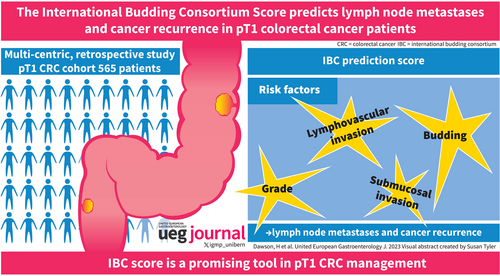
Lymph node metastases and recurrence in pT1 colorectal cancer: Prediction with the International Budding Consortium Score—A retrospective, multi-centric study
John-Melle Bokhorst and Linda Studer contributed equally to this paper.
Abstract
Background
The International Collaboration on Cancer Reporting proposes histological tumour type, lymphovascular invasion, tumour grade, perineural invasion, extent, and dimensions of invasion as risk factors for lymph node metastases and tumour progression in completely endoscopically resected pT1 colorectal cancer (CRC).
Objective
The aim of the study was to propose a predictive and reliable score to optimise the clinical management of endoscopically resected pT1 CRC patients.
Methods
This multi-centric, retrospective International Budding Consortium (IBC) study included an international pT1 CRC cohort of 565 patients. All cases were reviewed by eight expert gastrointestinal pathologists. All risk factors were reported according to international guidelines. Tumour budding and immune response (CD8+ T-cells) were assessed with automated models using artificial intelligence. We used the information on risk factors and least absolute shrinkage and selection operator logistic regression to develop a prediction model and generate a score to predict the occurrence of lymph node metastasis or cancer recurrence.
Results
The IBC prediction score included the following parameters: lymphovascular invasion, tumour buds, infiltration depth and tumour grade. The score has an acceptable discrimination power (area under the curve of 0.68 [95% confidence intervals (CI) 0.61–0.75]; 0.64 [95% CI 0.57–0.71] after internal validation). At a cut-off of 6.8 points to discriminate high-and low-risk patients, the score had a sensitivity and specificity of 0.9 [95% CI 0.8–0.95] and 0.26 [95% 0.22, 0.3], respectively.
Conclusion
The IBC score is based on well-established risk factors and is a promising tool with clinical utility to support the management of pT1 CRC patients.
Graphical Abstract
Key summary
Established knowledge on this subject
-
Risk factors in endoscopically resected pT1 colorectal cancer (CRC) are often reported descriptively and are used to guide patient management.
-
Most treatment guidelines do not take the actual probability of an adverse event (e.g. lymph node metastases) into account and may be too stringent, leading to overtreatment.
What are the significant findings of this study?
-
The International Budding Consortium (IBC) score delivers the probability of adverse events in pT1 colorectal cancers as an additional tool for individualised therapeutic management.
-
The IBC score includes well-established risk factors in pT1 CRC while minimising inter-observer variability and is a promising tool with clinical utility in daily practice.
INTRODUCTION
In the aera of personalised healthcare, interdisciplinary clinical management supports the optimisation of risk assessment in colorectal cancer (CRC) patients. Indeed, three clinical CRC scenarios highlight this statement: in pT1 CRC, the prediction of local lymph node metastases indicates a potential oncologic resection,1, 2 while in stage II CRC adjuvant therapy1, 2 and in rectal cancer neoadjuvant therapy should be considered.
Risk assessment is usually based on the Tumor Nodes Metastasis classification proposed by the union internationale contre le cancer3 and the additional evidence-based biomarkers suggested in the World Health Organization classification.3, 4 According to national and international CRC guidelines, the International Collaboration on Cancer Reporting (ICCR) proposes additional ‘core’ prognostic factors for pT1 CRC, such as histological tumour type, lymphovascular invasion, tumour grade, perineural invasion, extent of invasion, invasive carcinoma dimensions and margin status.5 In endoscopically resected pT1 CRC, the histological features of lymphovascular invasion, tumour grade and tumour infiltration depth are traditionally used to determine further therapy (observation vs. oncologic resection with en bloc removal of regional lymph nodes).1, 2 The incidence of lymph node metastases among all pT1 CRC is estimated to be about 7%–15% in the literature,6, 7 and lower for pedunculated polyps.8 However, if histological risk factors are considered, this probability varies from <1% (no risk factors present) to about 35% (several risk factors present).9 Typically, once a single unfavourable histological risk factor is present, oncologic resection is recommended.1, 2, 10 However, it has been suggested that this approach is too stringent, leading to over-treatment and potentially unnecessary clinical complications.11, 12 Therefore, a more refined risk assessment could be of great utility to aid patient management.
In the recent years, several studies have promoted the increasing prognostic evidence of two additional morphologic biomarkers, namely tumour budding and tumour immunology. Tumour budding is a tumour-related factor and is defined as single tumour cells or clusters of up to four cells at the invasive CRC front.13 Tumour budding is a strong marker of tumour progression and prognosis14, 15 including lymph node metastasis in pT1 CRC and can also be used among the above-mentioned histological risk factors for risk stratification.16-18 The prognostic role of immune response, especially by T-cell infiltrates, is supported by several studies19, 20 as well as its potential predictive value.19, 21
A promising approach for the integration of tumour budding and tumour inflammation into a predictive score for local lymph node metastases and cancer recurrence in pT1 CRC is based on the ‘attacker-defender model’ (referring to tumour buds as the ‘attacker’ and immune cells as the ‘defender’). This concept was first published in 200922 and reflects the pathogenetic dynamic of the tumour microenvironment (TME). Since then, several studies have shown the importance of this model, including the tumour budding grade and the cytotoxic T-lymphocyte count, in prognostic models.23, 24
The implementation of tumour budding in national and international guidelines was boosted by the standardised scoring system proposed by the International Tumour Budding Consensus Conference (ITBCC) in 2016.13 The International Budding Consortium (IBC) is a spinoff of the ITBCC and aims to validate the prognostic and predictive role of tumour budding in CRC and to decrease potential inter-observer variability using available artificial intelligence (AI) algorithms.
The aim of the present retrospective, multicentric IBC study is not to replace the already well-established additional risk factors in pT1 CRC but to investigate the potential role of the two pillars of the attacker-defender model, the tumour budding and immune response, in a predictive and reproducible scoring system for the clinical management of pT1 CRC patients.
MATERIALS AND METHODS
Study design
Cases of diagnosed pT1 CRC from 1991 until 2018 were retrieved from the pathology institutes of the University of Bern, Switzerland (n = 196), Radboud University Nijmegen, Netherlands (n = 105), Klinikum Bayreuth, Germany (n = 96), Cantonal Hospital Liestal, Switzerland (n = 92), Baylor Scott and White Health, Temple, Texas, USA (n = 44) and Medizinisches Versorgungszentrum am Bruderwald, Bamberg, Germany (n = 34). All participating centres complied with local ethics committee regulations (as applicable): Bern and Liestal: 2017-01803 (Kantonale Ethikkommission Bern); Nijmegen: 10,602 (Dutch Cancer Society); Bayreuth: 23-109-Br (Friedrich-Alexander-Universität).
Participants
Inclusion criteria were histopathological diagnosis of submucosally invasive (pT1) CRC with either (A) initial resection or completion oncologic resection (no salvage surgery following surveillance of endoscopic removal) and therefore with known lymph node status or (B) endoscopic specimens with at least 36 months follow-up with documented information on local/distant recurrence. Exclusion criteria included a history of inflammatory bowel disease, prior CRC or synchronous CRC and polyposis syndromes (Lynch syndrome only if first CRC). Patients with fragmented/piecemeal resection were included only if completion oncologic resection was performed. Participating centres confirmed compliance to corresponding local ethics regulations and provided a formalin-fixed, paraffin embedded (FFPE) block and corresponding haematoxylin and eosin (H&E) stained slides. In case of several blocks/case, the FFPE block with the most advanced tumour was selected. If no block with the most advanced tumour could be determined, all tumour blocks were provided.
A total of 773 patients were submitted to the study. During the study, a total of 209 cases were excluded due to reasons listed in Figure 1. In the final cohort consisting of 565 patients, 462 underwent either initial or subsequent resection (nodal status known) and 103 were treated by endoscopic resection alone. Seventeen patients with piecemeal resection included in the study were amenable to complete histologic review of all parameters and underwent oncologic resection.
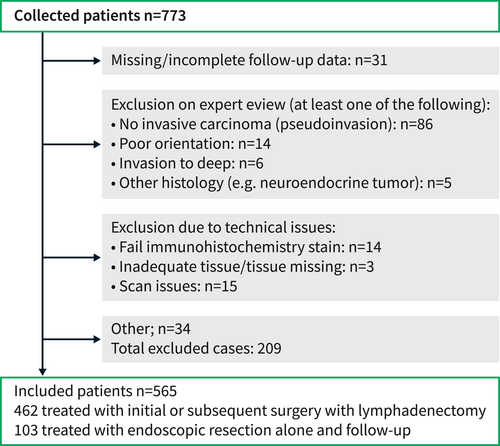
Flowchart of patients through the study.
Test methods
Histology review
A group of 8 gastrointestinal pathologists (AL, IDN, HD, RK, MV, SB, SKÖ, ASOE) were involved in a comprehensive histopathological review of all cases. Details concerning reviewed parameters (including histological risk factors of tumour grade, lymphovascular invasion and infiltration depth among others) and immunohistochemical assays including mismatch repair (MMR) proteins for a subset of patients can be found in Supporting Information S1. Of note, 86 cases (11%) were excluded due to reclassification as adenomas with epithelial misplacement (no invasive carcinoma). Only cases with full data for key predictor factors (n = 565) considered for the prediction model were included for further analysis.
Assessment of tumour budding
Tumour budding was assessed using an automated detection algorithm (Figure 2)25 in three different areas: in a manually selected hotspot, in an automatically detected hotspot and as a mean value among 10 automatically detected hotspots. All hotspots measured 0.785 mm2 according to the ITBCC guidelines.13 All manual tumour budding areas were selected using the Automated Slide Analysis Platform tool (https://computationalpathologygroup.github.io/ASAP/) with a square annotation (preprint available: https://www.researchsquare.com/article/rs-2093909/v1). The algorithm is available at https://grand-challenge.org/algorithms/colon-budding-in-ihc/.
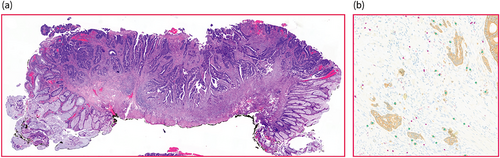
Scoring of cases. Haematoxylin stain of whole tissue slide (a, endoscopic resection specimen) used for the assessment of pathological features by an expert panel. Tumour budding and T-cells were analysed using an automatic algorithm on AE1/AE3 and CD8 double-stained slides (output in b; tumour buds marked green, T-cells marked pink).
Assessment of CD8+ lymphocytes
Colour deconvolution was used to separate the AE1/AE3 and CD8 stains. CD8-positive T-cells were automatically detected in the same areas as tumour buds (manually selected hotspot, automatically detected hotspot and mean value among 10 automatically detected hotspots). The algorithm used for lymphocyte detection has been described previously in detail.26 Manual quality control of the algorithm output revealed a precision of 89.9% and recall of 93%.27
Statistical analysis
Outcome and predictor variables
The primary outcome was the occurrence of an adverse event defined as either (1) lymph node metastasis in cases with known nodal status or (2) local cancer recurrence in cases undergoing endoscopic resection alone. To predict this outcome, we used information according to the following well-known risk factors based on guidelines1, 2: occurrence of lymphovascular invasion, tumour grade, invasion depth and depth classification and tumour buds. CD8+ T-cells were considered as an additional potential risk factor. Tumour grade was assessed as a numerical, a categorical (G1–G3), and a binary (i.e., G1–G2 vs. G3) variable; the invasion depth was measured from the submucosa and from the surface; the detected number of buds was considered in a manually selected hotspot, an automatically detected hotspot and mean value among the top 10 automatically detected hotspots. Due to their distribution, variables related to the number of buds were log10 transformed prior to the analysis, and null values were replaced by 0.1 prior to log10 transformation.
Prediction modelling
As detailed above, some risk factors were assessed using different methods/measuring techniques. In the current study, we decided to only consider the measurement with a stronger association with the outcome. To do so, we used a bootstrapping approach. For this, we built 500 bootstrap samples and in each sample, and for each risk factor, we regressed the outcome on the different measurements and selected the assessment with the best Akaike information criterion. Only the most commonly retained measurement was kept for the analysis (Table 1).
| Label | Level | Coef | OR |
|---|---|---|---|
| (Intercept) | −3.116 | ||
| Lymphovascular invasion | No | Ref. | Ref. |
| Yes | 0.239 | 1.27 | |
| Invasion depth, SM or Haggitt classification | Haggitt | Ref. | Ref. |
| SM | 0 | 1 | |
| log10 Nb of buds (manual hotspot) | 0.555 | 1.742 | |
| log10 invasion depth from the submucosa (mm) | 0.321 | 1.379 | |
| Tumour grade | 0.19 | 1.209 | |
| Invasion depth, SM or Haggitt classification x log10 invasion depth from the submucosa (mm) | Haggitt | −0.011 | 0.989 |
| SM | −0.011 | 0.989 |
- Note: Note that standard errors from penalised estimation methods such as LASSO are biased, generally leading to too small confidence intervals. As such, confidence intervals are not reported.
We used least absolute shrinkage and selection operator (LASSO) logistic regression analysis to predict adverse events as a function of the selected risk factors and the interaction between the invasion depth measurement and category. Alpha was set to one throughout. The optimum lambda penalty parameter was chosen using 10-fold cross-validation in a range between 0.01 and 0.37. To assess the performance of the obtained prediction model, we characterised the overall accuracy and the discriminatory power of the model by calculating the Brier score and the area under the receiver operating characteristic (AUROC) curve, respectively. Then, we evaluated the model calibration by using calibration curves.
The internal validity of the model was assessed using 1000 bootstrap samples, where samples were drawn with replacement from the derivation sample. The bootstrap-corrected performance estimates were calculated by subtracting the optimism from the performance of the original model (in-sample estimates are generally too high—too optimistic—so bootstrap samples are used to estimate how overly optimistic the model is and correct the statistics). The 95% confidence intervals for the boot-strapped performance measures were derived using the percentile method. Measurement selection (as described in the paragraph above) was included in the bootstrapping step.
In a second step, we used the prediction model to create a user-friendly point scoring system using the regression coefficient-based scoring method.28 The IBC score points were obtained by multiplying each beta-coefficient by 10 and rounding to the closest integer (what Mehta et al. 201629 refer to as Beta10/integer). Adding up the converted coefficients yielded the score of increasing risk of an adverse event.
Finally, we built a model predicting the risk of an adverse event as a function of the IBC score value. From this model, we estimated the predicted probabilities and associated 95% predicted interval and assessed the discriminatory power of the IBC score by calculating AUROC (ROC) curve (i.e., area under the curve [AUC]). The validity of the AUC value was assessed by performing internal validation as described above.
In the last step, we illustrated the distribution of the IBC score among all patients and split the patients into two (low and high-risk) categories. The reference point (cut-off) was chosen to maximise specificity while maintaining a minimum sensitivity of 0.9. To determine the accuracy of this method to predict an adverse event, we estimated sensitivity, specificity, positive and negative predictive values and likelihood ratios.
The R 4.2.1 software was used for statistical analysis.
The methods are based on the Standards for Reporting Diagnostic Accuracy Studies and Transparent Reporting of a multivariate Prediction model for Individual Prognosis Or Diagnosis30 guidelines.
RESULTS
Baseline characteristics
Baseline characteristics of patients included in the final dataset used for the prediction model with clinicopathological information and key variables for the predictive model are listed in Table 2. Of 462 patients who were treated with surgery, positive lymph nodes were found in 58 (13%) and 404 patients were nodal negative (87%). Of all patients treated with surgery, 371 (80%) had at least one of the following risk factors: tumour infiltration depth >1 mm/Haggitt level >2, presence of LVI and/or high tumour grade.
| Characteristic | Overall, N = 565 | No adverse event, N = 504 | Adverse event, N = 61 |
|---|---|---|---|
| Demographics | |||
| Sex–female, n (%) | 232 (41%) | 210 (42%) | 22 (37%) |
| Missing | 4 | 4 | 0 |
| Age at diagnosis | |||
| Median (interquartile range; IQR) | 69 (62, 76) | 69 (63, 76) | 67 (58, 75) |
| Missing | 4 | 4 | 0 |
| Surgery | |||
| Surgery, n (%) | 462 (81%) | 404 (80%) | 58 (94%) |
| Lymph nodes; LN (assessed on patients with surgery only) | |||
| Total number of LN | |||
| No. obs. | 462 | 404 | 58 |
| Median (IQR) | 16 (12, 23) | 16 (11, 23) | 16 (12, 21) |
| Missing | 37 | 34 | 3 |
| Tumour characteristics | |||
| Location, n (%) | |||
| Left | 257 (45%) | 228 (45%) | 28 (45%) |
| Rectum | 133 (23%) | 114 (23%) | 18 (31%) |
| Right | 128 (23%) | 117 (23%) | 11 (18%) |
| Transverse | 27 (5%) | 25 (5%) | 2 (3%) |
| Missing | 22 | 20 | 2 |
| Tumour type, n (%) | |||
| Adenocarcinoma | 533 (94%) | 479 (95%) | 54 (89%) |
| Mucinous adenocarcinoma | 28 (4.9%) | 23 (4.6%) | 5 (8.1%) |
| Other | 4 (0.8%) | 2 (0.4%) | 2 (3.2%) |
| Tumour grade, n (%) | |||
| 1 | 147 (26%) | 137 (27%) | 10 (16%) |
| 2 | 371 (66%) | 328 (65%) | 43 (71%) |
| 3 | 47 (8.3%) | 39 (7.7%) | 8 (13%) |
| Tumour precursor, n (%) | |||
| Conventional colorectal adenoma | 446 (79%) | 405 (80%) | 41 (67%) |
| Not observed | 67 (12%) | 57 (11%) | 10 (16%) |
| Sessile serrated lesion | 19 (3.4%) | 16 (3.2%) | 3 (4.8%) |
| Traditional serrated adenoma | 33 (5.8%) | 26 (5.1%) | 7 (11%) |
| Other pathological variables | |||
| Lymphovascular invasion, n (%) | 96 (17%) | 79 (16%) | 17 (28%) |
| Perineural invasion, n (%) | 10 (1.8%) | 5 (1.0%) | 5 (8.2%) |
| Invasion width (mm) | |||
| Median (IQR) | 7.1 (4.5, 9.8) | 7.0 (4.5, 9.7) | 7.9 (5.4, 10.2) |
| Missing | 1 | 1 | 0 |
| Invasion depth from the surface (mm) | |||
| Median (IQR) | 4.33 (3.08, 6.40) | 4.25 (3.05, 6.27) | 5.12 (3.20, 7.48) |
| Invasion depth from the submucosa (mm) | |||
| Median (IQR) | 2.83 (1.81, 4.36) | 2.79 (1.80, 4.18) | 3.85 (2.07, 5.58) |
| Invasion depth - Haggitt classification, n (%) | 154 (27%) | 136 (27%) | 18 (29%) |
| Haggitt 1 | 36 (6%) | 36 (7%) | 0 (0%) |
| Haggitt 2 | 83 (15%) | 71 (14%) | 12 (20%) |
| Haggitt ≥3 | 35 (6%) | 29 (6%) | 6 (9%) |
| Invasion depth—SM classification (Kikuchi level of submucosal invaion) | |||
| SM1 | 39 (7%) | 38 (8%) | 1 (1.6%) |
| SM2 | 112 (20%) | 104 (20%) | 8 (13.1%) |
| SM3 | 78 (14%) | 71 (14%) | 7 (11.5%) |
| Measurement not possible | 336 (59%) | ||
| No. of buds (manual hotspot) | |||
| Median (IQR) | 8 (2, 17) | 7 (2, 16) | 13 (7, 25) |
| No. of buds (automatic hotspot, top-1) | |||
| Median (IQR) | 16 (10, 27) | 15 (9, 26) | 21 (15, 34) |
| No. of buds (automatic hotspot, top-10) | |||
| Median (IQR) | 11 (7, 19) | 11 (7, 18) | 14 (9, 25) |
| No. of CD8 T-cells (manual hotspot) | |||
| Median (IQR) | 159 (81, 286) | 158 (80, 280) | 181 (88, 330) |
| Mismatch repair status | |||
| Proficient | 179 | 163 | 16 |
| Deficient | 16 | 16 | 0 |
| Missing | 373 | ||
Of the 103 patients treated by endoscopic resection alone, 3 patients had local cancer recurrence (3%).80 patients (76%) had at least one of the risk factors mentioned above. In 57 of the 80 patients with histologic risk factors, tumour infiltration depth was the sole risk factor.
Prediction model
Of the initially considered potential predictor variables (see ‘outcome and predictor variables’), four remain in the model after LASSO logistic regression analysis (Table 3): lymphovascular invasion, number of buds (manual hotspot), invasion depth and tumour grade (numerical). In our original model, which is shown in Table S2, CD8+ T-cell counts emerged as a risk factor. This result is surprising and may perhaps not reflect true biology due to the methodology (see discussion, paragraph 6). Considering this and the negligible effect of CD8+ T-cell counts on our model performance, we decided not to include this parameter in the final prediction model.
| Label | Level | Score |
|---|---|---|
| Lymphovascular invasion | No | |
| Yes | 2 | |
| No. of buds (manual hotspot), per log10-unit | 6 | |
| Invasion depth from the submucosa (mm), per log10-unit | 3 | |
| Tumour grade (1, 2, 3) | 2 |
Each included parameter was assigned a weight by multiplying each coefficient by 10 and rounding to the nearest integer, resulting in a scoring model (Table 3, see Figure 3 for detailed instructions on score calculation). The prediction model showed good accuracy and acceptable discrimination power, with a validated Brier score of 0.1 [0.08; 0.11] and a validated AUROC of 0.64 [0.57; 0.71] (Table 4).
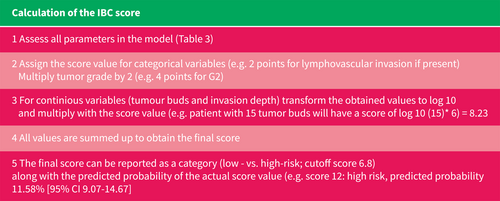
Calculation of the International Budding Consortium score.
| Raw | Corrected (internal validation)a | |
|---|---|---|
| AUC | 0.68 [95% CI 0.61–0.75] | 0.64 [95% CI 0.57–0.71] |
| Brier score | 0.09 | 0.1 [95% CI 0.08–0.12] |
- a Corrected by internal validation, 1000 bootstraps.
The distribution of the score among all patients is shown in Figure S4. The score, ranging from −6 to 24 points, increases with the probability of an adverse event (1.18%–36.2%; Table S3) and can be used to define high versus low-risk patients. As we wanted to maximise specificity and maintain a minimum of sensitivity of 0.9, we placed the cut-off value at 7 and obtained 141 low risk and 424 high-risk patients. With this cut-off we obtained score sensitivity of 0.9 [0.8, 0.95] and specificity of 0.27 [0.23, 0.31], positive and negative predicted values were estimated at 0.13 [0.1, 0.17] and 0.96 [0.91, 0.98], respectively (Table S5). Table S6 shows a comparison of low versus high-risk patients in terms of characteristics and clinical outcomes.
CD8+ T-cells, tumour budding and mismatch repair status
CD8+ T-cell infiltrates were examined in various tumour budding regions of the main tumour, namely in the manual and automated tumour budding hotspots and as an average among the 10 tumours budding hotspots of the main tumour. A weak positive correlation between increasing tumour budding and CD8 counts in the manual tumour budding hotspot was demonstrated (log transformed variables; R = 0.099; p = 0.018). Mismatch repair status was available for 195 cases, of which 16 (8.2%) demonstrated a loss of expression of at least one immunohistochemical marker (MLH1, MSH2, PMS2 and/or MSH6). Significantly higher CD8 counts were seen in MMR-deficient cases (p = 0.002).
DISCUSSION
This retrospective, international multicentric IBC study included 567 pT1 CRC patients and aimed to investigate established risk factors for lymph node metastases and cancer recurrence in pT1 CRC, including tumour budding and tumour inflammation. The resulting IBC score is a prediction model consisting of four histological parameters: lymphovascular infiltration, tumour grade, infiltration depth and tumour budding visualised by immunohistochemistry and assessed using AI. The score gives an estimate of the probability of an adverse event in pT1 CRC and also stratifies patients into a low- and high-risk groups.
In the last few years, several systematic reviews and meta-analyses have investigated the risk factors for pT1 CRC with partly different conclusions. Cracco et al. proposed in a review including eight studies (n = 1727 patients) the width and the area of submucosal invasion as reliable prognostic factors for cancer progression in pT1 CRC, but without agreement on ideal cut-offs.31 The systematic review and meta-analysis of Dykstra et al. included 60 studies (n = 10,000–13,128 patients) and confirmed established factors such as lymphovascular invasion, neural invasion, poor differentiation, and infiltration depth (1500 µm); additionally, poorly differentiated clusters and tumour budding were proposed.17 In the same year, Ebbehoj et al. published a further meta-analysis including 16 observational studies (n = 10,181 patients) confirming lymphovascular invasion, tumour budding, depth of submucosal invasion (at least 1000 mm), high tumour grade, polypoid growth pattern and rectal tumour location as potential risk factors for lymph node metastases in pT1 CRC.18 A further meta-analysis12 entered eight studies (n = 3621 patients) out of 67 included studies (n = 21,238 patients) into a multi-variable analysis showing a significant association between Lymp node metastases (LNM), poor differentiation, high-grade tumour budding and lymphovascular invasion. This contrasted with the deep submucosal invasion, which did not emerge as a significant independent predictor of LNM. Infiltration depth as a core element of the ICCR guidelines was considered a predictor of our model and contributed to predicting the outcome in our dataset. However, infiltration depth alone will virtually never classify a patient as ‘high-risk’ (see instructions on calculating the IBC score; Figure 3) Therefore, our results certainly support the recent conclusion that deep submucosal invasion should be reconsidered as a strong indicator of oncologic surgery.12
Despite the inclusion of risk factors in national and international guidelines, the question arises why a standardised predictive score system for the clinical management of pT1 CRC patients is still lacking. Therefore, considering the core risk factors proposed by the ICCR such as histological tumour type, lymphovascular invasion, tumour grade, perineural invasion, extent of invasion, invasive carcinoma dimensions and margin status, the following lines of thought need to be discussed. First, ‘histopathological’ factors such as tumour budding may be subject to high inter-observer variability32, 33; second, some factors may be assessed by measurements on the slides leading to suboptimal results; third, the description and listing of these factors itself may qualitatively help but does not optimally support the interdisciplinary team during clinic-pathological conferences or tumour boards, respectively. These points lead to the potential impact and limitations of the present study.
The proposed IBC score includes several strong points: (a) cost-effectiveness: Three out of four factors (lymphovascular invasion, invasion depth and tumour grade) can be assessed on H&E slides; (b) a potential inter-observer variability of the recently standardised factor ‘tumour budding’ can be avoided by the AI application which can be used for free; (c) the score mainly avoids arbitrary cut-offs normally based on single studies with small sample sizes and uses linear parameters; (d) the IBC score takes multiple histological risk factors into account to deliver a probability estimate of adverse events which can be taken as an additional tool for clinical management (e) the cohort is international and multicentric, reviewed by the IBC and the sample size seems to be quite competitive in comparison with other original studies.17 In contrast to a recently published risk stratification model,34 our cohort underwent rigorous review by a panel of gastrointestinal pathologists, which excluded 11% of initially identified cases due to reclassification as adenomas with epithelial misplacement (‘pseudoinvasion’), which is commonly mistaken for invasive carcinoma.35
Nevertheless, some limitations of the IBC score must be clarified: A two-tier risk category (low-/high) was established to facilitate clinical management with the cut-off at 90% sensitivity. This means that the low-risk category will still miss patients who have an adverse event. For this reason, it is important to report the estimated probability alongside the risk category for better identification of the extent of the risk (for instance, the model predicts around 7% probability of an adverse event for a patient with a score of 8%, and 39% respectively for a patient with a score of 24, yet both patients are in the high-risk category).
Other potential drawbacks are that the study is retrospective, hybrid by using partially conventional histology and AI and unexpectedly the impact of CD8+ cytotoxic T-cells was not a protective factor as expected in CRC. Indeed, the role of T-cell infiltrates in pT1 CRC is poorly characterised.36 Potential reasons may be the often observed focality of the tumour invasion in pT1 CRC and the specific TME in the tunica submucosa. Additionally, the approach of the ‘attacker-defender model’ used in the present study takes only lymphocytes in the tumour budding hotspot into account and not the lymphocyte number observed in the whole tissue section independently of the tumour budding hotspot. AI-based methods will continue to provide a promising approach for a more in-depth analysis of the role of CD8+ T-cells in submucosally invasive CRC. Also, in this cohort the measured depth of invasion (in mm from the submucosa) had no different effect in pedunculated or sessile polyps and was therefore used as the sole parameter for measuring the extent of tumour infiltration. External validation of the IBC score, which is required prior to widespread clinical use, may consider a separate analysis of pedunculated and sessile lesions. Also, MMR status was only available for a subset of cases and could therefore not be included in the model. As the role of MMR status in predicting adverse events may be better characterised in pT1 CRC in the future, further developments and refinements of the IBC score may also include this and other molecular parameters.
In conclusion, the IBC score is a solid, reproducible tool with potential clinical utility in daily practice and from the prospective point of view reflects an excellent basis for a future fully digitised IBC score.
ACKNOWLEDGEMENTS
We thank Femke Doubrava-Simmer (Radboud UMC, Nijmegen, the Netherlands) for her administrative and organisational support. We thank the Swiss Cancer Society (KFS-4108-02-2017) and the Dutch Cancer Society (KWF KUN2017-10602) for providing funding for this study.
CONFLICT OF INTEREST STATEMENT
None of the authors have potential conflicts of interest relevant to the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.