB to combined endoscopic treatment is generally justified if only to reduce rebleeding, and as long as classical contraindications for NSBB treatment of these complex varices are observed.
Author Contributions
Concept of the study: Shiyao Chen, Xiaoquan Huang; Data collection: Ling Wu, Xiaoquan Huang, Feng Li, Siyu Jiang, Liyuan Ni, Yingjie Ai; Statistical analysis: Ling Wu, Xiaoquan Huang, Feng Li, Siyu Jiang, Liyuan Ni, Yingjie Ai, Detlef Schuppan; Drafting the original manuscript: Ling Wu, Xiaoquan Huang; Revision of the manuscript: Ling Wu, Xiaoquan Huang, Feng Li, Siyu Jiang, Liyuan Ni, Yingjie Ai, Shiyao Chen, Detlef Schuppan; Approval of the final version of the manuscript: all authors.
Ethics Statement
The study protocol was approved by the Medical Ethics Committee of the Zhongshan Hospital, Fudan University, conforming to the ethical guidelines of the 1975 Declaration of Helsinki.
Consent
Informed consent was obtained from each patient included in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data will be made available upon reasonable request.