Widespread azendohsaurids (Archosauromorpha, Allokotosauria) from the Late Triassic of western USA and India
Abstract
Archosauromorph reptiles underwent rapid lineage diversification, increases in morphological and body size disparity, and expansion into new adaptive landscapes. Several of the primary early archosauromorph clades (e.g. rhynchosaurs) are easy to differentiate from others because of their characteristic body types, whereas the more lizard-like and carnivorous forms with long necks (e.g. tanystropheids) were historically all relegated to the groups Protorosauria or Prolacertiformes. However, it is now clear that these groups are polyphyletic and that a lizard-like, carnivorous form is plesiomorphic for Archosauromorpha, and multiple subclades started with that body plan. Among these early forms is Malerisaurus from the Upper Triassic of India (M. robinsonae) and the Upper Triassic of south-western USA (M. langstoni). In this paper, we critically re-evaluate the genus. We find both species of Malerisaurus as valid, and identify Malerisaurus as an early diverging, but late-surviving, carnivorous member of Azendohsauridae within Allokotosauria. Our histological analysis and assessment of ontogenetic changes of limb bones of small and large individuals demonstrate that the skeletons of the small forms grew slowly and became more robust through ontogeny, and that the larger recovered bones are at or near the maximum size of the taxon. Malerisaurus and Malerisaurus-like taxa were common members of the Otischalkian–Adamanian (late Carnian to mid-Norian) faunal assemblages from Upper Triassic strata of the south western USA, but they are absent from the younger Revueltian holochronozone. Specimens from western North America show that Allokotosauria had a near-Pangaean distribution for much of the Middle Triassic to Late Triassic.
The initial radiation of archosauromorphs (the group that contains birds, crocodylians, and all species closer to them than to lizards, snakes and tuataras) preceded the end of the Permian Period, but clear differentiation and proliferation of early archosauromorph subclades occurred in the Triassic Period (Benton 1985; Ezcurra et al. 2014; Ezcurra 2016; Foth et al. 2016; Ezcurra & Butler 2018). During the Triassic radiation, archosauromorphs rapidly diversified into various shapes and ecological niches including stocky-bodied herbivores (e.g. rhynchosaurs), possibly arboreal herbivores (e.g. trilophosaurids), dinosaur-convergent herbivores (e.g. azendohsaurids), some of the first large (>2 m total body length) reptile predators (e.g. proterosuchids, erythrosuchids, ‘rauisuchians’), and various other long-necked lizard-like forms (tanystropheids, prolacertids). Whereas inclusion into these clades using body and skull morphologies and their inferred ecology is generally clear for later members of these groups in the Middle Triassic and Late Triassic, earlier diverging and less specialized members of these groups are much more difficult to recognize and place along the archosaur stem or as early members of archosauromorph groups.
Over recent decades, detailed analyses of the relationships of archosauromorphs and archosauromorph subgroups (Dilkes 1998; Flynn et al. 2010; Ezcurra et al. 2014; Nesbitt et al. 2015; Pritchard et al. 2015; Ezcurra 2016) have helped clarify relationships between and within archosauromorph subclades. Most importantly, previously hypothesized groups such as ‘protorosaurs’ and/or ‘prolacertiforms’ have been instead recognized as non-monophyletic groups of taxa that were grouped together based on unexpected plesiomorphic traits (e.g. moderately long necks with elongated cervical vertebrae, which are plesiomorphic for many archosauromorph lineages). Taxa originally relegated to ‘protorosaurs’ and/or ‘prolacertiforms’ are now phylogenetically recovered at the base of Archosauromorpha (e.g. Protorosaurus speneri) or near the divergence with archosauriforms (e.g. Prolacerta broomi), and some represent early, less specialized members of archosauromorph subclades (e.g. Macrocnemus is an early diverging tanystropheid). Recent examples of these reassignments include Boreopricea funerea, now found to be closely related to allokotosaurians (Pritchard & Sues 2019, but see Ezcurra 2016) and Jesairosaurus lehmani, recently recovered as the sister taxon to Tanystropheidae (Ezcurra 2016). However, many forms have yet to receive the same analytical treatment because of poor preservation and lack of new specimens, among other reasons.
Malerisaurus robinsonae (Fig. 1) from the Upper Triassic of India and its proposed close relative M. langstoni (Fig. 2) from the opposite side of Pangaea (i.e. the Upper Triassic of western USA) are two challenging taxa that have not had a satisfying phylogenetic position established. First described by Chatterjee (1980), M. robinsonae was named from at least two individuals. Chatterjee (1986) later established M. langstoni (citing similarities with M. robinsonae) from associated remains from a multitaxic bonebed (Otis Chalk Quarry 2; TMM 31099) from the Upper Triassic Dockum Group of Texas. Beyond the initial description, few have included M. langstoni in discussions of broader taxonomic diversity studies, and in the latest revision of the taxon, the holotype of M. langstoni was interpreted to be a chimaera consisting of elements from trilophosaurids, rhynchosaurs, phytosaurs, and aetosaurs (Spielmann et al. 2006). Consequently, those authors concluded that the M. langstoni holotype should be restricted to the skull fragments and that these skull fragments should be considered a junior synonym of the trilophosaurid Trilophosaurus buettneri.
To date, Malerisaurus remains rarely have been recognized outside of the initial discoveries. There are no formally referred specimens of M. robinsonae from the (lower) Maleri Formation of India, and no other remains have been formally assigned to M. langstoni from western USA. For M. langstoni, assigning partial associated skeletons or isolated remains has been challenging because: (1) it is not clear if the holotype of M. langstoni represents a single individual, a collection of skeletal parts of the same species-level taxon, or a chimaera of archosauromorph clades; (2) the holotype is incomplete (e.g. fragmentary and broken skull, missing most trunk vertebrae, missing the distal elements of the hindlimb) and the preservation and state of the material is poor, making anatomical details difficult to interpret; (3) parts of the skeleton, such as the hindlimb, retain plesiomorphic character states and proportions of other early archosauromorphs; and (4) uncertainties of the phylogenetic placement of the taxon among the problematic ‘protorosaurs’ or ‘prolacertiforms’ made comparisons to other taxa difficult, and untangling plesiomorphies from apomorphies is incredibly difficult within the current evolutionary frameworks. Nevertheless, there have been Malerisaurus-like bones recorded informally in collections (TMM, TTU, UCMP) across western USA as well as enigmatic larger archosauromorphs with Malerisaurus-like features found in the same deposits (e.g. Otischalkia elderae, referred to rhynchosaurs; Elder 1978; Hunt & Lucas 1991a).
Here, we critically analyse Malerisaurus with a focus on the holotype of M. langstoni and other material from the same area (Otis Chalk localities) and discuss its taxonomic status. A reanalysis of the holotype was only possible because: (1) there is a general phylogenetic consensus of early archosauromorph relationships, including the vacating of the traditional, pre-cladistic ‘protorosaur’ or ‘prolacertiform’ wastebaskets (Rieppel et al. 2003; Pritchard et al. 2015; Ezcurra 2016; Spiekman 2018; Miedema et al. 2020; Spiekman et al. 2021); (2) the discovery of well-preserved comparative material (e.g. Azendohsaurus madagaskarensis, Shringasaurus indicus, new specimens of Teraterpeton hrynewichorum; Flynn et al. 2010; Nesbitt et al. 2015; Sengupta et al. 2017; Pritchard & Sues 2019) has been combined with phylogenetic information to confirm plesiomorphic or apomorphic character state determinations; (3) additional material has been recognized in collections; and (4) new material has been discovered at new localities. With these insights, we identify abundant Malerisaurus-like remains from both the Chinle Formation and the Dockum Group of western USA.
Institutional abbreviations
FMNH, Field Museum of Natural History, Chicago, Illinois, USA; GR, Ruth Hall Museum of Paleontology, Ghost Ranch, New Mexico, USA; ISIR, Indian Statistical Institute, Reptiles, Kolkata, India; MACN-Pv, Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’, Colección Nacional de Paleovertebrados, Buenos Aires, Argentina; MCZ, Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts, USA; MNA, Museum of Northern Arizona, Flagstaff, Arizona, USA; NMMNH, New Mexico Museum of Natural History and Science, Albuquerque, New Mexico, USA; PEFO, Petrified Forest National Park, Arizona, USA; PPHM, Panhandle-Plains Historical Museum, Canyon, Texas, USA; TMM, Vertebrate Paleontology Collections, The University of Texas at Austin, Texas, USA; TTU/MOTT, Texas Tech University Museum, Lubbock, Texas, USA; UA, Université d’Antannanarivo, Antannanarivo, Madagascar; UCMP, University of California Museum of Paleontology, Berkeley, California, USA; UMMP, University of Michigan Museum of Paleontology, Ann Arbor, Michigan, USA.
Material and method
Histological methods
Three humeri were chosen for sectioning to document the growth of small (TMM 31099-84 and TMM 31099-1488) and large (PEFO 38627) Malerisaurus-like forms from the Upper Triassic of western USA. Matrix was removed from all specimens with a carbide steel pin vice, digitally photographed, and a cast made prior to sectioning. The sections were taken as close to the midshaft as possible, and the removed midshaft was embedded in Castolite AP under vacuum and then cut into thin sections with an IsoMet 4000 saw equipped with a diamond wafering blade. Each section was ground with a 1200-grit grinding disc, then fixed onto a 0.3-mm-thick plastic slide with Aron Alpha (Type 201) cyanoacrylate. The slides were then ground by hand through a sequence of finer grits until final polishing. The medullary cavity of PEFO 38627 was filled with mudstone that expanded when wet, therefore Aron Alpha (Type 201) cyanoacrylate was used to replace the mudstone and fill the cavity during grinding. The slides were then imaged using a Nikon Eclipse LV100ND transmitted and reflected light microscope, a Nikon DS-Fi2 camera and a Digital sight DS-U3 interface, together with a Prior ProScan III automated microscope stage, and digitally assembled using Nikon NIS-Element Basic Research version 4.40.00 (Build 1084). These full-slide images were captured in plane-polarized and cross-polarized light with a gypsum plate (530 nm). High-resolution images of each section are available in Nesbitt et al. (2021a).
All of the larger bones from Otis Chalk Quarry 1 (TMM 31025) that overlap with the holotype of M. langstoni are morphologically consistent, with the exception of character state changes we ascribe to ontogeny. Furthermore, the wide and largely posterodorsally curved scapula of the holotype of M. langstoni is similar to the more complete and greatly expanded scapulae from Otis Chalk Quarry 1 (Fig. 3). The non-overlapping bones from Otis Chalk Quarry 1 and the holotype of M. langstoni include azendohsaurid character states that help solidify these specimens as azendohsaurids.

Phylogenetic analysis
To assess the phylogenetic position of M. langstoni, M. robinsonae and other Malerisaurus-like taxa, we used the dataset of Pritchard & Sues (2019) that extensively covers early archosauromorph diversity, including the densest sampling of members of Azendohsauridae and their closest relatives (Trilophosauridae). The Pritchard & Sues (2019) dataset is the latest version of a dataset started by Pritchard et al. (2015) and subsequently modified by Nesbitt et al. (2015), Pritchard et al. (2016), Pritchard & Nesbitt (2017), and Pritchard et al. (2018). The Pritchard & Sues (2019) dataset includes 337 characters and 68 terminal taxa with Petrolacosaurus kansensis selected as the outgroup. Here, two new characters informative among allokotosaur archosauromorphs were added to the dataset (Nesbitt et al. 2021a). The following characters were ordered: 1, 4, 7, 19, 20, 25, 28, 30, 40, 58, 60, 88, 97, 100, 113, 123, 152, 166, 183, 191, 196, 233, 276, 305, 316; 25 characters in total. We excluded Spinosuchus caseanus and Trilophosaurus jacobsi, and instead used Spinosuchus caseanus ‘combined’ (combining Sp. caseanus and Tr. jacobsi as a terminal taxon; see Nesbitt et al. 2015). Colobops noviportensis was also removed following the revision of the taxon by Scheyer et al. (2020). The matrix was analysed with equally weighted parsimony using TNT v. 1.5 (Goloboff & Catalano 2016). We used a heuristic search of 1000 replications of Wagner trees (with random addition sequence) followed by tree-bisection and reconnection (TBR) branch swapping (holding 10 trees per replicate). These resultant trees were used as the basis of a second round of TBR branch swapping. Zero-length branches were collapsed if they lacked support under any of the most parsimonious reconstructions.
Our scoring strategy for M. langstoni, M. robinsonae and other Malerisaurus-like taxa started by considering them as separate terminal taxa, and then we combined some terminal taxa. Originally, we scored the following terminal taxa as separate: ‘Malerisaurus langstoni’, which was the holotype of M. langstoni (TMM 31099-11), including all of the skeletal elements that were originally included by Chatterjee (1986); we scored the holotype (ISIR 150) and paratype (ISIR 151) of M. robinsonae together with the skeletal elements presented by Chatterjee (1980) here as ‘Malerisaurus robinsonae’; we scored the holotype (TMM 31025-263) and referred humerus (TMM 31025-262) of Otischalkia elderae with other similarly sized Malerisaurus-like remains from Otis Chalk Quarry 1 (TMM 31025) as ‘Malerisaurus-like form large’; and a Malerisaurus-like taxon from a monodominant bonebed (PFV 217) within Petrified Forest National Park represented by numerous individuals of various ontogenetic stages as ‘Malerisaurus-like taxon PEFO’. We subsequently combined all of the Dockum Group and Chinle Formation terminal taxa into a ‘Malerisaurus all NA’ (where ‘NA’ refers to North America) terminal taxon given that much of the anatomy of the taxon is represented across many specimens; there were only a few character conflicts caused by the combination, and these were treated as unknown (?) in the analyses. Thus, this phylogenetic analysis also allows us to test the hypothesis that O. elderae (‘Malerisaurus-like form large’) represents a larger Malerisaurus-like taxon, possibly even M. langstoni.
Measurement data
Measurements were taken using digital callipers and rounded to the nearest 0.1 mm (Tables S1, S2). Linear regressions with confidence intervals were conducted in RStudio(RStudio Team 2021).
Results
What is Malerisaurus?
Because of the complex taxonomic history of both species of Malerisaurus mentioned above, we briefly outline their history of study and the specimens assigned to the hypodigm of each. We also provide a brief overview of their anatomical features and character states that affect our scoring of these taxa in the phylogenetic analysis.
Reinterpretation of Malerisaurus robinsonae
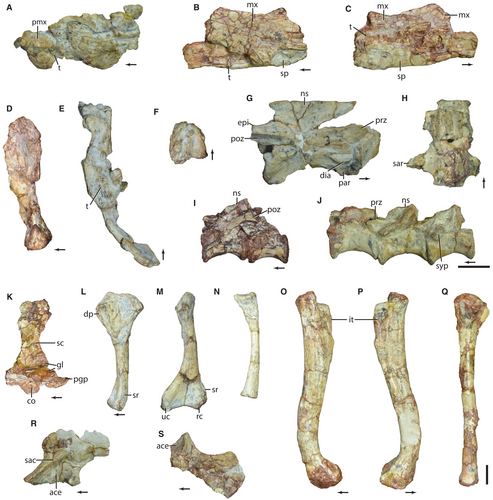
Malerisaurus robinsonae was named and first described by Chatterjee (1980) based on two partial skeletons (ISIR 150 and ISIR 151) found inside each of the rib cages of two complete and articulated skeletons of the phytosaur Parasuchus hislopi (Chatterjee 1978, 1980). These fossils come from the red mudstones of the early Upper Triassic (late Carnian to ?earliest Norian; Bandyopadhyay & Ray 2020) lower Maleri Formation, Pranhita–Godavari Basin, India (see Discussion). Chatterjee (1980) identified M. robinsonae as an ‘eosuchian’ reptile, within the Linnean suborder Prolacertiformes and the family Protorosauridae, based on the assumption that the lower temporal arcade was complete and the quadrate was not mobile. The genus was named after the Maleri Formation from which the taxon was collected, and the species-level epithet honours the pioneering work of Dr Pamela Lamplugh Robinson (Chatterjee 1980). Of the two skeletons, ISIR 150 was designated as the holotype specimen because its skull bones were better preserved, whereas ISIR 151 was made the paratype specimen (Chatterjee 1980).
The skull material of the hypodigm is fragmentary but does bear important anatomical information (Fig. 1A–F). The premaxilla lacks evidence of a prenarial process and has five tooth positions in both the holotype (ISIR 150) and the paratype (ISIR 151) specimens, contrasting with the six teeth reported by Chatterjee (1980). Premaxillary, maxillary, and dentary teeth are labiolingually compressed, but they lack evidence of denticles (which may have been present but removed by weathering), and all are ankylosed to the bone. There is at least one distally recurved mesial maxillary crown, and the preserved middle dentary crowns have convex mesial and distal margins. The ventral condyles of the quadrate are subequally developed ventrally and separated by a shallow groove, contrasting with the asymmetric ventral end of Azendohsaurus madagaskarensis and Shringasaurus indicus (Flynn et al. 2010; Sengupta et al. 2017). The posterior margin of the quadrate is continuously concave in lateral view. The supposed articulated right palatine and pterygoid were misinterpreted by Chatterjee (1980). This element corresponds only to the left pterygoid, whereas the putative palatine is actually the quadrate ramus of the pterygoid and lacks any teeth. The transverse ramus of the pterygoid is not preserved and the anterior ramus is dorsoventrally high, as in A. madagaskarensis (Flynn et al. 2010). The ventral surface of the palatal ramus lacks teeth at its base, but some are preserved on the most anteriorly preserved end of the bone. It is not possible to determine the pattern of arrangement of the pterygoid teeth, which are relatively large, and some preserve coarsely serrated margins.
The exoccipital and opisthotic are not co-ossified. The paraoccipital process is posterolaterally oriented, relatively short, and anteroposteriorly thickened. The exoccipital possesses two foramina for the passage of the 12th cranial nerve (CN XII). The ventral ends of the exoccipital contact each other on the midline. The occipital neck is moderately long and the occipital condyle subspherical. The basal tubera of the basioccipital are lateroventrally oriented and separated from each other at their bases. The parabasisphenoid has a deep, triangular depression on its ventral surface, but the posterior margin of this fossa is not well-defined, contrasting with A. madagaskarensis (Flynn et al. 2010). The foramina for the passage of the internal carotids cannot be recognized and these arteries probably did not enter into the basicranium, as in S. indicus and A. madagaskarensis (Flynn et al. 2010; Sengupta et al. 2017). The morphology of the braincase bones indicates that the parabasisphenoid was mostly oriented horizontally, but is slightly more ‘verticalized’ (the basipterygoid processes positioned ventral to the level of the basal tubera) in lateral view than in M. langstoni.
The cervical centra are parallelogram-shaped in lateral view and possess a low ventral keel (Fig. 1G). Epipophyses are present in at least the probable fourth to seventh cervical vertebrae. The neural spines are low, with a well-developed anterior overhang and a slightly transversely expanded dorsal edge. There is no transpostzygapophyseal lamina, nor evidence for postaxial cervical intercentra. The trunk vertebrae (Fig. 1I, J) lack a ventral keel on the centrum and laminae on the neural arch, with the exception of a paradiapophyseal lamina in the probable first trunk vertebra. Epipophyses are present in that vertebra, but they are absent more posteriorly in the dorsal series. The trunk vertebrae possess a single synapophysis, with the exception of the most anterior dorsal elements. The sacrum is composed of two sacral vertebrae that are fused to their respective ribs (Fig. 1H). The lateral ends of the sacral ribs are currently lost (illustrated by Chatterjee 1980). The anterior and middle caudal vertebrae have a longitudinal groove on the ventral surface of their centra. The middle caudal vertebrae are elongate, with a centrum 2.5-fold longer than dorsoventrally tall.
The scapular blade exhibits an anteroposteriorly expanded distal end (Fig. 1K). The subglenoid lip of the coracoid is developed as a shelf-like structure, as occurs in A. madagaskarensis (Nesbitt et al. 2015) and Pamelaria dolichotrachela (ISIR 316/1; Sen 2003). The postglenoid process is well developed, as in A. madagaskarensis (Nesbitt et al. 2015), but, in contrast with this taxon, there is no ridge on the lateral surface of the coracoid. The proximal and distal ends of the humerus are strongly transversely expanded, with their main axes rotated c. 70° to each other (Fig. 1L, M). The entepicondyle and the supinator process and groove are well developed, as in A. madagaskarensis (Nesbitt et al. 2015). The ulnar condyle is developed as a ball-shaped structure.
The ilium has a poorly developed preacetabular process (Fig. 1R), contrasting with that of A. madagaskarensis and S. indicus (Nesbitt et al. 2015; Sengupta et al. 2017). The transversely flat postacetabular process is better developed and tapers posterodorsally in lateral view. The ischium is a broad, plate-like bone with a prominent posterodorsal angle, unlike the gradually sloping bone in A. madagaskarensis (Fig. 1S). The femora are more gracile than those of A. madagaskarensis and S. indicus (Fig. 1O, P; Nesbitt et al. 2015; Sengupta et al. 2017). The internal trochanter reaches the proximal margin of the bone, contrasting with A. madagaskarensis and S. indicus (Nesbitt et al. 2015; Sengupta et al. 2017). The tibia (Fig. 1Q) is shorter than the femur and has the same depressions in the proximal end as in A. madagaskarensis (Nesbitt et al. 2015). The astragalus possesses a broad proximal non-articular gap, which is separated from the anterior hollow by a sharp edge. The preserved portion of the tibial facet is flat and the fibular facet is shallowly concave.
Reinterpretation of Malerisaurus langstoni
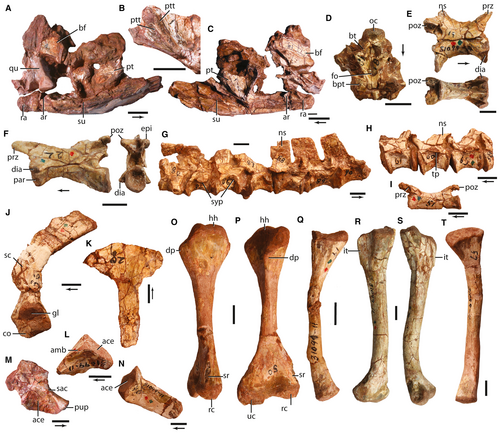
Malerisaurus langstoni was first based on a ‘mostly complete skeleton’ (TMM 31099-11) from Otis Chalk Quarry 2 (TMM 31099). The skeleton includes partial cranial material, forelimb and hindlimb bones, incomplete pectoral and pelvic girdle elements, and vertebrae from the cervical, trunk, sacral and caudal regions (Fig. 2). Some of the vertebrae of the holotype specimen remain in articulation (trunk and some of the now identified middle caudal vertebrae). Unfortunately, all of the other bones are now disarticulated, and the association of the skeletal material was not recorded. It is also clear that material of Trilophosaurus buettneri (e.g. TMM 31099-18, left maxilla) of a similar size was also mixed within the Otis Chalk Quarry 2 bonebed; therefore, others have doubted the validity of M. langstoni (Spielmann et al. 2006).
Here we support the original interpretation by Chatterjee (1986) that the holotype of M. langstoni is one species-level taxon, and that the material is probably all from a single individual based on the following lines of evidence: (1) osteological distinctions between the holotype (TMM 31099-11) from known association of Tr. buettneri skeletons (e.g. TMM 31025-140); and (2) non-overlap of the skeletal elements of the holotype, suggesting a single individual. This differs from the interpretation that M. langstoni is a chimaera composed of a variety of archosauromorphs (Spielmann et al. 2006). Those authors argued that the skull of M. langstoni is referable to a skull of Tr. buettneri based on rotating Chatterjee’s (1986, fig. 2C–D) interpretation 180° to make what Chatterjee identified as the back of the hemimandible into the skull roof of the specimen; Spielmann et al. (2006) identified tooth sockets in this part of their reinterpreted skull but they did not present any unique character states that assigned this partial skull to Tr. buettneri.
To test these vastly different interpretations, one of us (SJN) re-prepared the holotype specimen, removed the thick Glyptal cement and bits of plaster, and cleaned the surfaces. Similar to Chatterjee’s original interpretation, we find that this partial skull represents the posterior portion of the hemimandible with a long retroarticular process articulated with a poorly preserved quadrate and other fragments of probable skull elements. Our re-preparation also revealed other new details. For example, what was interpreted as a nasal–premaxilla by Spielmann et al. (2006, fig. 2b) and a postorbital–jugal by Chatterjee (1986, fig. 2D) is actually the ventral surface of a pterygoid with two parallel rows of small pterygoid teeth (Fig. 2B), a character state not present in Tr. buettneri, in which the palate is edentulous.
The braincase of M. langstoni was reassigned to an aetosaur by Spielmann et al. (2006) because of a hemispherical depression on the ventral surface of the parabasisphenoid. The presence of a fossa on the ventral surface of the parabasisphenoid is a widely distributed character state across tetrapods and is found in a variety of archosauromorphs (e.g. A. madagaskarensis; Flynn et al. 2010), so this character state is not restricted to aetosaurs. Spielmann et al. (2006) argued that there is a mismatch of the size of the cervical series and trunk vertebral series (Fig. 2G) so that more than one individual is present. We disagree with this interpretation based on the comparisons with other azendohsaurid vertebral proportions (e.g. A. madagaskarensis; Nesbitt et al. 2015). Additionally, Spielmann et al. (2006) stated that the vertebrae of the holotype of M. langstoni pertain to Tr. buettneri based upon a number of character states that are not diagnostic of Tr. buettneri. The cervical vertebrae of M. langstoni are 1.25 times longer proportionally and lack the strong procoely present in the cervical vertebrae of Tr. buettneri (TMM 31025-140), the articulated series of vertebrae lack intercentra that are present in Tr. buettneri (Gregory 1945), and the caudal vertebrae of M. langstoni are platycoelous, not strongly procoelous like those of Tr. buettneri (see below for more discussion).
The arguments that the pectoral girdle, radius, pelvic girdle, femur and tibia pertain to Tr. buettneri or, in the case of the pelvic girdle, to a phytosaur, were based on overall similarity, not apomorphic identifications with the reassigned taxa (Spielmann et al. 2006). Admittedly, the appendicular postcrania of M. langstoni appear similar to those of Tr. buettneri, but these similarities are a combination of plesiomorphic character states of archosauromorphs as a whole and plesiomorphies shared by other allokotosaurians (see below).
Last, the humerus (TMM 31099-11) was reassigned to the rhynchosaur Otischalkia elderae by Spielmann et al. (2006) based on morphological similarity. They reported that the humerus of M. langstoni lacks the hook-like supinator crest of the distal end present in Otischalkia elderae and rhynchosaurs, and concluded that the humerus of M. langstoni pertains to a small, adult rhynchosaur or a young rhynchosaur. Below, we argue that the holotype of M. langstoni represents a single small individual of the species. Through ontogeny, the humerus becomes more robust and changes in morphology (e.g. develops the hook-like supinator crest), and we propose that O. elderae is actually a larger, potentially ontogenetically more mature individual of M. langstoni or a closely related form (see growth trajectory graphs below).
Otis Chalk Quarry 1 (TMM 31025), within 50 m of the holotype locality of M. langstoni (Otis Chalk Quarry 2, TMM 31099), contains the remains of large Malerisaurus-like specimens among the dozens of disarticulated individuals of Tr. buettneri (Gregory 1945; Spielmann et al. 2008). The skeletal elements that we attribute to a Malerisaurus-like taxon have been attributed informally to an aetosaur (ulna with a large olecranon process, TMM 31025-586), dinosaurs (maxilla with recurved and serrated teeth, TMM 31025-271; long cervical vertebrae, TMM 31213-13), a captorhinid reptile (= ‘Polystrichodontos mandibularensis’ of Elder 1978; TMM 31025-265, TMM 31025-261; Elder 1978, fig. 14, 15; pterygoid fragments with multiple tooth rows, TMM 31025-261 and TMM 31025-268), a phytosaur (associated trunk vertebrae, TMM 31025-34), a proterosuchid (anterior end of dentary, TMM 31025-267; Elder 1978, pl. 4, fig. 1), an unknown reptile (right scapula, TMM 31025-154; Elder 1978, pl. 11, fig. 4), the holotype and paratype specimens of Otischalkia ‘dockumensia’ (sensu Elder 1978), and referred material of Tr. buettneri (nearly complete pelvis, TMM 31025-75; Spielmann et al. 2008, fig. 82). In addition to the previous identified material, we identified dozens more skeletal elements that possess Malerisaurus-like or azendohsaurid character states (Fig. 3). These latter specimens are particularly important because they are much larger than the holotype of M. langstoni, showing hypothesized ontogenetic variation, and are generally well preserved (see below).
Recent collection efforts also discovered a variety of Malerisaurus-like remains from previously known localities from the Dockum Group in Texas (e.g. Griffin Ranch, TMM 31213-11 and TMM 31213-12) and two monodominant bonebeds from the Chinle Formation of Arizona (Dinosaur Wash, PFV 217; Krzyzanowski bonebed, NMMNH L-3764; Fig. 4A, D). First attributed to Trilophosaurus, exceptionally well-preserved bones from Griffin Ranch (TMM 31213-11 and TMM 31213-12) in Borden County, Texas, pertain to a Malerisaurus-like taxon, and recent collection by one of us (BM) from the same locality (MOTT VPL3629) led to the discovery of dozens of diagnostic elements (see below) from the same taxon.
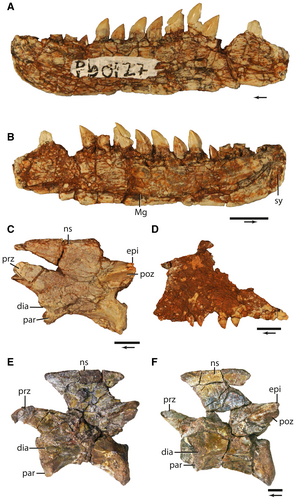
Two extensive bonebeds from the Chinle Formation produced an abundance of Malerisaurus-like specimens. Spielmann et al. (2013) referred the archosauromorph bones from the Krzyzanowski bonebed to Trilophosaurus but, as detailed above, their identifications were made on overall similarity to Trilophosaurus, not using unique character states of the taxon. For example, the cervical vertebrae from this site have lateral expansions at the dorsal margin of the neural spines forming a flattened platform, a character state present in Malerisaurus (known in M. robinsonae and other Malerisaurus-like specimens in western USA, although cervical neural spines are not preserved in M. langstoni) but absent in Trilophosaurus. We hypothesize that nearly all of these bones are referable to a Malerisaurus-like taxon. Furthermore, a number of cranial elements (Fig. 4A, B) that are similar to the Malerisaurus-like taxon from Petrified Forest National Park are also present at this locality (NMMNH L-3764). Dinosaur Wash (PFV 217) has produced hundreds of well-preserved elements of different ontogenetic stages of a Malerisaurus-like taxon (Marsh et al. 2017) that bridges the morphological gap between the holotype material of M. langstoni and that of O. elderae. A full description of this material will be given elsewhere.
Reinterpretation of Otischalkia elderae
First identified by Elder (1978), possible rhynchosaur remains including humeri, femora, and premaxillae were found in the Otis Chalk assemblage and then later recognized from other localities across the Upper Triassic deposits of western North America (Murry & Long 1989). That material later formed the basis of the taxon Otischalkia elderae Hunt & Lucas, 1991a (TMM 31025-263; Fig. 3K, L) with a left humerus as the holotype and all other previously identified material as referred specimens. The original interpretation of O. elderae as a rhynchosaur was largely supported by the ‘robustness’ of the humeri and femora and the assignment of two isolated elements identified as edentulous and grooved premaxillae (TMM 31185-92 and TMM 31185-93; Elder 1978; Hunt & Lucas 1991b; Long & Murry 1995), highly diagnostic cranial bones of rhynchosaurs. However, those purported premaxillae were re-identified as metoposaurid cleithra (Stocker 2013). Therefore, the identification of O. elderae as a rhynchosaur must rest entirely on the morphology of the humerus (Fig. 3K, L). TMM 31025-263 shares with rhynchosaurids (e.g. Stenaulorhynchus stockleyi, Huene 1938; Isalorhynchus genovefae, Whatley 2005; Hyperodapedon sanjuanensis, MACN-Pv 18185) the presence of a supinator process of the humerus, but it differs from members of this clade in the equal expansion of the proximal and distal ends of the humeri (proximal end more expanded in rhynchosaurids), the more massive and subquadrangular deltopectoral crest, and the presence of a capitulum that is larger than the trochlea on the distal end of the humerus (Hunt & Lucas 1991b). The humerus of the holotype of M. langstoni (TMM 31099-11) shares with the holotype humerus of O. elderae (TMM 31025-263) an enlarged deltopectoral crest, expanded proximal and distal ends as compared with the diameter of the humeral shaft, and, to the exclusion of Tr. buettneri, radial and ulnar condyles that are separated by a shallow sulcus (Spielmann et al. 2006). Therefore, there is no unambiguous evidence that the humerus of O. elderae is assignable to a rhynchosaur, and we hypothesize that the similar morphology of the humerus of M. langstoni and O. elderae indicates that the latter species is a larger version of a Malerisaurus-like taxon (see Discussion). We cannot confirm that the holotype of O. elderae is M. langstoni because there are no uniquely shared diagnostic character states. However, the holotype of O. elderae and other material of similar size occurs in the same geographical and stratigraphical proximity as the holotype and referred material of M. langstoni at the Otis Chalk localities, and, as we document here, both forms are almost always found together in the Dockum Group of Texas. Therefore, we consider that the holotype of O. elderae probably represents a growth stage of M. langstoni and, given that M. langstoni was named first, the material assigned to O. elderae should be referred to M. langstoni. Moreover, the humerus of O. elderae does not possess any unambiguous combination of character states or autapomorphies and should be a nomen dubium. Herein, we use quotes to refer to the ‘Otischalkia elderae’-sized elements.
Phylogenetic analysis
In our analysis that includes ‘Malerisaurus langstoni’, ‘Malerisaurus robinsonae’, ‘Malerisaurus-like form large’ and ‘Malerisaurus-like taxon PEFO’ as terminal taxa, we find that all of the ‘Malerisaurus’ terminal taxa fall in a clade within Allokotosauria and Azendohsauridae to the exclusion of other azendohsaurid species (most parsimonious trees (MPTs) = 12 with 1253 steps, consistency index (CI) = 0.29928, retention index (RI) = 0.64796, best score hit 821 times of the 1000 replicates; Fig. 5A). Within Azendohsauridae, the ‘Malerisaurus’ terminal taxa clade is recovered as the sister taxon to the group composed of Shringasaurus indicus and both species of Azendohsaurus. Malerisaurus robinsonae is the sister taxon to a polytomy composed of the North American ‘Malerisaurus’ terminal taxa. Pamelaria dolichotrachela is found as the earliest branching azendohsaurid. When ‘Malerisaurus robinsonae’ is removed and ‘Malerisaurus langstoni’, ‘Malerisaurus-like form large’, and ‘Malerisaurus-like taxon PEFO’ are combined into a ‘Malerisaurus all NA’ terminal taxon, this terminal taxon also falls within Azendohsauridae, again as the sister taxon to Azendohsaurus spp. + Shringasaurus indicus, and P. dolichotrachela represents the earliest branching azendohsaurid (MPTs = 6 with 1238 steps, CI = 0.30291, RI = 0.64573, best score hit 842 times of the 1000 replicates; Fig. 5B). When ‘Malerisaurus robinsonae’ is removed and ‘Malerisaurus langstoni’, ‘Malerisaurus-like form large’, and ‘Malerisaurus-like taxon PEFO’ are separate terminal taxa, the three Malerisaurus terminal taxa fall into an unresolved clade exclusive to other species, which is also the sister taxon of Azendohsaurus spp. + Shringasaurus indicus, and P. dolichotrachela is recovered as the sister taxon to all other azendohsaurid terminal taxa (MPTs = 12 with 1248 steps, CI = 0.30048, RI = 0.64699, best score hit 812 times of the 1000 replicates; Fig. 5C). When ‘Malerisaurus robinsonae’ and ‘Malerisaurus langstoni’ are the only ‘Malerisaurus’ terminal taxa, the two nest as sister taxa to each other and as the earliest branching azendohsaurids, in which P. dolichotrachela is the sister taxon to Azendohsaurus spp. + Shringasaurus indicus (MPTs = 6 with 1229 steps, CI = 0.30513, RI = 0.64725, best score hit 705 times of the 1000 replicates; Fig. 5D). Finally, when ‘Malerisaurus robinsonae’ is used and ‘Malerisaurus langstoni’, ‘Malerisaurus-like form large’, and ‘Malerisaurus-like taxon PEFO’ are combined into a ‘Malerisaurus all NA’ terminal taxon, the interrelationships within Azendohsauridae are the same as in the previous analysis, but P. dolichotrachela is found as the earliest branching member of the clade (MPTs = 6 with 1243 steps, CI = 0.30169, RI = 0.64687, best score hit 850 times of the 1000 replicates; Fig. 5E).
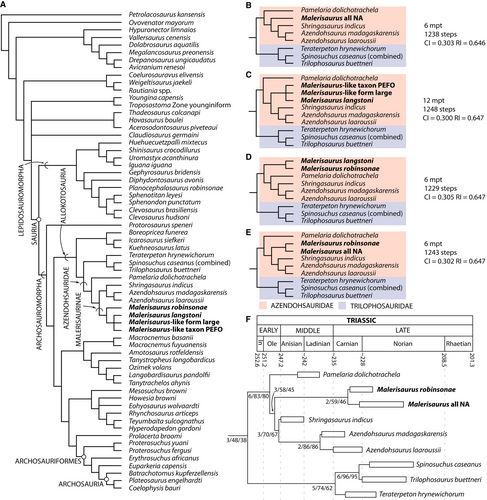
In the case of the analysis including M. robinsonae and the three North American ‘Malerisaurus’ terminal taxa independently, the branch supports are relatively high through most of the allokotosaurian region of the tree (i.e. Bremer support ≥3 or bootstrap frequencies ≥70%). In particular, the lowest Bremer supports (i.e. 2) are calculated for the Malerisaurus robinsonae + ‘Malerisaurus’ all NA clade and the genus Azendohsaurus. Similar branch supports are recovered when using M. robinsonae and the three ‘Malerisaurus’ NA terminal taxa independently. However, the resampling frequencies for the ‘Malerisaurus’ clade are slightly lower (<50%). The clade formed by the three North American ‘Malerisaurus’ terminal taxa has a minimum Bremer support of 1, and absolute and group present/contradicted (GC) bootstrap frequencies of 57% and 45%, respectively.
Under constrained suboptimal topologies, five additional steps are necessary to place the ‘Malerisaurus’ clade at the base of Azendohsauridae, and two apomorphies (chars 22:1→0, 337:0→1) support the placement of P. dolichotrachela as the sister taxon to Shringasaurus indicus + Azendohsaurus spp. Three extra steps force the positioning of the ‘Malerisaurus’ clade as the sister taxon of Azendohsaurus spp. and three apomorphies support this hypothesis (chars 21:0→1, 106:0→1, 231:0→1). Fourteen extra steps are required to place the ‘Malerisaurus’ clade at the base of Trilophosauridae, which is supported by one apomorphy (char. 233:0→1). Finally, 11 additional steps allow positioning of the ‘Malerisaurus’ clade as the sister taxon to Trilophosauridae + Azendohsauridae, and this topology is supported by five apomorphies (chars 37:0→1, 106:1→0, 121:0→1, 147:1→0, 326:0→1). Under this latter constraint, the ‘Malerisaurus’ group is paraphyletic and Boreopricea funerea and kuehneosaurids are found outside of Allokotosauria. As a result, these topologically constrained analyses show that the position of the ‘Malerisaurus’ clade in Allokotosauria and within this clade is well supported by our dataset.
Systematic palaeontology
REPTILIA Laurenti, 1768
ARCHOSAUROMORPHA Huene, 1946 sensu Benton, 1985
AZENDOHSAURIDAE Nesbitt et al., 2015
MALERISAURINAE nov.
LSID
urn:lsid:zoobank.org:act:96DEED1F-6EE3-48A5-86CE-9A45E6713807
Phylogenetic definition
The least-inclusive clade containing Malerisaurus robinsonae Chatterjee, 1986, but not Azendohsaurus madagaskarensis Flynn et al., 2010; Trilophosaurus buettneri Case, 1928; Tanystropheus longobardicus Bassani, 1886; Proterosuchus fergusi Broom, 1903; Protorosaurus speneri Meyer, 1830; Rhynchosaurus articeps Owen, 1842; or Passer domesticus Linnaeus, 1758.
Diagnosis
The skeletons of M. robinsonae and M. langstoni share a similar size and overall morphology of the forelimbs and hindlimbs, girdles, vertebrae, and overlapping skull elements, which prompted Chatterjee (1986) to refer both species to the same genus-level taxon. However, a combination of newly discovered taxa (e.g. Azendohsaurus spp., S. indicus) and an understanding of character state optimizations has rendered both existing diagnoses obsolete in the context of early archosauromorph relationships.
Malerisaurinae can be distinguished from other archosauromorphs based on the following combination of character states: maxillary teeth with distally recurved tips; teeth ankylosed to the bone of attachment; interdental plates present in the maxilla; anterior to mid-cervical centra length more than 1.75-fold the anterior articular facet height; lateral expansions of the dorsal end of the cervical neural spines (unknown in the holotype of M. langstoni, but consistent with referable material from the holotype locality); epipophyses present in the cervical vertebrae; intercentra absent; proximal portion of the scapula anteroposteriorly long compared with the distal portion; radius with a tall posteroproximal expansion on the proximal surface; postglenoid process of the coracoid present; supinator crest of the humerus present.
Remarks
We designate Malerisaurinae to encompass Malerisaurus-like taxa that are abundant in at least the Upper Triassic of western USA. Our phylogenetic analyses unambiguously recover a Malerisaurinae composed of both Malerisaurus species at present. If future studies find that Malerisaurus-like taxa form a grade at the base of Azendohsauridae, Malerisaurinae would have limited use and should be abandoned.
Malerisaurus robinsonae Chatterjee, 1980
Figure 1
| 1980 | Malerisaurus robinsonae Chatterjee, p. 163, pl. 1, figs 1b, 2–7, 8–10, 12. |
Holotype
ISIR 150 premaxillae, maxillae, right nasal, lacrimal, prefrontal, quadrates, left pterygoid, quadratojugal and articular-surangular, dentaries, splenial and coronoid, exoccipitals, opisthotic, basioccipital, parabasisphenoid, multiple vertebrae from all regions of the column, scapulae, humeri, right ulna, some carpal and manual bones, ilia, ischia, left pubis, femora, tibia, and indeterminate bone fragments. Note: the holotype and paratype are housed together and individual bones are not numbered, so we mainly rely on Chatterjee (1980) with a few modifications.
Paratype
ISIR 151, premaxilla, quadrate, quadratojugal, braincase, multiple vertebrae from all regions of the column, scapulae, left coracoid, humeri, radius, ilia, left ischium, femora, left tibia, several tarsal and pedal bones, and indeterminate bone fragments.
Locality & horizon
Lower Maleri Formation, Pranhita–Godavari Basin, India, upper Carnian to ?lowermost Norian (Chatterjee 1978).
Diagnosis
Malerisaurus robinsonae possesses the following unique combination of character states: marginal dentition ankylosed to the bone; premaxilla with five tooth positions; middle dentary tooth crowns with convex mesial and distal margins; ventral condyles of quadrate subequally developed ventrally; pterygoid teeth similar in size to marginal dentition and with coarsely serrated crown margins; basicranium anteroventrally–posterodorsally oriented; parabasisphenoid with a deep, triangular ventral fossa; postaxial intercentra absent; elongated postaxial cervical centra with a length : anterior height ratio of 1.84–3.02; fourth cervical to first trunk vertebrae with epipophyses; anterior postaxial cervical neural spines with anterior and posterior overhangs, and a low transverse expansion with a flat dorsal surface; trunk vertebrae with a single rib facet and lacking laminae on the neural arch, with the exception of an anterior centrodiapophyseal lamina on one of the most anterior trunk vertebrae; anterior and middle caudal vertebrae with a ventral longitudinal groove; coracoid with a shelf-like subglenoid lip and without a lateral anteroposteriorly oriented ridge; humerus with ball-shaped distal ulnar condyle; radius with a tall posteroproximal expansion on the proximal surface; ilium with poorly developed preacetabular process; and femur with internal trochanter that reaches the proximal articular surface.
Malerisaurus langstoni Chatterjee, 1986
Figure 2
Holotype
TMM 31099-11, partial skull including the right pterygoid, quadrate, articular and surangular, braincase, cervical, trunk, sacral and caudal vertebrae, left scapulocoracoid, interclavicle, left humerus, left radius, right ilium, left pubis, left ischium, femora and tibiae.
Locality and horizon
Otis Chalk Quarry 2, Colorado City Formation, Dockum Group, Howard County, Texas, USA, early Norian (Lucas & Hunt 1993; Stocker 2013; Martz & Parker 2017; Nesbitt et al. 2021b).
Diagnosis
Malerisaurus langstoni bears the following unique combination of character states: two parallel rows of teeth on the medial edge of the pterygoid; deep fossa on the ventral surface of the parabasisphenoid with the absence of the entrance of the internal carotid foramina; posteriorly expanded retroarticular process of the articular and surangular with a medial process; epipophyses on the postzygapophyses of the cervical vertebrae; elongated cervical centra with a ratio of 2 relative to the anterior articular surface height; weak procoelous articulations in the cervical vertebrae; intercentra in the postaxial presacral vertebrae absent; scapula greatly expanded posterodistally (anterodistal portion unknown); posterolaterally directed glenoid of the scapulocoracoid; long axes of the proximal and distal ends of the humerus twisted 75° from each other; radius with a tall posteroproximal expansion on the proximal surface; ventral margin of the ilium straight in lateral view; femur with an internal trochanter that reaches the proximal articular surface.
Malerisaurus langstoni can also be clearly differentiated from the contemporary Tr. buettneri from the same locality and area. The cervical vertebrae of M. langstoni lack interpostzygapophyseal laminae with a slit at the midline and have less well developed procoelous articulations; the caudal vertebrae of M. langstoni lack procoelous articulations; M. langstoni lacks intercentra in the postaxial presacral vertebrae; the ventral end of the quadrate is more convex in Malerisaurus; the scapula is more posterodistally expanded and proximodistally shorter in Malerisaurus; the humerus shaft is proportionally shorter (especially in large Malerisaurus-like forms); the humerus lacks a sharp angle to the proximal edge of the entepicondyle in Malerisaurus; and the humerus distal condyles are more weakly differentiated in Malerisaurus.
Discussion
Phylogenetic position among allokotosaurians
Our phylogenetic analysis and our comparative description (below) place Malerisaurus-like taxa from the Upper Triassic of western USA as stem archosaurs within Azendohsauridae, within the larger clade Allokotosauria. This phylogenetic position is well supported by a wealth of data across the holotype of M. langstoni, larger specimens that we refer to Malerisaurus from Otis Chalk Quarry 1 (including the holotype and paratype of Otischalkia), partial skeletons from monodominant bonebeds, and some isolated specimens with azendohsaurid synapomorphies (see Results).
The skull of Malerisaurus-like taxa possesses synapomorphies (indicated with character number and state transformation) and a unique combination of character states uniquely shared with other azendohsaurids. The premaxilla of Malerisaurus-like taxa (PEFO 43914, Fig. 6C; TTU-P 11212, Fig. 6D) lacks an anterodorsal (=nasal) process on the anterior portion of the premaxilla, similar to that of Azendohsaurus madagaskarensis (Flynn et al. 2010), Shringasaurus indicus (Sengupta et al. 2017) and Pamelaria dolichotrachela (Sen 2003), thus resulting in a single midline narial opening. The posterodorsal process is mediolaterally flat and posteriorly elongate in the Malerisaurus-like taxon, like that of A. madagaskarensis (Flynn et al. 2010) and S. indicus (Sengupta et al. 2017). Malerisaurus-like taxa (Fig. 6C) and A. madagaskarensis (Flynn et al. 2010) share four premaxillary teeth that decrease in apicobasal length distally. The maxilla of the Malerisaurus-like taxa (Fig. 6A) bears a dorsal process that is posteriorly recurved at its tip, more than that of tanystropheids (e.g. Macrocnemus bassanii, Miedema et al. 2020; Tanystropheus hydroides, Spiekman et al. 2020, 2021), but not as distinct and elongate as the dorsal process of A. madagaskarensis (Flynn et al. 2010). The maxilla of the Malerisaurus-like taxon bears interdental plates, and the teeth are ankylosed to the bone once fully erupted (TTUP P10516; Fig. 6B), as in A. madagaskarensis (Flynn et al. 2010), P. dolichotrachela (ISIR 316/1) and S. indicus (ISIR 795). As in other azendohsaurids (Flynn et al. 2010; Sengupta et al. 2017), the teeth of the Malerisaurus-like taxa have pronounced serrations (char. 145:0→1; possibly absent in M. robinsonae, but may be related to poor preservation) (Fig. 6B, C). The quadrate of the Malerisaurus-like taxon has a clear posterior hook of the quadrate head, a flexed shaft, and importantly, a medial condyle of the ventral end expanded posteriorly and ventrally to create a distinct, mediolaterally asymmetric articulation with the articular–surangular (PEFO 43938; TTUP P19378 Fig. 6E); all character states possessed by azendohsaurids (Nesbitt et al. 2015). The distinct ventral condyles of the quadrate translate into a deep glenoid at the posterior portion of the hemimandible (M. langstoni holotype TMM 31099-11; Fig. 1A, C), a character state clearly present in A. madagaskarensis (Flynn et al. 2010), P. dolichotrachela (ISIR 316/1) and S. indicus (ISIR 797). The braincase of the M. langstoni holotype (TMM 31185-11; Fig. 1D) possesses a deep midline fossa on the ventral surface of the parabasisphenoid, like that of A. madagaskarensis (Flynn et al. 2010) and S. indicus (ISIR 798). Malerisaurus langstoni, Malerisaurus-like taxa and M. robinsonae lack an entrance of the internal carotid arteries from the ventral surface of the parabasisphenoid (char. 107:1→2, absent in P. dolichotrachela). The dentary of the Malerisaurus-like taxon has parallel dorsal and ventral margins for much of the length of the element, and anteriorly the ventral margin angles anteroventrally to the anterior tip of the element (PEFO 44246; TMM 31025-267); this is also present in A. madagaskarensis (FMNH PR 2751) and P. dolichotrachela (ISIR 316/1; Sen 2003), but contrasts with the more tapering anterior end of the dentary of S. indicus (ISIR 802).

The cervical vertebrae of the Malerisaurus-like taxon share a number of proportion-based character states with Azendohsauridae, but also more broadly with other allokotosaurs (e.g. Tr. buettneri) and other archosauromorphs (e.g. Protorosaurus speneri; Gottmann-Quesada & Sander 2009) with elongated cervical vertebrae. Neck elongation appears to have increased independently in many early archosauromorph lineages (Ezcurra 2016), so the distribution of these character states is not clear and is dependent on optimizations based on particular phylogenetic hypotheses. The preserved presacral vertebrae of Malerisaurus-like taxa lack intercentra, and this absence is supported by the lack of any bevelling of the ventral edges of the centra. The presence of presacral intercentra is common in early archosauromorphs (Ezcurra 2016), including Trilophosauridae within Allokotosauria; in contrast, the presacral intervertebral elements are absent in all members of Azendohsauridae (Nesbitt et al. 2015; Sengupta et al. 2017).
The coracoid of the Malerisaurus-like taxon (TMM 31025-803; Fig. 3P) bears a distinct postglenoid process that is also mediolaterally expanded into a horizontal shelf, as in S. indicus (ISIR 941) and A. madagaskarensis (FMNH PR 3822; Nesbitt et al. 2015). The postglenoid process of the Malerisaurus-like taxon and other azendohsaurids is as proportionally elongate as that of Tr. buettneri (TMM 31025-68b) or Spinosuchus caseanus (NMMNH P-44279). The humeral proportions of larger specimens of the Malerisaurus-like taxa have similarly expanded proximal and distal ends, with a highly constricted midshaft, like those of S. indicus (ISIR 951), P. dolichotrachela (ISIR 316/1) and A. madagaskarensis (FMNH PR 2755) (char. 243:0→1). An isolated metacarpal I (TMM 31025-139; Fig. 3O) from Otis Chalk Quarry 1 shares a similar width : length ratio (c. 0.66 width to length) and is asymmetric proximally and distally in exactly the same way as A. madagaskarensis (UA 9-8-98-498). In contrast, most early archosauromorph first metacarpals are proportionally more elongate (e.g. Protorosaurus speneri, Gottmann-Quesada & Sander 2009; Tr. buettneri, TMM 31025-141).
The pelvic girdle of the Malerisaurus-like taxon is composed of three similarly sized elements like that of other archosauromorphs (TMM 31025-75; Fig. 3Q). The ilium tapers to a distinct point posteriorly like that of S. indicus (ISIR 991), P. dolichotrachela (ISIR 316/1) and A. madagaskarensis (FMNH PR 2794), but also similar to Tr. buettneri (TMM 31025-77). The pubis bears a flange-like ambiens process that contacts the acetabulum, as in A. madagaskarensis (FMNH PR 2794) and S. indicus (ISIR 1007) (char. 339:0→1), unlike the clear separation of these structures in Tr. buettneri (TMM 31024-140). The ventrolateral portion of the pubis is expanded posteriorly into a distinct tubercle like that of A. madagaskarensis (FMNH PR 2794) and P. dolichotrachela (ISIR 316/1), but unlike the uniformly thin, sheet-like ventrolateral portion of Tr. buettneri (TMM 31025-140). The ischium of the Malerisaurus-like taxon (TMM 31025-75) also has a dorsoventrally expanded medial surface for articulation with its antimere, which is a feature shared with A. madagaskarensis (Nesbitt et al. 2015) and P. dolichotrachela (Ezcurra 2016).
Differences between azendohsaurids
Malerisaurus-like taxa bear numerous plesiomorphic archosauromorph character states absent in the larger, bulkier, probably herbivorous, Azendohsaurus + Shringasaurus clade. The maxilla of Malerisaurus-like taxa is proportionally long and dorsoventrally low like those of early archosauromorphs (e.g. Macrocnemus bassanii, Protorosaurus speneri, Prolacerta broomi), in contrast to that of the foreshortened elements of P. dolichotrachela, Azendohsaurus spp. and S. indicus, and the teeth of Malerisaurus-like taxa are recurved at their tips with much finer denticles (10 serrations/mm in TMM 31025-270) than in the former taxa. The braincase of Malerisaurus-like forms is nearly horizontally oriented (i.e. the basitubera are in nearly the same horizontal plane as the basipterygoid processes), whereas the braincase in A. madagaskarensis (Flynn et al. 2010) and S. indicus (Sengupta et al. 2017) is much more vertically oriented, with the basitubera located more dorsally than the basipterygoid processes. Pamelaria dolichotrachela possesses a ventrally positioned entrance of the internal carotid arteries on the basicranium (ISIR 316/1), but contrasting with the absence of an ossified entrance in the basicrania of M. langstoni (TMM 31185-11; Fig. 2D), M. robinsonae (ISIR 151), S. indicus (Sengupta et al. 2017) and A. madagaskarensis (Nesbitt et al. 2015).
All of the Malerisaurus-like taxa from western USA and M. robinsonae from India possess low lateral expansions across the entire dorsal margins of the neural spines in the cervical vertebrae, unlike those of A. madagaskarensis (Nesbitt et al. 2015), S. indicus (Sengupta et al. 2017), P. dolichotrachela (Sen 2003) and trilophosaurids (Gregory 1945; Sues 2003; Spielmann et al. 2008, 2013; Pritchard & Sues 2019). These expanded neural spines also occur in the trunk region (TMM 31025-43; Fig. 3G), but their distribution through this region is not clear. Mid- to posterior caudal vertebrae of Malerisaurus-like taxa are much longer proportionally and more gracile (TMM 31085-11; Fig. 3M, N) than those of the more foreshortened caudal vertebrae of A. madagaskarensis (Nesbitt et al. 2015) and S. indicus (Sengupta et al. 2017).
The scapula of the Malerisaurus-like taxa (TMM 31025-254; Fig. 3J) is about equally anteroposteriorly wide proximally and distally, with little constriction distal to the glenoid. This scapula shape is similar to that of P. dolichotrachela (Sen 2003), but in contrast to the much more constricted scapulae of A. madagaskarensis (FMNH PR 2798) and S. indicus (ISIR 929).
Although the stylopodial limb elements are similarly robust within Azendohsauridae (see below), the zeugopod and autopod elements are more gracile in Malerisaurus-like taxa and in P. dolichotrachela (Sen 2003). The more distal limb elements are more like those of other archosauromorphs (e.g. Tr. buettneri, tanystropheids, Prolacerta broomi) to the point that isolated limb bones from these regions of the Malerisaurus-like taxa are highly difficult to distinguish from other archosauromorphs when found isolated or in mixed assemblages.
Growth and size
Proportions
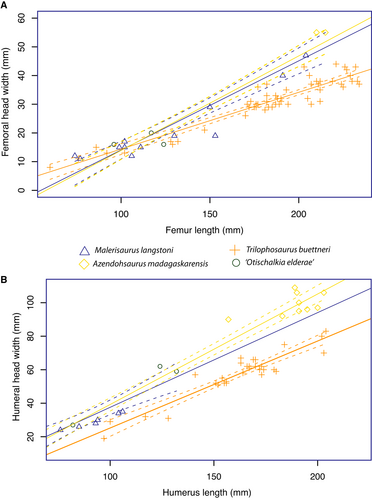
The overall similarity in the gracile proportions of the humeri and the femora of Tr. buettneri and M. langstoni has led to misattribution to each taxon. However, the larger specimens of Tr. buettneri (TMM 31025-140) and the Malerisaurus-like form (TMM 31025-263) do show clear proportional differences. The proximal and distal ends of stylopodial bones of the Malerisaurus-like taxon are much more expanded relative to that of Tr. buettneri. To demonstrate this difference, we measured variously sized specimens of humeri and femora of Tr. buettneri (from Otis Chalk Quarry 1), those of the large Malerisaurus-like taxon (TMM 31025-263), the holotype specimen of M. langstoni (TMM 31185-11), other identical femora from the holotype locality, and those of A. madagaskarensis (from Nesbitt et al. 2015) (Tables S1, S2).
Overall, we find that the stylopod elements of Tr. buettneri and the Malerisaurus-like taxon slightly diverge through growth and are on different growth trajectories relative to each other (Fig. 7). This is clearer with the humeri (Fig. 7B) of the Malerisaurus-like taxon and A. madagaskarensis, which have proximal and distal ends that expand more than those of Tr. buettneri. The smaller, and probably ontogenetically younger, specimens of the Malerisaurus-like taxon and Tr. buettneri are not as easily differentiated as those of the larger specimens, thus indicating that the proportions of the two are likely to have been more similar earlier in ontogeny. The femora (Fig. 8A) of the Malerisaurus-like taxon and A. madagaskarensis are typically ‘more robust’ than those of Tr. buettneri, but the biological and taphonomic variation of the femora of Tr. buettneri overlaps with that of the Malerisaurus-like taxon. Therefore, differentiating the femora of the Malerisaurus-like taxa from Tr. buettneri is nearly impossible given our data, particularly in the smaller forms.
Ontogenetic changes
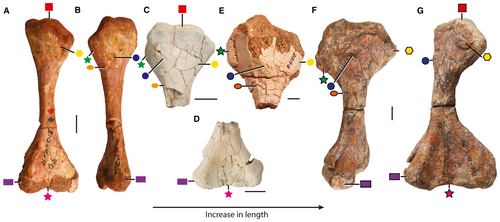
Our hypothesis that small M. langstoni specimens (such as the holotype individual TMM 31099-11) develop throughout ontogeny into more robust forms more similar to A. madagaskarensis (e.g. ‘Otischalkia elderae’) is supported by changes in limb bone morphology and proportions. This is clearest in the humerus, where the smaller specimens are gracile and the larger referred specimens are more robust (Fig. 8); this hypothesis relies on a growth series from the Dinosaur Wash locality (PFV 217) and on humeri found associated with other Malerisaurus-like bones across the Upper Triassic of western USA. The smaller humeri (length < 100 mm), like that of the holotype of M. langstoni (TMM 31099-11), are proportionally longer relative to the maximum dimensions across the proximal and distal ends, they lack well defined proximal and distal articulation surfaces (e.g. humeral head, distal condyles), and they lack distinct bone/muscle scars (Fig. 8A). In contrast, the larger specimens (e.g. estimated length >120 mm, TMM 31025-263) possess greater expansions of the proximal and distal ends relative to the length (see below), well-developed proximal and distal articulation surfaces, and bone scars that are clearly differentiated from the rest of the surface (Fig. 8E–G). These changes occur throughout ontogeny in other reptiles (e.g. Griffin & Nesbitt 2016).
We have identified a number of intermediate-sized humeri (e.g. TMM 31213-8, TMM 31213-12) that demonstrate that the ontogenetic transformation occurs in a series of steps (Fig. 8C–E). Throughout this ontogenetic series, the humeral head, epicondyles, and distal condyles develop prominent crests and tuberosities (Fig. 8E–G). For example, a bone scar on the anterolateral edge near the deltopectoral crest (Fig. 8E, green star) is a pit on smaller specimens (TMM 31213-12, TMM 31099-11), and develops into a convex and striated bone scar in larger individuals (TMM 31025-263, TMM 31213-8). Additionally, all the humeri of the larger specimens of A. madagaskarensis (Nesbitt et al. 2015) and S. indicus (Sengupta et al. 2017), and the holotype of P. dolichotrachela (ISIR 316/1), have this rounded scar (= ridge in Nesbitt et al. 2015). The greater expansion of the proximal end of the humerus of the large forms (TMM 31025-263) is in part a consequence of the lateral expansion of the deltopectoral crest relative to the smaller forms (TMM 31099-11; Fig. 8A, B). An intermediate-sized specimen (TMM 31213-8) has the enlarged deltopectoral crest, but it is clear from the lack of co-ossification with the rest of the humeral surface that this part of the deltopectoral crest ossified just prior to the individual’s death (Fig. 8E). A proximodistally oriented ridge on the dorsolateral surface is absent in small forms (TMM 31099-11) but is clearly present in the larger forms (TMM 31025-263; TMM 31213-8). A prominent bone scar on the dorsal surface of the proximal portion near the medial edge in TMM 31025-263 (Fig. 8F, G) is absent in all of the small forms. Thus, all of the differences between the smallest and largest forms appear to be explained by ontogenetic shifts.
Histology
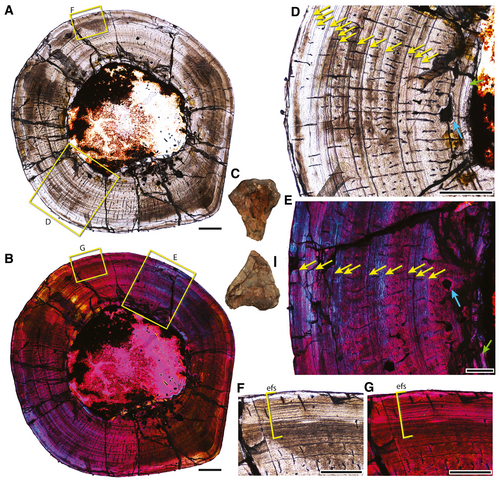
We sectioned one large humerus (PEFO 38627: estimated length, 110 mm; circumference at midshaft, 37.1 mm; minimum diameter, 11.8 mm; Fig. 9) and two small and similarly sized humeri (TMM 31099-84: estimated length, 61.8 mm; circumference at midshaft, 16 mm; minimum diameter, 4.9 mm; and TMM 31099-1488: estimated length, 60.2 mm; circumference at midshaft, 16.1 mm; minimum diameter, 5.1 mm; Fig. 10) of Malerisaurus-like taxa to examine growth patterns and test our hypothesis that these may represent different ontogenetic states of the same or closely related forms. The large specimen (PEFO 38627; Fig. 9) has an exceptionally well-preserved bone microstructure. The section has a hollow medullary cavity with a thin layer of endosteal laminae delimiting the cavity (i.e. the internal circumferential layer). Secondary remodelling is minimal with only a few secondary osteons located in the inner cortex. Zones of parallel-fibred and laminar bone are present throughout the cortex, with a relative increase of laminae at the outer edge. Vascularization is poor, and the longitudinal canals are largely limited to the space between the growth marks. Radial canals vary in length through the cortex and many of these transect growth marks; there are only a few short anastomoses associated with radial canals. The number of growth marks is prominent throughout the cortex and we estimate that there are five distinct lines of arrested growth (LAGs) followed by more densely vascularized tissue. The LAGs occur in ‘packets’ (sensu Werning 2013) that consist of 2–5 closely spaced laminae. Osteocyte density is high throughout the cortex, but no pattern of arrangement is clear. The outer cortex has an external fundamental system (EFS) like that present in other reptiles, indicating the near cessation of growth (Werning 2013). The outer zone of the cortex is nearly avascular, but a few longitudinal and short radial vascular canals do occur in this zone (Fig. 9F, G).
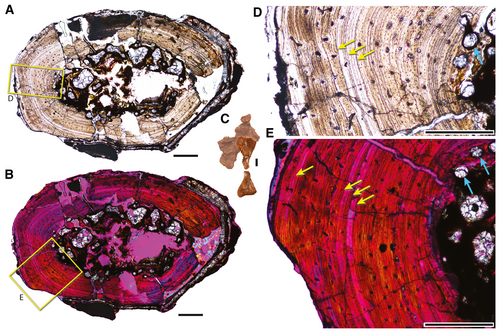
The smaller specimens (TMM 31099-84, TMM 31099-1488) have a similar histological signature as the large specimen, but do preserve some differences that are expected in ontogenetically less mature specimens. The medullary cavity of TMM 31099-84 (Fig. 10) possesses large erosion rooms, and early growth tissues still bridge the cavity, unlike the open medullary cavity of the larger specimen. Proportionally, the number of longitudinal canals is greater than the radial canals in the smaller specimens compared with the larger specimen. The cortices of the smaller specimens are marked by numerous laminae that can be traced around the cortex, and it appears that only one clear LAG is present in the middle of the cortex in each. A second LAG may be present near the medullary cavity in TMM 31099-1488. The outer cortex of both smaller specimens shows no sign of changing pattern compared with the inner cortex (i.e. no EFS is present; Fig. 10D, E).
The growth patterns in all three specimens indicate that Malerisaurus-like taxa were slow-growing, as shown by closely spaced laminae throughout the cortex with no woven bone, few longitudinal vascular canals that are largely simple, and regions of avascular bone deposition; these features are typical for slow-growing reptiles (Enlow & Brown 1957; de Buffrénil et al. 2008; Werning 2013). Compared with the humeral histology of close relatives, the histology of the humerus of Malerisaurus-like taxa is similar to that of the humerus of the larger azendohsaurid Azendohsaurus laaroussii (Cubo & Jalil 2019). The Malerisaurus-like taxa and A. laaroussii share features consistent with slow growth (e.g. laminar and parallel-fibred bone, zones of avascularity, simple longitudinal canals). This is also consistent with the growth pattern of another allokotosaurian, Tr. buettneri (Werning & Irmis 2010; Werning 2013). The bony tissues of the humeri share all of the characteristics present in A. laaroussii and Malerisaurus-like taxa. Additionally, the Malerisaurus-like taxa and Tr. buettneri share a similar pattern of vascularity (the primary vascular canals are linked by short anastomoses) that is absent in other limb bones of similar size (see Werning 2013). Among other non-archosaurian archosauromorphs, rhynchosaurs (e.g. Stenaulorhynchus stockleyi; Werning & Nesbitt 2016), the probable earliest branching species Aenigmastropheus parringtoni (at least during most of its life; Ezcurra et al. 2014), tanystropheids (Jaquier & Scheyer 2017), and the archosauriforms Vancleavea campi (Nesbitt et al. 2009) and Euparkeria capensis (Botha-Brink & Smith 2011) share similar growth signatures in their limb bones. In contrast, the archosauromorph Prolacerta broomi possesses relatively fast, uninterrupted growth, which is even faster in the early archosauriforms Proterosuchus fergusi, Chanaresuchus bonapartei and Erythrosuchus africanus (Botha-Brink & Smith 2011). As a result, the long bone microstructure of non-archosauriform archosauromorphs generally suggests slower growth compared with archosauriforms (Werning & Nesbitt 2016), but there are deviations from this pattern such as in the cases of Prolacerta broomi, Vancleavea campi and Euparkeria capensis (Botha-Brink & Smith 2011).
We find that the growth characteristics are consistent between the larger and smaller specimens of the Malerisaurus-like forms. Based on the similarity of histological tissues, the presence of fewer LAGs and absence of an EFS, the smaller specimens were still actively growing, in contrast with the larger specimen. Therefore, these observations support that small specimens near the size of the holotype of M. langstoni could grow into the larger, more robust forms that share additional similarities with the large bodied Azendohsaurus spp. + Shringasaurus indicus clade. It appears that M. langstoni and closely related forms had moderately to poorly vascularized parallel-fibred and/or lamellar bone for a significant portion of their growth.
Distribution in the Chinle Formation and Dockum Group
Malerisaurus-like azendohsaurids are relatively common in Upper Triassic strata across the Dockum Group of Texas and central New Mexico and the Chinle Formation of eastern Arizona (Fig. 11). Otischalkian occurrences are currently restricted to the Colorado City Formation of the Dockum Group of Texas. The holotype specimen of M. langstoni (TMM 31099-11, partial skeleton) is from Otis Chalk Quarry 2 (TMM 31099) (Chatterjee 1980). Otis Chalk Quarry 1 (TMM 31025), from which the holotype and paratype specimens of ‘Otischalkia elderae’ (TMM 31025-263 and TMM 31025-262, respectively) were recovered (Hunt & Lucas 1991a), is usually treated as part of a composite Otis Chalk assemblage (type Otischalkian land vertebrate faunachron of Lucas & Hunt 1993; see also Hunt & Lucas 1991a, b; Long & Murry 1995; Lucas 1998; Stocker 2013) because of a lack of superpositional data from the original excavations and a lack of detailed lithostratigraphy that encompasses all Otis Chalk localities. These localities form the basis of the Otischalkian because of the presence of non-leptosuchomorph phytosaurs (Parasuchus bransoni; TMM 31025-172 in Otis Chalk Quarry 1; TMM 31100-101 in Quarry 3; Hunt & Lucas 1991b; Long & Murry 1995; Stocker 2013; Martz & Parker 2017). Outside of the Otis Chalk localities, the Griffith Ranch Site (MOTT 3629/TMM 31213) in the ‘pre-Tecovas horizon’ of Borden County, Texas, is probably from a similar horizon in the Colorado City Formation, and it also preserves a Malerisaurus-like taxon (e.g. TMM 31213-11, humerus; TMM 31213-12, humerus; TMM 31213-13, cervical vertebra) and remains referred to ‘a new species of Otischalkia’ (Voss 2018). The Boren/Neyland Quarry (MOTT 3869) in Garza County, Texas, also preserves Malerisaurus-like azendohsaurids (e.g. TTU-P10346, cervical vertebra; TTU-P10563, femur; TTU-P10565, humerus). This site can also be placed in the Otischalkian (e.g. TTU-P9423, Paleorhinus cf. P. sawini; Martz 2008) and occurs just above the Boren Ranch sandstone that has been dated at c. 225 Ma (Rb–Sr age; Long & Lehman 1993, 1994; Long 2009). Adamanian occurrences of Malerisaurus-like azendohsaurids in western Texas are from the Post (Miller) Quarry (MOTT 3624) in Garza County and the Rotten Hill/Sierrita de la Cruz Creek area further north in Potter County (Long & Murry 1995, fig. 15e–g). The Post Quarry, the upper part of the lower unit of the Cooper Canyon Formation, preserves a cervical vertebra (TTU-P11338) of a Malerisaurus-like taxon. That site is bracketed by <225 Ma and c. 211 Ma Rb–Sr ages (Long & Lehman 1993, 1994; Long 2009), and is Adamanian owing to the presence of non-mystriosuchine leptosuchomorph phytosaurs (e.g. TTU-P9234; Martz et al. 2013; Martz & Parker 2017). The Rotten Hill/Sierrita de la Cruz Creek azendohsaurid specimens (UMMP 12972, humerus; PPHM WT 3206, humerus) are from the lower part of the Tecovas Formation (Dockum Group). The Rotten Hill bonebed of the metoposaurid temnospondyl Anaschisma browni (NMMNH L-8997) preserves a non-mystriosuchine leptosuchomorph phytosaur attributed to Smilosuchus gregorii (e.g. PPHM WT 3217; Lucas et al. 2016).
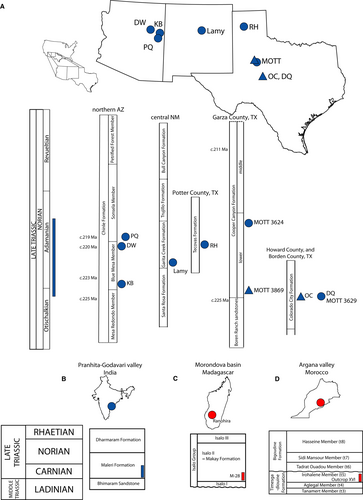
The only occurrence of a Malerisaurus-like azendohsaurid in New Mexico is from the Lamy Quarry (NMMNH L-1176; Fig. 4E, F) in the Garita Creek Formation (Dockum Group) of Santa Fe County (Hégron et al. 2020). The Lamy Quarry preserves a hemimandible (MCZ 101531) and cervical vertebra (MCZ 101539; Fig. 4E) of a Malerisaurus-like azendohsaurid among the numerous remains of the metoposaurid temnospondyl Anaschisma browni (Gee et al. 2020). The Lamy Quarry has been hypothesized to be Adamanian (Lucas et al. 2010); we have not reviewed phytosaur specimens that would substantiate that designation.
Three localities in the Chinle Formation of Apache County, Arizona, preserve Malerisaurus-like azendohsaurids, and all are in the Adamanian teilzone. A monodominant bonebed of a new Malerisaurus-like taxon occurs in the upper part of the Blue Mesa Member at Petrified Forest National Park (Marsh et al. 2017). This taxon from Dinosaur Wash (PFV 217) is represented by hundreds of bones (e.g. PEFO 43914, associated premaxilla and maxilla; PEFO 43992, cervical vertebra; PEFO 38627, humerus) from individuals of varying ontogenetic stage. A similar assemblage occurs at the Krzyzanowski bonebed (NMMNH L-3764) lower in the Blue Mesa Member near St Johns, Arizona (Spielmann et al. 2013), and includes a dentary (NMMNH P-60127) and cervical vertebra (NMMNH P-60208), among other elements (Fig. 4). The Blue Mesa Member above the Newspaper Rock bed at Petrified Forest National Park is Adamanian (e.g. Smilosuchus adamanensis UCMP 26699); however, the Newspaper Rock bed facies does not occur at NMMNH L-3764, and the quarry does not preserve diagnostic phytosaur remains. High-resolution U–Pb ages from outcrops bracket the Blue Mesa Member; the age of the lower part of the member near Petrified Forest National Park is between 225.185 ± 0.079 Ma and 223.036 ± 0.059 Ma (Ramezani et al. 2011), and the upper part of the Blue Mesa Member at Petrified Forest National Park is 220.124 ± 0.068 Ma in age (Atchley et al. 2013). The second youngest single crystal U–Pb age from the uppermost part of the Blue Mesa Member in a recent core (age model 1; Rasmussen et al. 2021) is 220.54 ± 0.96 Ma.
Malerisaurus-like azendohsaurids may occur in the Lot’s Wife beds or equivalent strata in the lower part of the Sonsela Member of the Chinle Formation near St Johns (219.39 ± 0.12 Ma; Ramezani et al. 2014) at the Placerias Quarry (UCMP A269/MNA 207-2). Two ilia (MNA V2956 and MNA V2928) and the distal end of a femur (MNA V2969) may belong to early diverging allokotosaurs, but they may also be identified as Trilophosaurus, which is represented by articulated sacral vertebrae (MNA V2894, originally referred to Desmatosuchus by Long & Murry 1995). No azendohsaurids are known from Revueltian rocks in western USA, and indeed, the only allokotosaur to occur in Revueltian strata in the area is Trilophosaurus phasmalophos from the Jim Camp Wash beds (Sonsela Member, Chinle Formation, c. 214.08 ± 0.20 Ma; Kent et al. 2018; Kligman et al. 2020; Rasmussen et al. 2021) at and near Petrified Forest National Park, which contains mystriosuchine phytosaurs at localities in similar stratigraphic horizons (e.g. PEFO 34042, Machaeroprosopus pristinus; Parker & Martz 2011).
Biogeography
Malerisaurus-like taxa were widely distributed in the early part of the Late Triassic, with a much richer fossil record than previously appreciated. Specimens of these taxa are now recognized from multiple localities across western North America and India. This distribution pattern across western North America and India is paralleled by some Triassic clades (e.g. the phytosaurs Parasuchus spp., the protopyknosians Triopticus primus and Kranosaura kuttyi) but not others (e.g. sauropodomorphs; Ezcurra 2010; Novas et al. 2011). The majority of these common clades are Otischalkian in age (by definition, the Parasuchus material is Otischalkian), although Kranosaura is known from the Adamanian upper Maleri Formation (Nesbitt et al. 2021b). However, the upper Maleri Formation also has at least two sauropodomorph species that are probably closely related to South American taxa, with no confirmed allokotosaur material from those South American sites. Thus, the tetrapod assemblages of both the lower and upper Maleri Formation appear to have some biogeographical affinities with South American and North American and European tetrapod assemblages (Ezcurra 2010; Novas et al. 2011). Upper Triassic deposits in both India and western North America were formed within the ‘summer wet’ biome with monsoonal conditions, differing from central South America, which is reconstructed as arid (Sellwood & Valdes 2006), so a possible explanation of this pattern of taxon distribution is that Malerisaurus-like taxa may have preferred wetter environments.
Implications for stem archosauromorph diversity
Our supported hypothesis that small, slender M. langstoni holotype-sized forms grew into larger stocky forms (previously ‘Otischalkia’) more similar to Azendohsaurus has a number of important implications for the diversity, biogeography, and biochronology of archosauromorphs from the Upper Triassic deposits of the Chinle and Dockum basins. First, our re-identification of ‘Otischalkia’ as an azendohsaurid, and possibly a large individual of M. langstoni, eliminates the rhynchosaur record (see Hunt & Lucas 1991a, b) from the entire Chinle Formation and Dockum Group basins for their duration, and consequently, this implies that the type Otischalkian teilzone is not necessarily late Carnian like other rhynchosaur-bearing deposits across Pangaea (Hunt & Lucas 1991a, b; Lucas 1998; Lucas et al. 2002). Rhynchosaurs are known from Triassic strata from Wyoming (Lucas et al. 2002), and a specimen reported from Natrona Country in eastern Wyoming was used as additional evidence for correlation of the Popo Agie Formation with the Otischalkian. However, the rhynchosaur specimen reported by Lucas et al. (2002) was not found associated directly with Parasuchus, and its original location was probably not in Natrona County (MRS & D. Lovelace unpub. data; Stocker 2013). The Triassic exposures in and around Natrona County, including the Heptasuchus locality, are nearly all exposures of stratigraphically lower units in the Chugwater Group, rather than in the Popo Agie Formation as was reported by Dawley et al. (1979). Recent Sr87/Sr86 isotopic data support a Spathian to earliest Anisian age for the Alcova Limestone (Lovelace 2012). The Heptasuchus locality lies c. 10 m stratigraphically above the Crow Mountain Formation in the ‘unnamed redbeds’, and below the few exposures of the Popo Agie Formation (D. Lovelace & MRS unpub. data; Stocker 2013), and the age of the Heptasuchus locality is poorly constrained (Nesbitt et al. 2020). Therefore, correlations to the Otischalkian based on the Wyoming Hyperodapedon occurrence or using Heptasuchus are questionable and likely to be incorrect, and any correlations should be restricted to be with the type locality of Parasuchus bransoni in the Popo Agie Formation of Fremont County, Wyoming. The exact age of these deposits is not clear and could be entirely older than that of the bone-bearing localities of the Chinle Formation and Dockum Group (Stocker 2013; Nesbitt et al. 2020).
The remains of Malerisaurus-like taxa have been assigned to various archosauromorph clades (see above), and early phylogenetic studies of Malerisaurus considered the taxon to be a member of the long-necked ‘Protorosauria’ or ‘Prolacertiformes’. However, recent phylogenetic studies have shown that ‘protorosaurs’ represent a wide range of plesiomorphic early branching archosauromorphs, masking the phylogenetic diversity of lineages in the Triassic (e.g. Pritchard et al. 2015; Ezcurra 2016). Our interpretation demonstrates that Malerisaurus-like taxa were abundant up through the Adamanian. Furthermore, the identification of Malerisaurus-like taxa using apomorphies has shown that specimens once assigned to Trilophosaurus are actually Malerisaurus-like taxa, or else that the two taxa cannot be separated based on some isolated bones. Consequently, the abundance and distribution of non-dental specimens of Trilophosaurus within the Adamanian is lower than previously thought.
The presence of carnivorous, long-snouted, and long-necked azendohsaurids in the Adamanian (early Norian) represents a relict or plesiomorphic lineage of early diverging azendohsaurids that apparently survived well after highly divergent, large, and herbivorous azendohsaurids disappear from the fossil record. To date, these smaller and carnivorous forms have not been found with their larger and herbivorous relatives anywhere in Pangaea. The Malerisaurus-like taxa are the last known members of the azendohsaurid lineage and are among the last non-archosaurian archosauromorphs with tanystropheids (Pritchard et al. 2015) and trilophosaurids (Sidor et al. 2018; Kligman et al. 2020). However, Malerisaurus-like taxa went extinct at or near the Adamanian–Revueltian boundary in the mid-Norian at c. 216 Ma, well before the disappearance of tanystropheids (Pritchard et al. 2015) and trilophosaurids (Robinson 1957; Skinner et al. 2020) in North America and Europe.
The small head, slender neck, elongate cervical vertebrae, and sprawled limbs of Malerisaurus all recall the bauplan of many non-archosauriform archosauromorphs ranging from the late Permian to the Middle Triassic. These anatomical traits were long-used to arrange these long-necked forms into a grouping variably named ‘Prolacertiformes’ or ‘Protorosauria’ (e.g. Huene 1954; Gow 1975; Carroll & Currie 1991). However, cladistic analyses, beginning with Dilkes (1998), began to recognize that this grouping was polyphyletic, with lineages spread throughout Archosauromorpha (e.g. Gottmann-Quesada & Sander 2009; Ezcurra et al. 2014; Pritchard et al. 2015; Ezcurra 2016; Pritchard & Nesbitt 2017; Spiekman et al. 2021). Indeed, Protorosaurus speneri, one of the oldest known and earliest diverging archosauromorphs, bears all of these traits, as do a range of taxa that were globally distributed during the first 10 myr of the Triassic (e.g. Gow 1975; Bartholomai 1979; Colbert 1987; Benton & Allen 1997; Borsuk-Bialynicka & Evans 2009). Derived members of Tanystropheidae represent an extreme case, with additions to the numbers of cervical vertebrae, further elongation of the centra, and (in some taxa) procoely. However, it is Malerisaurus that represents the last of the Protorosaurus-like bauplan known in the fossil record. Most ecological considerations of ‘protorosaurs’ envision them as secondary consumers, preying upon small vertebrates and possibly insects (e.g. Gow 1975), although conifer ovules have been identified in the gut region of specimens of Protorosaurus speneri (Karlsruhe & Sues 1993), and remains of teleost fishes and cephalopod hooks have been identified as stomach contents in the highly specialized tanystropheids Dinocephalosaurus and Tanystropheus, respectively (Renesto 2005; Liu et al. 2017). Regardless of their specific dietary preferences or ecological roles, archosauromorphs with a ‘protorosaur’ bauplan persisted on multiple continents and in a wide range of environments through to the Late Triassic, with Malerisaurus-like taxa as the latest known representatives in the fossil record.
Conclusion
Malerisaurus is a valid taxon containing both M. langstoni from the Dockum Group (Upper Triassic of western USA) and M. robinsonae from the lower Maleri Formation (lower Upper Triassic of India). Within Archosauromorpha and Allokotosauria, Malerisaurus and closely related taxa comprise the clade Malerisaurinae and this group is an early diverging member of Azendohsauridae. All members of Malerisaurinae were carnivorous and much smaller than other members of Azendohsauridae. Malerisaurus langstoni and a number of Malerisaurus-like taxa from the Late Triassic of southwestern USA are abundant in the Otischalkian and Adamanian teilzones but disappear before the start of the Revueltian teilzone. Using histology and documented growth changes, we demonstrate that various growth stages of the clade are present, ranging from the smaller holotype of M. langstoni to larger forms that have been previously identified as other taxa; for example, we find that the purported rhynchosaur ‘Otischalkia elderae’ is nomen dubium and probably a larger form of M. langstoni. The similarity of early diverging azendohsaurids in south-western USA and India strengthen a faunal connection across the summer wet biome of Pangaea in the Late Triassic.
Acknowledgements
This paper is dedicated to Bill Mueller, who passed away prior to the completion of the project. We thank Matthew Smith (PEFO), Jessica Cundiff (MCZ), Chris Sagebiel (TMM), Kendra Dean Wallace (TTU), Sankar Chatterjee (TTU), and Matthew Brown (TMM) for specimen curation help and for allowing us to access the collections under their care. We thank Christopher Griffin and Sarah Werning for help imaging the histology slides. Kenneth Bader (TMM) prepared large Malerisaurus material from Otis Chalk Quarry 1. We thank Gretchen Gürtler (GR) for collecting and cataloguing a number of the TTU specimens. We thank Christopher Griffin for help and code for the R analyses. Christian Kammerer is thanked for discussing the taxonomy of Malerisaurus. Access to the free version of TNT 1.5 was possible due to the Willi Henning Society. SJN was funded by the National Science Foundation CAREER program (NSF EAR 1943286) and MDE’s research is funded by Agencia Nacional de Promoción Científica y Técnica in the context of the Complete Archosauromorph Tree Project (CoArTreeP) (PICT 2018–01186). This is PEFO contribution number 83. The conclusions presented here are those of the authors and do not represent the views of the US Government. We thank Felipe Pinheiro and an anonymous reviewer for providing helpful feedback and thank Daniel Field for handling the paper.
Author contributions
Conceptualization S. J. Nesbitt, M. R. Stocker, B. Mueller, N. C. Fraser; Investigation S. J. Nesbitt, M. R. Stocker, M. D. Ezcurra, N. C. Fraser, A. B. Heckert, W. B. Parker, B. Mueller, S. Sengupta, S. Bandyopadhyay, A. C. Pritchard, A. D. Marsh; Phylogenetic Analysis S. J. Nesbitt, M. R. Stocker, M. D. Ezcurra, A. D. Marsh; Data Curation S. J. Nesbitt, M. D. Ezcurra; Visualization S. J. Nesbitt, M. R. Stocker, M. D. Ezcurra, A. D. Marsh; Writing – Original Draft Preparation S. J. Nesbitt, M. R. Stocker, M. D. Ezcurra, S. Sengupta, S. Bandyopadhyay, A. C. Pritchard, A. D. Marsh; Writing – Review & Editing S. J. Nesbitt, M. R. Stocker, M. D. Ezcurra, N. C. Fraser, A. B. Heckert, W. G. Parker, B. Mueller, S. Sengupta, S. Bandyopadhyay, A. C. Pritchard, A. D. Marsh.
Open Research
Data archiving statement
The data matrix for this paper is archived in MorphoBank: http://morphobank.org/permalink/?P3970 This published work has been registered in ZooBank: http://zoobank.org/References/4E9E652E-97B5-4988-876A-1A49B9160816




