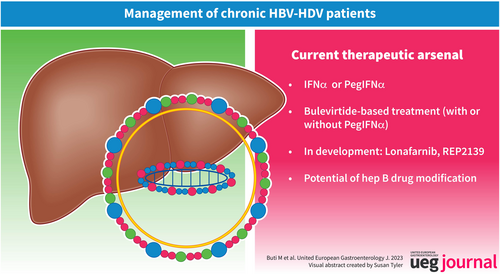
Management of chronic HBV-HDV patients chronic HBV-HDV infection: A review on new management options
Abstract
Hepatitis D virus was first described by Mario Rizzeto in 1977, and it is considered chronic viral hepatitis with the poorest prognosis. Despite its discovery almost 50 years ago, progress in its diagnosis and treatment has been scarce until recent years. The approval of bulevirtide has shed some light for patients with Chronic Hepatitis D, although important gaps regarding its use in therapy as well as about the epidemiology and diagnosis of the disease need to be addressed.
Graphical Abstract
EPIDEMIOLOGY OF HEPATITIS D VIRUS INFECTION
Hepatitis D virus (HDV) prevalence vary according to geographic regions, with the highest rates of infection found in Mongolia, the Republic of Moldova and countries in Western and Middle Africa.1-3 Hitherto, the real global burden of HDV infections remains elusive. Many factors contribute to this fact. In low-income countries, individuals with chronic HBV infection are frequently not tested for HDV infection, leading to a gap of information in some areas such as central Africa where HDV may affect as much as 26% of the HBsAg population.4
Three recent meta-analyses have estimated that the overall prevalence of HDV infection ranges from 0.16%–0.98% among the general population and from 4.5% to 14.57% among HBsAg carriers.3, 5, 6 Therefore, a total of 12-72 million individuals could be infected by HDV worldwide.3, 5, 6
NATURAL HISTORY OF CHRONIC HEPATITIS D INFECTION
Chronic Hepatitis D (CHD) represents the most severe form of chronic viral hepatitis. The risk of developing cirrhosis, hepatocellular carcinoma (HCC) and decompensated liver disease is 2 to 3 times greater in CHD than in HBV monoinfection.7
Several studies have confirmed the poor prognosis of subjects with CHD. A retrospective study in 337 patients with antibodies against the hepatitis D virus (anti-HDV) positivity, 233 with Hepatitis C Virus RNA detectable, revealed a 3.8-fold higher risk of developing liver-related events and 2.6-fold of HCC than those with undetectable HDV-RNA.8 Similar results have been reported in a multicenter Spanish study in 118 anti-HDV-positive individuals, with a higher rate of developing either cirrhosis and/or liver events among those with detectable HDV RNA (31% vs. 0%, p = 0.002; and 28% vs. 3%, p = 0.019, respectively).9
Because of the accelerated development of liver cirrhosis and decompensation, CHD accounts for an important number of HBV liver transplantations. For instances, in a study carried out in Italy, the ratio of HDV to the overall HBsAg transplants was 50.2% in 2010–2019, rate even higher than the observed in previous years at the same centers (38.5% in 2000–2009).10 Furthermore, age at liver transplantation is lower among CHD patients compared with HBV monoinfected patients.11
Although persistent HDV viremia is the most important risk factor for decompensation and HCC development, it has been suggested that the HDV genotype may also influence disease prognosis, with European HDV-1 and African HDV-5 genotypes associated with a higher risk of developing cirrhosis.12
Spontaneous HBsAg loss and seroconversion to anti-HBs have been described, with a rate ranging from 0.25% to 1% for year according to two Italian cohorts including 487 patients.13, 14 Importantly, in patients with cirrhosis even after HBsAg loss there is still a risk of developing HCC. This highlights the need to maintain HCC surveillance in patients with liver cirrhosis even after HBsAg is eliminated.
SCREENING
Screening for hepatitis D involves a combination of laboratory tests and medical history assessment. HBsAg determination is important since hepatitis D can only occur in individuals with HBV infection. The first test for screening HDV is the evaluation of anti-HDV, which can determine whether a person has been exposed to the virus or if they are currently infected. After, testing for serum/plasma HDV RNA is needed to confirm an ongoing HDV infection. Recent 2023 Clinical Practice Guidelines on Hepatitis Delta Virus by the European Association for the Study of the Liver recommends that screening for anti-HDV antibodies should be performed least once in all HBsAg-positive individuals and re-testing for these antibodies should be performed in HBsAg-positive individuals whenever clinically indicated (e.g., in case of aminotransferase flares, or acute decompensation of chronic liver disease) and may be performed yearly in those remaining at risk of infection.15
This recommendation differs from 2018 American Association for the Study of Liver Diseases guidelines that only recommend anti-HDV testing in those HBsAg-positive who are at risk (people who inject drugs, men who have sex with men, those at risk for sexually transmitted diseases and immigrants from high HDV endemic areas).16
Testing for HDV RNA still has some issues. Several in-house and commercial reverse transcription-polymerase chain reaction assays are available for the quantitative detection of HDV RNA, but still some harmonization of the different assays optimizing and standardizing HDV RNA detection protocols are needed. For those patients with undetectable HDV RNA, particularly if Alanin aminotransferase (ALT) levels are elevated it is recommended to retest HDV RNA due to the possibility of spontaneous fluctuation on the levels of viremia.15 Currently there are no other serum markers of HDV replication: Hepatitis D Antigen can only be detected for a short period in the serum of patients with acute HDV infection and IgM anti-HDV antibodies are usually detected in the acute phase of the infection and are associated with disease activity in chronic HDV infection but they are not a direct marker of HDV replication and are not suitable for its monitoring. The best way of increasing screening for HDV is performing reflex testing of anti-HDV antibodies in the new HBsAg positive cases.17 Even the screening and diagnosis of Hepatitis D can be optimized by doing double reflex testing that means first testing for anti-HDV antibodies and in positive cases for HDV RNA. This is the most effective method to generate estimates of the prevalence of anti-HDV and HDV RNA positivity and to identify undiagnosed individuals.18
In patients with CHD, it is also important to test regularly for HBV markers, particularly HBV DNA and HBsAg.
DIAGNOSIS
For the diagnosis of the presence and degree of liver damage, it is important to determine liver function and fibrosis assessment by non-invasive markers, but specific cut-off values are not well established like in Hepatitis B and C. Liver imaging tests, such as ultrasound, computed tomography scan, or magnetic resonance imaging, can be useful for the diagnosis of liver cirrhosis. Liver biopsy is recommended in certain situations such as when clinical signs or indirect evidence of cirrhosis is absent and if it may contribute to the patient's management.15
TREATMENT OF CHRONIC DELTA HEPATITIS
The aim of CHD treatment is to prevent the development of complications of liver disease, such as cirrhosis, decompensation, and HCC, and ultimately death. The ideal endpoint of treatment efficacy is to achieve both HDV RNA undetectability and HBsAg clearance or seroconversion, which would prevent the risk of HDV reactivation. However, this aim was rarely achieved with any HDV treatment.19, 20 Therefore, surrogate endpoints of treatment efficacy have been developed.21, 22 “Virological response” is defined by > 2 log decline of HDV RNA including HDV RNA undetectability at any time on treatment or off treatment. “Combined response” is defined by > 2 log decline in HDV RNA combined with normal ALT levels”. This was based on the long-term clinical results of 9 patients treated with high dose IFN -alfa23 in the 1990s using an in-house HDV RNA assay of weak sensitivity. Additional confirmatory studies are needed to assess the validity of this “combined response” using HDV RNA assays with excellent sensitivity, specificity, and reproducibility. This “combined response” is currently used to evaluate new treatments.
INTERFERON ALFA OR PEGYLATED INTERFERON ALFA TREATMENT
For 30 years, CHD treatment was based on the off-label use of Interferon alfa (IFNα) or (peg)-IFNα for their antiviral and immunomodulatory effects.16, 24, 25 Treatment was usually given for 48 weeks, sometimes during several years: Viral response defined by HDV_RNA undetectable was obtained in 20%–30% of patients 6 months post treatment, with more than 50% late relapse.26 IFNα tolerance is poor, with numerous adverse effects. The use of (peg) IFNα is even more complex in patients with cirrhosis, which explains the frequency of treatment discontinuations or dose reductions. Anti-HBV nucleos(t)ide analogues have no effect on HDV replication, as demonstrated in two prospective studies (HIDIT-1 and 2).27 However, anti-HBV nucleos(t)ide analogue (NUCs) in CHD must be discussed in case of HBV replication persisting or liver cirrhosis. The limited results of (peg)IFNα in CHD patients demonstrate the urgent need for new treatments. Currently, new drugs are under development They target various steps of the HBV and HDV life cycle and one of them, bulevirtide (BLV), was approved since July 2020 in Europe.
BULEVIRTIDE-BASED TREATMENT
The first in class of entry inhibitor myrcludexB, also named BLV, inhibits high affinity binding of HBV and HDV to the entry receptor sodium taurocholate co-transporting polypeptide.
In the proof-of-concept phase 1b/IIa study,28 BLV with and without PegIFNα, has showed favorable efficacy and safety profile, that was confirmed in two phase 2 studies: Myr202 that evaluated 3 doses of BLV (2, 5 and 10 mg) for 24 weeks) y and Myr203 that evaluated the combination of PegIFNα and 3 doses of BLV 2, 5 or 10 mg, monotherapy with BLV 2 mg or 10 mg and PegIFNα, all administered for 48 weeks.29, 30 The results of these studies have led the European Medicines Agency on 31 July 2020 to grant BLV conditional marketing authorization at a dose of 2 mg daily, alone or in combination with a nucleos(t)ide analogue for the treatment of compensated CHD. Since this authorization, several phase 2 (Myr204) and 3 (Myr301) studies have been undertaken to evaluate the efficacy of long-term BLV treatment for 96 or 144 weeks (Myr301) and of combined BLV plus PegIFNα treatment for 48 weeks followed by 48 weeks of BLV (Myr204). In addition, data from real-life studies on BLV treatment as monotherapy or in combination with PegIFNα have been reported.
Bulevirtide monotherapy
The main results of clinical trials with BLV monotherapy are summarized in Figure 1. In the phase 2 Myr202 study, a dose-dependent decline of HDV RNA and ALT levels was observed in all BLV arms. However, HDV RNA relapse occurred in 60%–83% of viral responders' patients during follow-up (FU).29 Two treatment arms in the Myr203 study received BLV monotherapy 2 mg or 10 mg for 48 weeks. At the end of treatment, rates of HDV RNA undetectability were 13% versus 47% in the 2 versus 10 mg arm, respectively. After FU, relapse was frequent and only 7% and 33% of the patients remained with HDV RNA undetectability indicating the need for prolonged treatment duration.30
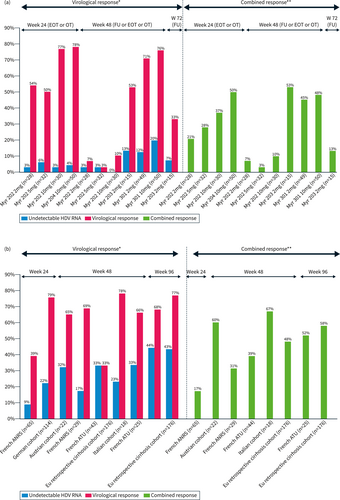
(a) Virological and combined response rates of bulevirtide (BLV) monotherapy in clinical trials. (b) Virological and combined response rates of BLV monotherapy in real-life.
(a) Virological and combined response rates of bulevirtide (BLV) monotherapy in clinical trials. (b) Virological and combined response rates of BLV monotherapy in real-life.
The phase 3 Myr301 study investigated BLV monotherapy.31 The interim analysis at week 96 showed similar virological response rates 76% and 82% with HDV RNA undetectability rates of 20% and 36% for the 2 and 10 mg, respectively.32 Rates of response were similar across all patient subgroups including cirrhosis in all treatment arms. A dose-dependent elevation in serum bile acids was observed in both BLV arms. Headache, pruritus, and injection site reactions were reported in 19%, 14% and 23% of cases, respectively, mostly mild to moderate in severity and at a higher frequency in BLV 10 mg dose.31 To date study results did not support an efficacy advantage between 10 mg and 2 mg dosage.
The results of real studies with BLV monotherapy are summarized in Figure 1b.33-37 These studies confirm the results of the clinical trials, with an increasing in virological, biochemical and combined response with treatment prolongation up to 96 weeks.33, 38 In the French ATU cohort, patients with cirrhosis and HDV RNA >6.5 log IU/mL at baseline appear more difficult to treat.39 Analysis of virological response on treatment suggests that patients with a <1 log reduction in HDV RNA levels at month 6 of treatment have no chance of a virological response at 72 weeks of treatment, suggesting a futility rule to be confirmed in ongoing clinical trials (Myr301).40 In addition, increasing the dose of BLV to 10 mg in 15 non virological responders after at least 3 months of treatment did not result in virological benefit at year 1 of therapy.41 Another potential option suggested by an Austrian study should be to add PegIFNα in patients with no virological response at 24 weeks of monotherapy with BLV based on 8 patients, who showed a decline in HDV RNA levels after adding PegIFNα. These strategies should be evaluated in further studies.35 Data are scarce regarding treatment discontinuation after long-term BLV monotherapy. In the French ATU cohort, stopping treatment after 72 weeks of BLV monotherapy results in (3/5) 60% early relapse, indicating the need for more prolonged treatment33 and in the Austrian cohort, virological relapse was seen in 4 patients (3 within the first 24 weeks and one after almost 1 year of FU) out of 7 who discontinued therapy including 2 treated with BLV plus PegIFNα, indicating the need for more prolonged treatment.42 An Italian CHD patient with compensated cirrhosis treated with BLV for 3 years with more than 2 years of HDV RNA undetectability remained HDV RNA undetectable after 48 weeks of FU despite the persistence of HBsAg. Liver biopsy showed significant improvement of fibrosis and HBcAg and HDAg stained negative.43 The safety and efficacy of stopping BLV need to be further studied in larger cohorts.
Off label BLV monotherapy for CHD in 15 patients with decompensated cirrhosis has been recently reported.44 14 patients Child-Pugh B and one Child-Pugh A were treated. Liver function and ascites improved in four patients under BLV treatment. The virologic response was achieved in 66% and biochemical response in 47% of the cases. Controlled trials are urgently needed for confirmation of safety and efficacy in this population.
Bulevirtide in combination with PegIFNα
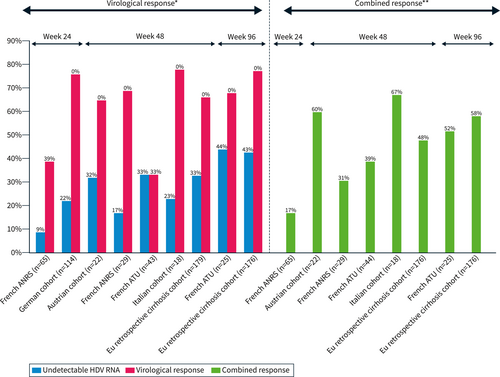
The main results of clinical trial studies with PegIFNα plus BLV combination are summarized in Figure 2a. In the phase II (MYR 203), the best response was observed with the combination of PegIFNα + 2 mg BLV with a 4.81log10 decline in HDV RNA at the end of treatment and 4.04log10 decline at week 24 r FU. Hepatitis D virus RNA was undetectable in 60% (9/15) and 53% (8/15) of the patients at the end of treatment and week 24 FU respectively. ALT normalization was observed in 27% (4/15) and 47% (7/15) of patients at the end of treatment and week 24 FU respectively. HBsAg loss or > 1log decline from baseline at week 24 FU was observed, only in patients treated with BLV combined with PegIFNα, in 27% (4/15) or 40%, 7% (1/15) or 13%, 0% or 13% of patients for BLV 2 mg, 5 and 10 mg, respectively.30
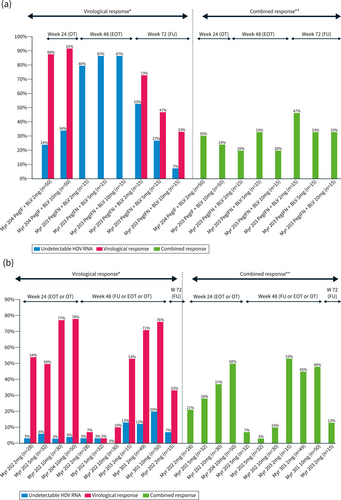
(a) Virological and combined response rates of bulevirtide (BLV) + PegIFN in clinical trials. (b) Virological and combined response rates of BLV + PegIFN in real-life.
In the Phase 2 Myr204 study, the interim analysis at week 24 reported a median −2.68 log IU/mL HDV RNA decline in BLV monotherapy lower than the decline observed with combination therapy −3.78log IU/mL for BLV 2 mg + PegIFNα and −4.11 log IU/mL for BLV10 mg + PegIFNα, while ALT normalization was higher with BLV monotherapy. These results suggest that the BLV plus PegIFNα combination appears to be more virologically potent than BLV monotherapy. Virological efficacy and combined response rates were higher in all BLV ± PegIFNα arms compared to PegIFNα arm.45
Real-life studies confirm the results of clinical trials, with faster virological efficacy with the combination than with BLV monotherapy.33 The results are shown in Figure 2b. In the Agency National Research SIDA and Virakl hepatitis (ANRS) cohort, in multivariate analysis, having been treated with PegIFNα for 12 weeks was associated with virological response (OR 8.4; 95% CI 3.4–20.8, p < 0.001).37 As expected, side effects were more frequent during combined treatment, as were PegIFNα discontinuations (10% in the ATU cohort and 14% in the ANRS cohort).
OTHER THERAPEUTIC OPTIONS UNDER DEVELOPMENT
Lonafarnib
Lonafarnib (LNF) is an oral farnesyl transferase inhibitor, which blocks assembly and secretion of virions in the cells through the delta antigen prenylation LFN is given booster with tonavir (RTV) to increase LNF levels in the liver and reduce gastrointestinal side effect.46 In the phase 2 dose ranging study, the best results were obtained using either the oral combination of LNF 50 mg twice a day plus ritonavir in patients with low viral load or with the triple combination of LNF 50 mg twice daily plus ritonavir plus weekly PegIFNα in a patient with high viral load.47 In the phase 3 randomized control study, 407 patients were randomized in four groups: placebo (n = 52), LNF 50 mg x2/d + RTV 100 mg x2/d (n = 178), LNF 50 mg x2/d plus RTV 100 mg x2/d plus PegIFNα-2a weekly (n = 125) and PegIFNα (n = 52) for 48 weeks. The primary end point “combined response” rates at week 48 were achieve in 1.9%, 10.1%, 19.2% and 9.6% of the patients in the placebo, LNF + RTV, LNF + RTV + PegIFNα and PegIFNα arms, respectively. Virological response rates at week 48 were achieved in 3.8%, 14.6%, 32% and 36.5% of the patients in placebo, LNF + RTV, LNF + RTV + PegIFNα and PegIFNα arms respectively. 36.6% of patients had LNF dose modification during treatment. The combined responses at week 72 were available among 313 patients and it was achieved in 0%, 15.6%, 29.3% and 15.4% of patients in the placebo, LNF + RTV, LNF + RTV + PegIFNα and PegIFNα arms respectively. The histological response with improvement >2 points in necro-inflammatory activity without worsening fibrosis was observed in 53% of the LFN/RTV/PegIFNα group (p = 0.014).48 Both LNF arms achieved both composite primary endpoint and key secondary virological endpoints versus placebo. Triple therapy with PegIFNα seems to give the best results in terms of combined response. Prolonged FU is necessary to ensure the persistence of response, which would make it possible to envisage a treatment of finite duration.
IFN lambda
IFN λ acting on a single site highly expressed on hepatocytes should be better tolerated than PegIFNα. In a phase II study, 33 patients with HDV received 48 weeks 120 μg or 180 μg per week of PegIFNλ. 180 μg per week produced the best response, with a reduction of 2log HDV RNA by 50% at the end of treatment and 36% 6 months after the end of treatment. 36% of patients had undetectable HDV RNA at the end of treatment and 6 months after discontinuation. The combined response virologic and ALT normalization was observed in 14% and 29% of patients at the end of treatment and 6 months after discontinuation respectively. Tolerance appeared to be better than with PegIFNα but, grade 3 bilirubin was elevated in 30% of patients.49
In addition, an open-label phase II study investigated the efficacy and safety of the combination of LFN 50 mg twice a day and ritonavir with IFNλ 180 μg/week for 24 weeks in 26 patients with chronic delta hepatitis. Per protocol analysis, a reduction of >2 log HDV RNA was observed in 77% (17/22), undetectable, HDV RNA in 50% of patients at the end of treatment and in 23% (5/22) at week 24 of FU. Hepatis improvement in inflammatory activity was observed in 55% (11/20) of patients with paired biopsies. However, 5 patients had to discontinue treatment due to hyperbilirubinemia4 and the appearance of ascites.1, 50 A phase 3 study will be crucial to understand the hyperbilirubinemia issue observed in phase 2 studies.
Nucleic acid polymers:
Nucleic acid polymers act by targeting the Host HSP40 chaperone DNA JB12; they block the inhibition of HBV Sub Viral Particle assembly and blocks the envelopment of HDV ribonucleoprotein. Moreover, with continued dosing, they target S-HDAg and L-HDAg and inhibit the replication and morphogenesis of HDV upstream of RNA envelopment. In a phase II study, 12 patients with compensated liver disease received 15 weeks of REP 2139-Ca as an intravenous infusion once weekly, followed byREP 2139 and PegIFN and then PegIFN alone for 33 weeks. At the end of treatment, 6/12 patients were HBsAg negative with HBs seroconversion.51 After 3.5 years of FU, HBV functional cure was maintained in 4/11 patients, HDV RNA was undetectable in 7/11 and normal ALT in 6/11 patients had a normalization.52 Asymptomatic and transient ALT/AST elevations during REP 2139 monotherapy or bitherapy have been reported in 8/12 patients, correlated with the decrease in HBsAg titer, with no sign of liver failure.52 A subcutaneous formulation of REP 2139 has been developed and used under an ATU in France in BLV failure patients. 18 NUCS suppress patients received 250 mg REP 2139 and 90 μg PegIFN alfa-2a both weekly (only in case of compensated cirrhosis) for 48 weeks. Among the 6 patients who have more than 24 weeks of treatment, 5 have a virological response of which 3 were HDV RNA undetectable. Two patients have HBs seroconversion and one patient has an HBsAg titer <10 IU/mL.53 Three patients with decompensated cirrhosis were treated with tenofovir disoproxil fumarate plus REP2139 with good tolerability and response, with undetectable HDV RNA in 2 patients (w6 and w20), HBs seroconversion in one patient and reversal of ascites in 2 patients.54 Additional phase 2 and 3 studies are urgently needed with REP 2139.
IN SUMMARY
Hepatitis D still needs better available screening and diagnostic tests, particularly for low income countries that allow diagnosis of CHD in early stages. The therapy of CHD is evolving with the introduction of BLV for patients with compensated liver disease. However, many questions remain unanswered, such as the optimal duration of BLV treatment and the potential role of combining BLV with PegIFNα. The ongoing Phase 2 and 3 studies should provide some answers. Besides, other drugs in development such as LNF and REP2139 or even drugs in development for hepatitis B could modify the hepatitis D therapeutic arsenal in the coming years.
CONFLICT OF INTEREST STATEMENT
MB Advisory Board of Gilead. No other COIB of Gilead.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.