Comparison of various strategies to define the optimal target population for liver fibrosis screening: A population-based cohort study
Abstract
Background & Aims
Liver fibrosis screening is recommended in high-risk populations, but the optimal definition of “high risk” remains to be established. We compared the performance of several risk-stratification strategies in a population-based setting.
Methods
Data were obtained from the Finnish population-based health examination surveys Health 2000 and FINRISK 2002–2012. The Chronic Liver Disease Risk Score (CLivD) was compared to previously published risk-stratification strategies based on elevated liver enzymes, alcohol use, diabetes, fatty liver index, body mass index, and/or metabolic risk factors for their ability to detect either advanced liver fibrosis or incident severe liver events. Advanced fibrosis was defined as an Enhanced Liver Fibrosis (ELFTM) score >9.8 in the Health 2000 study (n = 6084), and incident liver events were ascertained from registry linkage in the combined FINRISK 2002–2012 and Health 2000 cohort (n = 26,957).
Results
Depending on the cohort, 53%–60% of the population was considered at risk using the CLivD strategy (low-intermediate-high risk, excluding the minimal-risk category), compared to 30%–32% according to the other risk-stratification strategies. The CLivD captured 85%–91% of cases in the population with advanced liver fibrosis and 90% of incident severe liver events within 10 years from baseline. This compares to 33%–44% and 56%–67% captured by the other risk-stratification strategies, respectively. The 10-year cumulative incidence of liver events varied by risk-stratification strategy (1.0%–1.4%).
Conclusions
Compared to previously reported traditional risk factor-based strategies, use of the CLivD captured substantially more cases with advanced liver disease in the population and may be superior for targeting further fibrosis screening.
Graphical Abstract
Key summary
Summarise the established knowledge on this subject
-
Liver fibrosis screening is recommended in high-risk persons, but the most optimal strategy to define high risk in the population is unknown.
-
The Chronic Liver Disease Risk Score (CLivD) risk prediction score was recently introduced to simultaneously account for a combination of several liver-related risk factors, which could be used for targeting further liver fibrosis screening.
What are the significant and/or new findings of this study?
-
In a general population sample, we found that the CLivD score captured ∼9 out of 10 cases with estimated advanced liver fibrosis and of those who developed clinical liver-related outcomes during follow-up.
-
Traditional risk factors (harmful alcohol use, diabetes, obesity etc.) considered in isolation captured only 3–7 out of 10 such cases, which means that many patients with advanced liver fibrosis would not be subject to liver fibrosis screening under such strategies.
INTRODUCTION
To reduce liver-related morbidity and mortality, subclinical advanced liver fibrosis needs to be diagnosed early through simple and effective pathways.1-3 Guidelines and expert opinion recommend liver fibrosis screening using widely available non-invasive fibrosis tests (NITs), such as Fibrosis-4 (FIB-4) or aspartate aminotransferase to platelet ratio index (APRI), in subjects with risk factors.2, 3 In this two-step strategy, high-risk individuals are targeted for fibrosis screening. In contrast, the performance of NITs is considerably poorer in unselected general populations (no risk factors) due to low disease prevalence (i.e., low pretest probability)4-6 related to the spectrum effect,7 and unselected liver fibrosis screening is currently not recommended.2, 3
With a paucity of evidence, clear guidance is lacking on how to define high-risk individuals, in whom fibrosis screening is warranted, in practice. Although previous studies have shown that liver fibrosis screening in subjects in the community with elevated liver function tests, harmful alcohol use, or type 2 diabetes can improve case-finding for cirrhosis and reduce unnecessary referrals to secondary care,8, 9 the number of subjects with advanced liver fibrosis that are missed by this strategy due to the way the target screening population is defined has not been evaluated. While many studies focus on non-alcoholic fatty liver disease (NAFLD), these studies rely on a preceding diagnosis of NAFLD, which excludes many active alcohol drinkers, and may not represent a large proportion of individuals with advanced liver disease in the general population.
The rationale behind a two-step screening strategy advocated by guidelines is to comprehensively capture an at-risk population with high enough pretest probability to minimize the spectrum effect related to NITs, increasing the gain from NITs. Therefore, the first step, defining the at-risk population, should have high sensitivity (low false negative rate) with reasonably high pretest probability. The second step (fibrosis screening by NITs) should ideally have high specificity (low false positive rate). Low specificity of the first step is less of a concern when the subsequent NIT comes without harm.
The recently developed and validated Chronic Liver Disease Risk Score (CLivD) simultaneously considers multiple individual risk factors.10 Based on age, sex, alcohol use, waist-hip ratio, diabetes, smoking, and, if available, gamma-glutamyltransferase (GGT), the CLivD provides an estimate for the 15-year risk of incident severe liver disease. Compared to traditional risk factors (e.g., heavy alcohol use or diabetes) considered in isolation, use of the CLivD has the potential to capture more high-risk individuals and improve the definition of pretest probability to identify who should be offered screening by NITs.
The Enhanced Liver Fibrosis (ELFTM) test is a direct marker of fibrosis that has been shown to outperform indirect NITs in detecting advanced liver fibrosis in both NAFLD and alcohol-related liver disease (ArLD).11-13 As a blood-based marker, the ELF test does not require special equipment, such as transient elastography, and is potentially well-suited for screening high-risk individuals as well as for population studies.
In this study, we evaluated the first step of the two-step screening strategy, the sensitivities and pretest probabilities of various approaches used previously to define the at-risk population. Specifically, in a large and representative sample from the general population, we compared the performance of traditional risk factors and the CLivD in detecting individuals with advanced fibrosis as assessed by the ELF test. Furthermore, utilizing linked electronic healthcare registry data, we also compared the performance of the various risk-stratification strategies for discriminating incident severe liver disease.
MATERIAL AND METHODS
Cross-sectional sample for advanced liver fibrosis
For the purpose of estimating the number of individuals with advanced liver fibrosis, we extracted data from the Health 2000 Survey, conducted in 2000–2001 and coordinated by the Finnish Institute for Health and Welfare (THL). The survey originally comprised 8028 adults aged ≥30 years.14 The participation rate in the full examination was 80%. The cohort is considered to be representative of the entire Finnish population through a regional, two-stage, stratified, cluster sampling procedure. Data were collected from each participant at baseline via interviews, questionnaires, and health examination using standardized procedures as described previously.14 Blood samples were collected at baseline for a wide spectrum of laboratory measurements and handled using a standardized protocol.
Of the original sample of 8028 adults, 6084 subjects (76%) had blood samples available for ELF analyses. For the ELF test, tissue inhibitor of matrix metalloproteinase-1 (TIMP-1), hyaluronic acid, and amino-terminal peptide of pro-collagen III (PIIINP) were analyzed in accordance with the manufacturer's instructions (Siemens Healthineers) using the ADVIA Centaur XPT analyzer (Siemens Healthineers) at the Biomarkers Team, Finnish Institute for Health and Welfare (THL, Helsinki, Finland). Serum samples kept in −70 ℃ were used. Advanced liver fibrosis was defined as an ELF test >9.8, in line with previous studies and manufacturer recommendations.15
Longitudinal cohort for incident severe liver disease
For the purpose of analyzing incident severe liver disease through registry linkage, we extracted data from the national FINRISK Studies from 2002, 2007, and 2012, and the Health 2000 survey.14, 16 FINRISK studies are cross-sectional health-examination surveys conducted in a systematic and standardized fashion by THL in Finland every 5 years since 1972. The surveys provide data on adults (age 25–74 years) from four to six regions in Finland. The samples were randomly drawn from the Finnish National Population Register and stratified by region, sex, and 10-year age groups. The methods, measurements, and protocols used in the FINRISK studies are similar to those of the Health 2000 survey and were previously described elsewhere.16
Follow-up data were obtained from several national registers through linkage using the unique personal identity code assigned to all Finnish residents. Data for hospitalizations were obtained from the Care Register for Health Care (HILMO), which covers all hospitalizations in Finland since 1969. One or several International Classification of Diseases (ICD) diagnoses are assigned to each hospitalization at discharge; these diagnosis codes are systematically recorded in the HILMO register. Data on malignancies were obtained from the Finnish Cancer Registry, which has held nationwide cancer records since 1953. Vital status and cause of death data were obtained from Statistics Finland. Data collection in these registries is mandatory and virtually 100% complete with generally consistent quality.17, 18
Study endpoints were fatal and non-fatal incident severe liver disease (requiring hospital admission or causing liver cancer or liver-related death) as defined by the ICD codes shown in Table S1, in line with a recent consensus paper.19
Of 27,236 individuals, we excluded those with baseline liver disease (ICD-10: K70–K77, C22.0; ICD-8/9: 570–573, 155.0; n = 228) or chronic viral hepatitis (ICD-10: B18; n = 51). Thus, the final sample comprised 26,957 individuals.
All participants provided signed informed consent, and the studies were approved by the Coordinating Ethical Committee of the Helsinki and Uusimaa Hospital District. Previously, the studies were also approved by the institutional review board of the National Public Health Institute in Helsinki, Finland. The FINRISK and Health 2000 sample collections were transferred to THL Biobank in 2015 after approval from the Coordinating Ethical Committee of the Helsinki and Uusimaa Hospital District.
Risk-stratification strategies
We compared several strategies to define the population at risk for liver disease. First, we used the recently published CLivD based on age, sex, alcohol use, waist-hip ratio, diabetes, smoking status, and GGT.10 The CLivD equation is shown in the Supplementary Appendix. This score provides the 15-year risk of incident severe liver disease, and we defined the at-risk population as those with a CLivD above the “minimal risk” based on the cutoffs reported previously10: −0.258 in the model that includes GGT (CLivDlab) and −0.412 in the model that excludes GGT (CLivDnon-lab). Second, we considered alternative risk-stratification applying the criteria used in previous population-based or community-based studies identified through literature search. Specifically, we selected real-world studies where liver fibrosis screening had been targeted to individuals in the community with risk factors, excluding studies from secondary care and those restricted to selected patients with a pre-existing NAFLD or ArLD diagnosis or other specific patient groups. We identified the following four studies: El-Gohary et al.8 Harman et al.9 Chalmers et al.20 and Caballeria et al.21 These different criteria, referred to here by the first author of the publication, were based on the presence of elevated liver enzymes, alcohol use, diabetes, fatty liver index, body mass index, and/or metabolic factors. The exact definitions used in the various studies are detailed in Table S2. El-Gohary et al.8 and Harman et al.9 used virtually the same criteria, which are referred to here as the El-Gohary/Harman criteria.
Statistical analysis
Considering that the main purpose of defining the population at risk for future clinical liver disease is to capture as many of the cases with advanced liver fibrosis as possible while minimizing the proportion of the general population that needs to undergo further liver fibrosis screening, we used a bubble plot to plot the proportion of advanced liver fibrosis cases captured (sensitivity) against the size of the population considered at risk, and thus in need of further liver fibrosis screening, in the cross-sectional Health 2000 sample according to the various risk definition strategies. The size of the bubbles was then defined by the net benefit of the specific risk-stratification strategy. Net benefit provides an estimate of clinical value, namely the tradeoff between benefit (number of advanced liver fibrosis cases captured; true positives) and harm (number of individuals subject to unnecessary liver fibrosis screening; false positives). Net benefit is calculated as (true positives/N) − (false positives/N) × (p1/[1 − p1]), where N is the total sample size and p1 is the threshold probability.22 In this case, threshold probability refers to the minimum probability of a subject having advanced liver fibrosis for which fibrosis screening would be considered warranted. As it is universally agreed that patients with NAFLD should undergo liver fibrosis screening, and approximately 6% of NAFLD patients have advanced liver fibrosis according to large population studies,23, 24 we set the threshold probability to 6% in our net-benefit calculations.
In sensitivity analyses, we defined advanced fibrosis as ELF >10.51 (instead of >9.8), and we assessed the characteristics of the risk stratification strategies in subgroups of individuals with either diabetes or alcohol risk use (>168 g/week for men or >112 g/week for women).8, 9
In the longitudinal cohort, we considered incident cases of severe liver disease occurring within 10 years after study baseline as the outcome and calculated 5- and 10-year cumulative incidence estimates for the various risk-stratification strategies using the nonparametric cumulative incidence function considering death without liver disease as a competing-risk event. Finally, in the Health 2000 sample, we estimated the cumulative incidence of severe liver disease after stratifying the population into three groups: (a) risk-stratification criteria not fulfilled, (b) risk-stratification criteria fulfilled and ELF <9.8, and (c) risk-stratification criteria fulfilled and ELF ≥9.8. Data were analyzed with R software version 3.6.1.
RESULTS
The Health 2000 sample with ELF data available comprised 6084 individuals, and the combined FINRISK and Health 2000 cohort with linked outcome data comprised 26,957 individuals (Figure 1). These samples had similar demographics at baseline (Table 1).
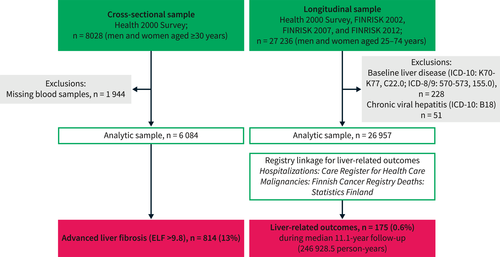
Flow chart with inclusion and exclusion criteria for the cross-sectional and longitudinal study samples.
| Cross-sectional sample | Longitudinal sample | |
|---|---|---|
| Individuals | 6084 | 26,957 |
| Age (years) | 52.7 ± 14.9 | 50.8 ± 14.3 |
| Sex, male/female | 2773 (46)/3311 (54) | 12,474 (46)/14,483 (54) |
| Body mass index (kg/m2) | 26.9 ± 4.7 | 27.0 ± 4.8 |
| Diabetes | 606 (10) | 2396 (9) |
| Metabolic syndrome | 2726 (45) | 9422 (35) |
| Hypertensiona | 3149 (52) | 13,948 (52) |
| Statin treatment | 445 (7) | 3469 (13) |
| Smoking | ||
| Current | 1600 (26) | 6229 (23) |
| Former | 1324 (22) | 6218 (23) |
| Never | 3132 (52) | 14,349 (54) |
| Alcohol use (g/week) | 74.7 ± 144.0 | 78.25 ± 138.5 |
| Gamma-glutamyltransferase (U/L) | 36.4 ± 47.6 | 34.8 ± 49.7 |
| Alanine aminotransferase (U/L) | 25.0 ± 18.1 | 26.6 ± 19.0 |
| Aspartate aminotransferase (U/L) | 28.2 ± 12.9 | 28.2 ± 17.8 |
| Total cholesterol (mmol/L) | 5.9 ± 1.1 | 5.5 ± 1.1 |
| LDL cholesterol (mmol/L) | 3.7 ± 1.1 | 3.4 ± 1.0 |
| HDL cholesterol (mmol/L) | 1.3 ± 0.4 | 1.4 ± 0.4 |
- Note: Values are given as mean ± SD or n (%).
- Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein.
- a Systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or antihypertensive medication at baseline.
Cross-sectional analysis
In the Health 2000 sample, the proportion of individuals fulfilling the various risk-stratification criteria were as follows: CLivDlab 53.2%, CLivDnon-lab 59.2%, El-Gohary/Harman criteria 29.8%, Chalmers criteria 31.7%, and Caballeria criteria 31.1% (Figure 2). In the Health 2000 sample, 814 (13.4%) individuals had an ELF test >9.8 and were considered to have advanced liver fibrosis. Of all cases with an ELF test >9.8, the CLivDlab approach captured 85.4% and CLivDnon-lab captured 90.9%; the corresponding capture rates were 33.4% for the El-Gohary/Harman criteria, 44.3% for the Chalmers criteria, and 44.0% for the Caballeria criteria (Figure 2). The net benefit of the various strategies was highest for CLivDnon-lab (9.2%), followed by CLivDlab (8.2%), Chalmers criteria (4.4%), Caballeria criteria (4.3%), and El-Gohary/Harman criteria (3.0%).
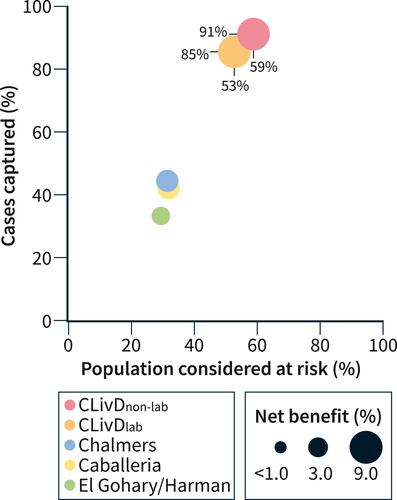
Bubble plot showing the proportion of cases with advanced liver disease that were captured (y-axis), proportion of the population considered at risk (x-axis), and the net benefit (bubble size) of the various risk-stratification strategies.
In sensitivity analysis using the ELF cutoff >10.51, 278 (4.6%) individuals were considered to have advanced liver fibrosis. With this higher ELF-cutoff, the capture rates improved for the CLivD score (90.4% for CLivDlab and 94.0% for CLivDnon-lab) but remained unchanged for the other strategies (35.2% for the El-Gohary/Harman criteria, 44.9% for the Chalmers criteria, and 42.4% for the Caballeria criteria).
In the subgroup of 606 individuals with diabetes, 184 (30.4%) had an ELF test >9.8. In the subgroup of 916 individuals with alcohol risk use, 71 (7.8%) had an ELF test >9.8. The El-Gohary/Harman criteria considered all diabetes patients and alcohol risk users at risk. The CLivD score considered 90%–95% of diabetes patients and 92%–97% of alcohol risk users at risk, while Chalmers and Caballeria criteria considered 35%–60% at risk (Table S3). Capture rates for ELF >9.8 were 100% for the El-Gohary/Harman criteria (all individuals considered at risk), 97%–100% for CLivD, and 53%–76% for Chalmers and Caballeria criteria (Table S3).
Longitudinal analysis
In the combined FINRISK and Health 2000 cohort, the median follow-up until first liver event, death, or end of follow-up was 11.1 years (IQR 6.2–12.9, range 0.02–13.3, mean 9.2, person-years of follow-up 246,928.5). During follow-up, there were 131 incident severe liver events within 10 years and 175 during the entire follow-up. The proportions of individuals fulfilling the various risk-stratification criteria in this combined cohort were comparable to those in the Health 2000 sample alone (Table 2).
| CLivDlab | CLivDnon-lab | El-Gohary/Harman8, 9 | Chalmers20 | Caballeria21 | |
|---|---|---|---|---|---|
| Incident severe liver disease events within 10 years from baseline | |||||
| True negative | 12,479 | 10,644 | 18,781 | 18,466 | 18,283 |
| False negative | 13 | 13 | 45 | 43 | 58 |
| False positive | 13,685 | 15,739 | 8045 | 8360 | 8543 |
| True positive | 116 | 118 | 86 | 88 | 73 |
| Proportion of incident liver events captured | 89.9% | 90.1% | 65.6% | 67.2% | 55.7% |
| Proportion of population considered at risk | 52.5% | 59.8% | 30.2% | 31.3% | 32.0% |
| Cumulative incidence of severe liver disease (95% CI) | |||||
| Criteria not fulfilled | |||||
| At 5 years | 0.03% (0.00–0.07) | 0.05% (0.00–0.10) | 0.13% (0.08–0.19) | 0.10% (0.05–0.15) | 0.16% (0.10–0.23) |
| At 10 years | 0.13% (0.06–0.21) | 0.15% (0.07–0.23) | 0.30% (0.21–0.39) | 0.31% (0.21–0.30) | 0.41% (0.30–0.52) |
| Criteria fulfilled | |||||
| At 5 years | 0.50% (0.37–0.63) | 0.66% (0.50–0.82) | 0.59% (0.42–0.77) | 0.64% (0.46–0.82) | 0.50% (0.34–0.66) |
| At 10 years | 1.12% (0.92–1.33) | 1.00% (0.81–1.18) | 1.41% (1.11–1.72) | 1.34% (1.06–1.63) | 1.10% (0.85–1.37) |
- Abbreviation: CLivD, Chronic Liver Disease Risk Score.
The CLivDlab captured 89.9% and CLivDnon-lab 90.1% of incident severe liver events within 10 years, compared to 65.6%, 67.2%, and 55.7% by the El-Gohary/Harman, Chalmers, and Caballeria criteria, respectively (Table 2). The cumulative incidence of severe liver disease among those who fulfilled the risk-stratification criteria ranged from 0.50% to 0.66% at 5 years, and from 1.00% to 1.41% at 10 years when considering death without liver disease as a competing risk event (Table 2). Figure 3 shows the empirical cumulative distribution of the CLivD, that is, the proportion of the entire population that will be considered at risk and the proportion of incident liver events within 10 years that will be captured depending on what CLivD cutoff is chosen.
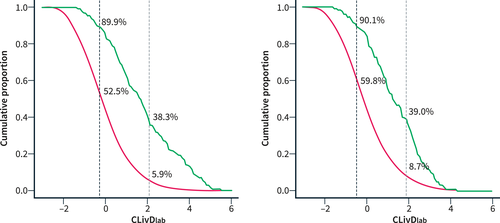
The empirical cumulative distribution of the proportion of the population (FINRISK 2002–2012 and Health 2000) with a Chronic Liver Disease Risk Score (CLivD) greater than or equal to each score. The blue curve represents the entire population, and the red curve represents those with an incident liver event within 10 years. If the CLivD with laboratory measurements (CLivDlab) cutoff is set at the previously defined level to exclude minimal risk (−0.258; black dotted line), 52.5% of the entire population will be considered at risk and 89.9% of incident liver events will be captured. If the cutoff is instead set at the level to exclude minimal and low risk (2.066; gray dotted line), 5.9% of the entire population will be considered at risk and 38.3% of incident liver events will be captured. The panel on the right shows the same distributions for CLivDnon-lab using its corresponding cutoffs.
In the Health 2000 sample with available ELF data, there were 67 incident cases of severe liver disease during a median follow-up of 13.1 years (IQR 12.9–13.2, range 0.04–13.3, mean 12.0, person-years of follow-up 72,387.5). Cumulative incidence estimates of severe liver disease at 10 years ranged between 0.25% and 0.66% by risk-stratification strategy when risk criteria were not fulfilled, between 0.44% and 1.11% with risk criteria fulfilled but ELF <9.8, and between 2.6% and 5.3% with risk criteria and ELF ≥9.8 both fulfilled (Table 3).
| Liver events/individuals | CLivDlab | CLivDnon-lab | El-Gohary/Harman8, 9 | Chalmers20 | Caballeria21 |
|---|---|---|---|---|---|
| Risk criteria − | 7/2719 | 6/2441 | 15/4244 | 18/4130 | 27/4105 |
| Risk criteria +, ELF <9.8 | 21/2461 | 22/2815 | 17/1530 | 13/1558 | 7/1584 |
| Risk criteria +, ELF ≥9.8 | 18/615 | 18/706 | 14/266 | 15/352 | 12/351 |
| 10-year cumulative incidence (95% CI) | |||||
| Risk criteria − | 0.26% (0.07–0.45) | 0.25% (0.05–0.44) | 0.35% (0.17–0.53) | 0.44% (0.23–0.64) | 0.66% (0.41–0.91) |
| Risk criteria +, ELF <9.8 | 0.85% (0.49–1.22) | 0.78% (0.46–1.11) | 1.11% (0.59–1.64) | 0.83% (0.38–1.29) | 0.44% (0.12–0.77) |
| Risk criteria +, ELF ≥9.8 | 2.60% (1.34–3.86) | 2.55% (1.39–3.71) | 5.26% (2.58–7.95) | 4.26% (2.15–6.37) | 3.42% (1.52–5.32) |
- Abbreviations: CLivD, Chronic Liver Disease Risk Score; ELF, enhanced liver fibrosis.
DISCUSSION
A clear definition of the appropriate target population is a critical prerequisite for any disease screening program. We found that defining the at-risk population using traditional risk factors considered in isolation as reported previously8, 9, 20, 21 fails to capture 56%–67% of individuals with advanced liver fibrosis as defined by the ELF test, and 33%–44% of the individuals that will go on to develop severe clinical liver disease within the next 10 years. These individuals would not be subject to liver fibrosis screening by NITs in these scenarios. The CLivD provided a better trigger for liver fibrosis screening in the sense that it reduced the proportion of missed cases of advanced liver fibrosis to 9%–15% and of incident cases to 10%. Using the higher ELF cutoff of 10.51, only 6%–10% of cases of advanced liver fibrosis were missed by the CLivD score. Furthermore, the CLivD provided superior net benefit in defining the at-risk population. Although use of the CLivD increased the proportion considered at risk to 53%–60%, compared to 30%–32% when using the other risk-stratification strategies, this can be considered acceptable because the NITs, such as FIB-4, that are commonly recommended as the next step are simple, cheap, widely available, and without harm. To the best of our knowledge, this study is the first population-based study to compare various strategies to define the at-risk population for liver fibrosis screening.
The CLivD is an externally validated tool based on simple and widely available variables that works without a single blood test, and as such is well suited for large-scale first-line risk stratification of the general population.10 For example, the non-lab version of the CLivD score can be used as part of liver-oriented public health campaigns to raise awareness of the risk for liver disease, and as part of nurse-led health check-ups. GGT improves the performance of the CLivD, but the CLivD can be calculated without GGT by anyone online or using color-coded scoring sheets provided in the original publication.10 Of all standard liver function tests, GGT is recognized as the best predictor of future clinical liver disease.5, 10, 25 The CLivD includes the waist-hip ratio, which can be self-measured using a simple tape measure or by mobile applications using digital photography technology.26-28 In contrast to the other risk-stratification strategies, which are dichotomous in nature, the CLivD is a continuous variable with a linear association with incident liver disease risk. Therefore, one may set the cutoff anywhere along the CLivD depending on the desired pretest probability.
The strengths of our study include the representative general population samples with data on both a direct marker of fibrosis, the ELF test, and linked outcome data from national registries. ELF is considered one of the best blood-based markers of liver fibrosis with AUCs of 0.78–0.97 for discriminating advanced liver fibrosis in NAFLD and ArLD samples.11, 12 The discrimination performance of ELF is reportedly close to that of transient elastography.11, 13, 29 Liver biopsies are practically impossible in large-scale population studies. Due to the lack of platelet data, we were unable to calculate indirect markers of fibrosis, such as FIB-4 and APRI. Although FIB-4 is often recommended as the first-line liver fibrosis screening test, it has poorer discrimination performance than ELF.13 In the NICE guidelines,30 after considering both accuracy and cost, the ELF test is recommended as the first-line test to screen for advanced liver fibrosis in NAFLD. Regarding clinical outcomes, we included all types of liver-related outcomes considered clinically relevant and representative of severe liver disease (hospitalization, death, liver cancer). The national registries are considered a source of high-quality data with high coverage.17, 18, 31
The limitations of the present study include the fairly low number of liver-related events, precluding analyses on specific ICD codes. As the study was based on the Finnish population, the findings need to be validated in other countries.
The EASL-Lancet commission recognizes that simple care pathways focused on the detection of progressive liver fibrosis are needed to pick up more patients in time to intervene.1 We aimed to determine the best strategy to define the at-risk population in which further liver fibrosis screening should be targeted, that is, the first step of a two-stage screening process. Due to the spectrum effect,7 the performance of NITs is generally impaired at low pretest probability, such as in the unselected general population.2, 5, 6 As a marker of pretest probability, 5- and 10-year cumulative incidence rates of severe liver disease were similar in the various risk-stratification strategies in the present study. Therefore, it would be expected that the performance of various NITs will be similar regardless of which of the tested risk-stratification strategies are being used as the trigger for fibrosis screening, but this remains to be demonstrated in larger studies with data available to calculate FIB-4 and other liver fibrosis tests and compare their performance to liver histology. Although the CLivD increased the proportion of the population considered at risk compared to the other risk-stratification strategies, this did not seem to markedly compromise the subsequent cumulative incidence estimates. In addition, in the Health 2000 cohort, 10-year cumulative incidences for severe liver disease were 2.6%–5.3% among individuals simultaneously fulfilling the various risk-stratification criteria and ELF ≥9.8. Therefore, though our study suggests that the CLivD is a valid and straightforward tool for risk stratification of the general population, further decision-analysis studies and a health economic evaluation are needed to confirm the best screening approach for liver fibrosis. Specifically, the present study only assessed the definition of the at-risk population, whereas future decision analyses should address the whole screening process, including the choice of the best fibrosis test in various scenarios under different pretest probabilities.
In conclusion, the CLivD shows high sensitivity and reasonable pretest probability in first-line risk-stratification of the general population regarding chronic liver disease and could be used as part of community care pathways for selecting individuals to undergo liver fibrosis screening.
AUTHOR CONTRIBUTIONS
Fredrik Åberg: Study concept; Study design and enrollment of participants and data collection; Data analysis; Data interpretation; First draft; Critical revision for important intellectual content. Antti Jula: Study design and enrollment of participants and data collection; Data interpretation; Critical revision for important intellectual content. Martti Färkkilä: Study design and enrollment of participants and data collection; Data interpretation; Critical revision for important intellectual content. Veikko Salomaa: Study design and enrollment of participants and data collection; Data interpretation; Critical revision for important intellectual content. Iris Erlund: Study design and enrollment of participants and data collection; Data interpretation; Critical revision for important intellectual content. Satu Männistö: Study design and enrollment of participants and data collection; Data interpretation.; Critical revision for important intellectual content Terhi Vihervaara: Study design and enrollment of participants and data collection; Data interpretation; Critical revision for important intellectual content. Markus Perola: Study design and enrollment of participants and data collection; Data interpretation; Critical revision for important intellectual content. Annamari Lundqvist: Study design and enrollment of participants and data collection; Data interpretation; Critical revision for important intellectual content. Ville Männistö: Study concept; Study design and enrollment of participants and data collection; Data analysis; Data interpretation; Critical revision for important intellectual content.
ACKNOWLEDGMENTS
The samples/data used for the research were obtained from THL Biobank (study numbers: BB2016_98, BB2017_101 and BB2019_31). We thank all study participants for their generous participation at THL Biobank and the National FINRISK Study (years 2002, 2007, 2012) and the Health 2000 and 2011 Surveys. We thank Siemens Healthineers for supplying reagents and consumables needed to conduct ELF testing. Dr. Åberg was supported by the Mary and Georg Ehrnrooth Foundation, Medicinska Understödsföreningen Liv och Hälsa, Finska Läkaresällskapet, Academy of Finland (#338544), and Sigrid Jusélius Foundation. Dr. Salomaa was supported by the Finnish Foundation for Cardiovascular Research and by the Juho Vainio Foundation. Dr. Männistö was supported by the The Finnish Medical Foundation, and Mary and Georg Ehrnrooth Foundation. The researchers are all independent of the funders. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest regarding the content of this manuscript.
ETHICS APPROVAL
The studies were approved by the Coordinating Ethical Committee of the Helsinki and Uusimaa Hospital District. Previously, the studies were also approved by the institutional review board of the National Public Health Institute in Helsinki, Finland.
PATIENTS CONSENT STATEMENT
All subjects provided informed consent for the study and for registry linkage.
Open Research
DATA AVAILABILITY STATEMENT
FINRISK and Health 2000 data are available from the THL biobank based on a research application, as explained on the website of the THL biobank (https://thl.fi/en/web/thl-biobank/for-researchers).