An early Cambrian mackenziid reveals links to modular Ediacaran macro-organisms
Abstract
Many Ediacaran macrofossils are apparently composed of unique pneus (similar, repetitive tubular modules) forming a variety of body shapes by combinations of modular growth regimes. The large-scale disappearance of Ediacaran macro-organisms at the end of the Precambrian leaves this now extinct modular body plan only rarely reported from the Cambrian onwards. Mackenzia costalis is an enigmatic and poorly understood soft-bodied organism from the middle Cambrian, and is a possible surviving member of the Ediacaran macrobiota. Here, we describe a new mackenziid, Paramackenzia canalifera, from the early Cambrian Chengjiang biota, Yunnan Province, south-west China. It has a body plan comprising longitudinal tubular modules that are radially arranged to form a cylindrical body with a basal attachment. Each tubular module is enclosed by an inner and outer concentric membrane, separated from adjacent modules by a thin lamellar sheet. Infilling sediment and preservation of dark, basal stains at the inner and outer membranes reveal a pore–canal system within the lamellae, connecting the internal cavity to the exterior. This arrangement of tubular modules is similar to that of several macroscopic Ediacaran species, including erniettomorphs and dickinsoniomorphs, which were constructed of serial hydrostatic modules referred to as ‘pneus’. The mackenziid pore system is similar to that of sponges and archaeocyathids in function and feeding strategy, but the overall anatomy precludes affiliation to this phylum. We suggest that mackenziids are most likely to be a blastic or diploblastic anatomical grade of stem eumetazoans, similar to other modular Ediacaran macro-organisms.
Ediacaran organisms document, from a neontological perspective, a range of bizarre body plans that increasingly are considered to be mostly representatives of the first macroscopic animals (Xiao & Laflamme 2009; Droser & Gehling 2015). They exhibit diverse body architectures and probably represent a polyphyletic or paraphyletic group, but their unusual body plans and the poor preservation of soft tissues mean that only a few fossils currently could be affiliated with living taxa, such as the fossil Kimberella, a potential bilaterian with several possible molluscan synapomorphies (Fedonkin & Waggoner 1997; Vinther 2015). The diverse macrobiota largely disappeared around the boundary between the Ediacaran and the Cambrian (Laflamme et al. 2013; Darroch et al. 2018).
In contrast, the Cambrian fortuitously has a number of deposits preserving soft tissues, most importantly the Burgess Shale-type (BST) Lagerstätten. The fossils preserved in these deposits record the early diversification of animal phyla (e.g. Briggs et al. 1994; Hou et al. 2017; Lerosey-Aubril et al. 2018), with stem lineages preserving characters that can help resolve anatomical evolution (e.g. Vinther & Parry 2019; Zhao et al. 2019; Nanglu et al. 2020) and phylogenetic topology (Mongiardino Koch et al. 2021). The Cambrian is also a particular interval in evolutionary history when extant animal phyla became established and the major branches in the animal tree of life diversified (Erwin et al. 2011; Budd 2013; Shu et al. 2014).
However, the understanding of the nature and affinity of Cambrian fossils is not without its challenges and controversies. The scarcity of fossils, the inadequacy of the morphological characters available, or the large anatomical gaps between extinct and extant organisms are some of the sources of these controversies. Enigmatic fossils may have been equivocally placed in different parts of the tree due to (some of) these reasons and remain categorized as ‘Problematica’ (Butterfield 2003; Donoghue & Purnell 2009). Of these Cambrian Problematica, some fossils have been considered as the surviving members of Ediacaran macro-organisms (Budd & Jensen 2017). Because of the different mechanisms and style of preservation, those Cambrian fossils could offer novel insights into the nature and affinity of their clades. Such an example is Stromatoveris, a Cambrian Problematicum initially affiliated to ctenophores (Shu et al. 2006), but more recently linked to the Ediacaran Petalonamae and placed in the eumetazoan stem lineage (Hoyal Cuthill & Han 2018).
Another proposed Ediacaran holdover is Mackenzia costalis Walcott, 1911 initially described from the Burgess Shale. Mackenzia costalis was described as an atentaculate, bag-shaped body that bears 8–10 longitudinal bands radially arranged into a hollow cylinder (Walcott 1911). It was latterly noted that the elongate body of M. costalis has superficial similarities to a few of the Ediacaran genera, including Platypholinia pholiata, Inaria karli and Protechiurus edmondsi (Conway Morris 1993). However, the detailed anatomy of M. costalis remains to be fully understood, especially with regard to whether the three-dimensional architecture is consistent with the modular and functional arrangement observed in the Ediacaran comparative taxa.
Here, we report a new mackenziid from the early Cambrian (Epoch 2, Age 3) Chengjiang Lagerstätte from the Yunnan Province in south-west China, and hence extend the geological range further back in time. The material is well preserved and, due to infilling sediment and organic preservation, reveals novel aspects of the internal anatomy. We show that the mackenziid body plan, which is composed of tubular modules, resembles the modular organization of hollow ‘pneus’ in the Ediacaran erniettomorphs and dickinsoniomorphs. The new body reconstruction provides novel functional insights into the mackenziid body plan and its possible phylogenetic position.
Method
All specimens were examined and prepared under a Leica M205C stereomicroscope. Cross-polarized high-angle and low-angle light was used for photography with either a Leica DFC 5000 camera mounted on a Leica M205C microscope or a Canon EOS 5DS R digital camera mounted with Canon EF 100 mm or Canon MP-E 65 mm (1–5×) macro lenses. Fluorescence imaging was captured with a Leica M205 FA fluorescence microscope mounted with a Leica DFC7000 T digital camera. The interpretative drawings were made using camera lucida tracings and photographs. All figures were processed in Adobe Photoshop CC 2019. The body plan reconstruction was created in Adobe Illustrator CC 2019.
Material and preservation
Seven specimens were collected on a field trip in 2013, from the Erjie section, Jinning in the Kunming area, eastern Yunnan, China (see Hou et al. 2017, p. 22 for locality information). The fossil strata correspond to the Eoredlichia–Wutingaspis trilobite Biozone of the Yu’anshan Member of the Chiungchussu Formation, lower Cambrian (Series 2, Stage 3).
The Chengjiang biota is preserved in a unit consisting of mudstone bearing two distinct sedimentological regimes, namely the background beds and the event beds (Zhu et al. 2001; Forchielli et al. 2014). The event beds are mainly sourced for exceptional fossils and represent abrupt deposition over a short interval into beds of one to several inches thick, probably caused by episodic storm events onto a sea floor close to the oxycline (Zhu et al. 2001).
All studied specimens were from the event beds: fine-grained, well-weathered yellowish mudstone. The rapid deposition implies a regime in which the specimens experienced limited transportation and are generally preserved in high fidelity. Several specimens have lost parts of the body, due to the elongate body size and the irregular splitting of slabs. All specimens are isolated, with no sign of association to any debris at their basal attachment site. They are laterally compressed with the body parallel/sub-parallel to bedding surfaces. The body does not appear to have been sclerotized, but maintains a relatively straight orientation, with some being slightly curved in the distal region (as in YKLP 13348a). The body is preserved as a reddish-brown thin film consisting of aluminosilicate and iron oxide minerals. Evident pyritization (now weathered iron oxides) is prevalent at the lateral margin due to compaction and at the most proximal region of the body. The dark grey, sometimes black parts as seen in certain parts of the body largely represent carbonaceous films of more oxidation-resistant organic matter (Zhu et al. 2005). The fossils occasionally exhibit minor relief due to sediment infilling of the internal cavity and digitate pockets that are observed in the longitudinal strands (see description below).
Institutional abbreviation
YKLP, Yunnan Key Laboratory for Palaeobiology, Yunnan University, Kunming, China.
Systematic palaeontology
Kingdom ANIMALIA Linnaeus, 1758
Total Group EUMETAZOA Bütschli, 1910
Phylum INCERTAE SEDIS
Family MACKENZIIDAE Conway Morris, 1993
Type genus
Mackenzia Walcott, 1911
Amended diagnosis
(Amended after Conway Morris 1993, p. 606.) Bag-like metazoan with an atentaculate, elongate body, characterized by the radial arrangement of longitudinal tubular modules enclosed by an inner and outer membrane and septal lamellae. A basal stalk is present.
Remarks
Mackenziidae was erected by Simon Conway Morris when he re-described Mackenzia costalis from the Burgess Shale (Conway Morris 1993). The new taxon described from the Chengjiang confirms the body shape of Mackenziidae as being atentaculate, elongate and cylindrical. One specimen, ?Mackenzia sp. from the Cambrian Stage 4 in the Three Gorges area, China (Chang et al. 2018), has also been referred to this genus (but see below). Based on specimens from Chengjiang, the longitudinal folds of M. costalis are re-interpreted as hollow tubular modules, while the intervening ridges refer to the imprints of septal lamellae. Thus, the mesentery-lining columnar body is reconstructed as a modular body composed of repetitive tubular modules. A cnidarian affinity of Mackenziidae is rejected accordingly. We tentatively treat the family Mackenziidae as a non-bilaterian metazoan, probably in the eumetazoan total group.
Genera included
Mackenzia Walcott, 1911; Paramackenzia gen. nov.
Occurrence
Cambrian unnamed Series 2, Stage 3 (Chengjiang biota, south-west China) and Miaolingian Series, Wuliuan Stage (Burgess Shale, Canada).
Genus PARAMACKENZIA nov.
LSID
urn:lsid:zoobank.org:act:4771DB33-3E11-4822-974A-F8FAF9DD994E
Derivation of name
Greek para-, similar, plus Mackenzia, the name of the type genus of mackenziids, referring to the similarities to the type genus.
Type species
Paramackenzia canalifera sp. nov.
Diagnosis
As for type and only species.
Remarks
Paramackenzia shares with Mackenzia from the Burgess Shale an elongate bag-like body, consisting of longitudinal tubular modules separated by septal lamellae, indicating that the new taxon can also be assigned to the family Mackenziidae Conway Morris, 1993. However, the proximal end of Mackenzia is blunt and attaches to a holdfast; occasionally a short stalk can be discerned (Conway Morris 1993). In contrast, the proximal region of Paramackenzia has a more distinct stalk (as in YKLP 13348, 13350). The number of tubular modules is different between Paramackenzia and Mackenzia, with the latter having fewer modules (Table 1). In addition, the pore–canal system identified in Paramackenzia is lacking in Mackenzia, although this could be a preservational artefact. Given the difference in the total number of axial modules and the c. 10 myr of stratigraphic age separation, we treat the new taxon as a separate new genus, Paramackenzia gen. nov.
| Specimens (species) | Occurrence | Recorded length | Maximum width | Number of modules exposed | Number of total modules |
|---|---|---|---|---|---|
| YKLP 13345a | Cambrian Stage 3 | 72 mm* | 26 mm | ≈6 | ≈17–20# |
| YKLP 13346a | 109 mm* | 18 mm | 6 | 16–20# | |
| YKLP 13347a | 87 mm* | 20 mm | 8–9 | 24–28# | |
| YKLP 13348a | 116 mm | 18 mm | 6 | 16–18# | |
| YKLP 13350a | 21 mm | 13 mm | 9 | 26–28# | |
| YKLP 13482a | 88 mm* | 27 mm | ≈9 | ≈21–24# | |
| YKLP 13483 | 21 mm* | 8 mm* | 4* | / | |
| Mackenzia costalis | Cambrian Wuliuan Stage | 25–200 mm (mean: 86.5 mm) | ≈9–34 mm | / | 8–10 |
- *Incomplete specimen, the recorded value is less than the actual value.
- #The number is inferred by dividing the calculated circumference (diameter multiplied by π) by the width of a medially exposed module.
- /No exact data available.
Another possible mackenziid, ?Mackenzia sp., was described based on one small and incomplete specimen (7.4 mm in length) (Chang et al. 2018). The specimen is narrower (0.98–1.54 mm in width) than either Mackenzia or Paramackenzia (Table 1) and its preserved state possibly suggests a more robust body wall (see below). With those distinct differences, its assignment to Mackenziidae remains uncertain.
Paramackenzia canalifera sp. nov.
Figures 1-3, 4?
LSID
urn:lsid:zoobank.org:act:B8C81F20-FE02-4DF0-9723-84B48D6E502B
Derivation of name
Latin canalis, means tubular passage, referring to the interlamellar pore–canal system in the new taxon.
Type material
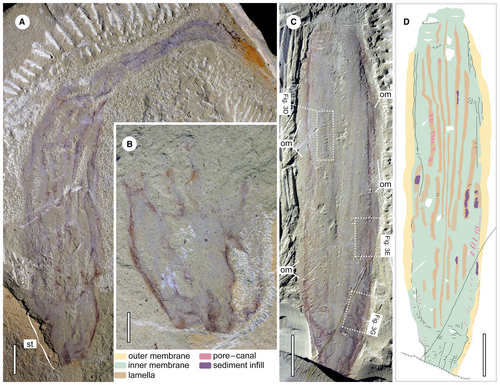
Holotype, YKLP 13348 (Fig. 1A, B), a nearly complete specimen with part and counterpart. The part exposes a prominent stalk and most of the elongate body, which is folded on itself in the distal region. The counterpart retains only the stalk due to irregular splitting of the slab. The paratype, YKLP 13347 (Fig. 1C, D), is a specimen with high fidelity preservation of the main body, showing the two-layer membranes and the pore–canal system, but lacking the stalk region.
Other figured material
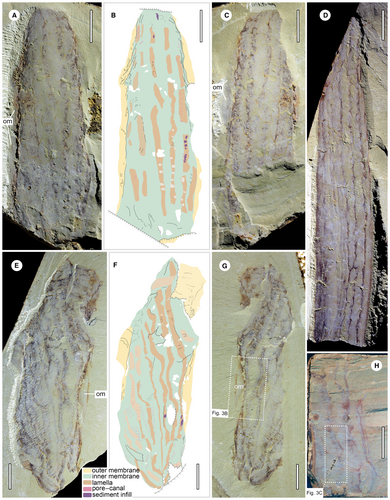
YKLP 13345a, b (Fig. 2A–C); 13346a (Fig. 2D); 13482a, b (Figs 2E–G, 3B); 13483 (Figs 2H, 3C) and 13350a, b (Fig. 4).
Diagnosis
A bag-shaped mackenziid with an elongate body and a prominent stalk. The body consists of hydrostatic tubular modules, with a regular pore–canal system in the septal lamellae, separating the modules.
Description
Paramackenzia canalifera is elongate, cylindrical in shape with a prominent stalk and a slender body. In the holotype, YKLP 13348, the stalk is robust with a trapezoidal outline in lateral view (Fig. 1A, B), being c. 15 mm in width and decreasing to 6 mm at the proximal end. The membrane along the margin is thicker, probably due to compactional folding, making the margin darker in colour. The proximal end of the stalk is blunt, with no apparent holdfast or similar structure (Fig. 1B).
Paramackenzia canalifera appears to have been smaller than Mackenzia costalis (Table 1), with a maximum length of 116 mm in YKLP 13348a. In other studied material, the preserved dimensions range from 72 to 109 mm in length, with a small, incomplete individual of 21 mm in length (YKLP 13483). Albeit incomplete, we do not consider it likely that any specimen would reach lengths of up to 200 mm, as observed in the largest available specimens of M. costalis (e.g. ROM 61115; https://burgess-shale.rom.on.ca). The body is usually straight, without strong curvature or twisting, except for in the holotype YKLP 13348, in which the distal area is curved and folded on itself (Fig. 3A) where it sharply decreases in width from 18 to 3–4 mm (due to folding).
The central body in some specimens is preserved with more relief due to sediment infill, while the outer margin of the body collapses to a thin film. Thicker folded membrane can be observed along the lateral margins in YKLP 13345 (Fig. 2A, C), 13347 (Fig. 1C) and 13482 (Fig. 2E, G), and the other smooth membrane extends outwards from the folded parts (Fig. 3B). Given these observations, the presence of an inner and outer membrane is surmised.
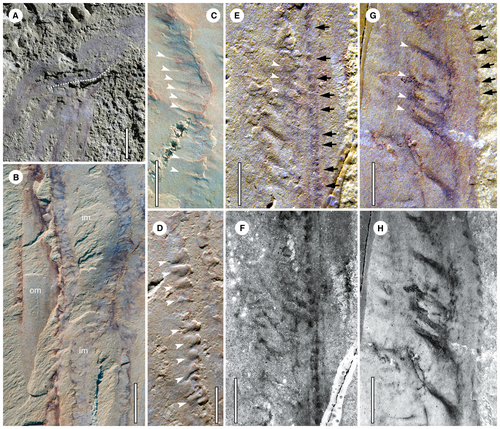
Longitudinal dark strands, sometimes preserved as furrows (Fig. 2D), emerge along the stalk region and extend distally (Fig. 1A). Based on the topological position, size and shape they are considered the same structure as the so-called ‘reflective strands’ in M. costalis (Conway Morris 1993). Some of the dark strands in P. canaliferum are obscure (Figs 1A, 2D) and appear to have twisted or displaced themselves laterally (Fig. 2E, G) upon compaction. In most cases, they are observed to be continuous along the body. The width of the dark strands varies between different specimens and within the same specimen (Figs 1C, D, 2E, F). The exact number of dark strands is difficult to determine due to the incomplete preservation and overlap. In a few specimens conflation with modules exposed from the opposite side further complicate the assessment. When counting the modules clearly exposed on the top surface of the fossil, the number ranges from a maximum of 9 to a minimum of 6 (Table 1). This would suggest a total number of 12–18 modules. However, it is also feasible that some modules along the margins are compacted into an undiscernible layer, which would give rise to a higher total number. BST fossils have been shown to retain their lateral dimensions during sediment compaction (Briggs & Williams 1981). Therefore, given that the circumference of a cylindrical organism can be calculated by multiplying its diameter by π, then, by dividing that by the width of a single module exposed in the middle of the specimen, a much higher number of modules, upwards of 18–28 can be estimated (Table 1).
In most specimens, small transverse tubular structures (STTSs) can be recognized in association with the dark strands. They have a regularly spaced arrangement along the length of the associated dark strands (Fig. 3C). They are preserved generally with a dark colour with variable widths (Figs 1C, D, 2, 3E–H). Some STTSs are infilled with teardrop-shaped sediment (Fig. 3D), indicating an originally hollow, tapering nature. In the lower part of the paratype (YKLP 13347), each STTS corresponds to a dark spot (DS) arranged in longitudinal rows alongside the dark strands (Fig. 3E, F). Some DSs are preserved organically along their length (Fig. 3E–H). On average, the diameter of each DS is 0.26 mm, equal to, or slightly smaller than, the inferred maximum width of associated STTSs preserved by infilling.
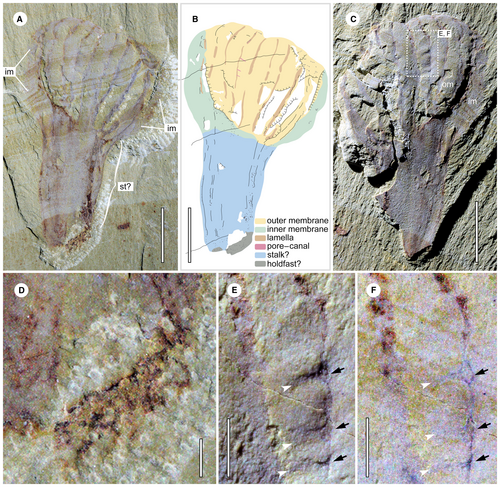
YKLP 13350a (Fig. 4) is the smallest but most complete specimen available. It is 21 mm long and up to 13 mm wide. The body appears very short compared with all known mackenziid specimens, but its complete distal end has a bilobate outline. At least 9 dark strands can be recognized on the exposed surface of the body, with the middle one delineating the separation of the two lobes (Fig. 4A–C). The dark strands form slightly concave furrows (Fig. 4A, C). This specimen has a distinctive stalk (Fig. 4A–C), which is up to 11 mm long, comprising about half of the total length of the specimen. The stalk gently tapers to 4 mm at the proximal end. The lateral edges of the stalk are slightly curved, and the surface is almost smooth, contradicting the slight relief in the section comprising the body (Fig. 4A, C). It seems that the body strands can extend into the stalk, as indicated by the weathered pyrite strip at the right side. Alternatively, this narrower ‘stalk’ can be less confidently interpreted as the main body given the poorer preservation of the dark strands. Weathered pyrite is present at the right bottom of the stalk as well, which may reflect the holdfast of the animal (Fig. 4A, B, D). STTSs are also clearly present. Intriguingly, the DSs connected with STTSs form small circles, with size evidently varying (Fig. 4E, F). Although this specimen has a relatively larger stalk and distinct proximal body end, given its small body size compared with other specimens, we tentatively treat it as a juvenile individual of P. canalifera, although the morphological differences may suggest that it is a new taxon.
The modular body plan
The outer and inner membranes as well as the sheet-shaped longitudinal strands among them indicate that the body wall of Paramackenzia canalifera is composed of longitudinal tubular modules (Fig. 5). Each module is interpreted as a hollow structure, enclosed by the outer and inner membranes and the intervening septal lamellae (preserved as the observed dark strands). The variable size of each lamella is interpreted as a consequence of the varying degrees of decay of the outer membrane during diagenesis (Fig. 5A–D). In the case that the outer membrane is poorly preserved and disappearing, rendering the lamellae fully exposed (Fig. 5A, C), this is exemplified in YKLP 13345, 13347, 13482 and 13483 (Figs 1C, 2A, C, E, G, H). In the case that the outer membrane is retained and in good preservation, the lamellae would be preserved with narrower and somewhat concave furrows, many of which are unclear because of the covering of an outer membrane (Fig. 5B, D), as seen in YKLP 13346, 13348 and 13350 (Figs 1A, 2D, 4A, C).
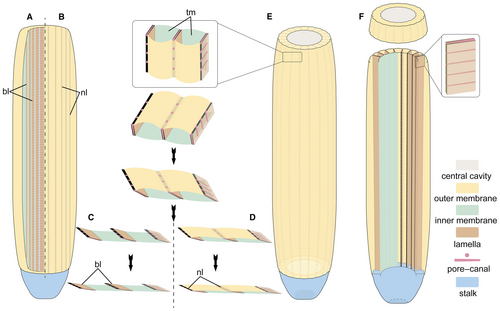
The STTSs are evenly distributed along the lamella (Fig. 3C). Some of the STTSs are infilled with sediment (Fig. 3D), indicating that they are hollow in nature. The DSs correspond well with the STTSs (Figs 3E–H, 4E, F), thus they can be interpreted as the openings of STTSs. These together are supporting evidence of a simple pore–canal system developed within each lamella (Fig. 5E, F).
With this new information, the mackenziid body plan is reconstructed as a bag-shaped, macroscopic organism, of which the elongate body forms a hollow cylinder with a wall consisting of radially arranged longitudinal tubular modules (Fig. 6). Between each module is a septal lamella harbouring an internal canal system consisting of regularly arranged pores, connecting the internal cavity to the exterior (Figs 5E, F, 6). The central cavity was probably infilled with fluid (seawater). In addition, degradational collapse and the lack of sediment infill into the tubular modules suggest that they were hollow units with no major connection to the exterior. Such a construction indicates that these modules should serve as a hydrostatic skeleton facilitating an erect shape of the large and slender body.

Comparison
The presence of a body consisting of axial longitudinal modules that serve as a hydrostatic skeleton is noteworthy. There is no apparent equivalent of such a body plan in living phyla. It is, however, widespread among many Ediacaran macro-organisms. When first identified, this type of Ediacaran construction was termed ‘a quilted pneu’ and defined as an inflatable tubular module (Seilacher 1989) (Fig. 7A). Pneus are identified as the core component of the body architecture of some Ediacaran macro-organisms (e.g. Grazhdankin & Seilacher 2002; Brasier & Antcliffe 2004; Narbonne 2004). Due to the variation in size (inflation), ontogenetic addition and arrangement between different taxa, the simple pneus can be assembled into a variety of modular body plans, such as foliate, bag-shaped, discoid, and radial (Seilacher 1989, 1992; Xiao & Laflamme 2009). Two principal types of ontogenetic combination mode have been recognized: the serial and fractal modes (Seilacher 1992, 2007).
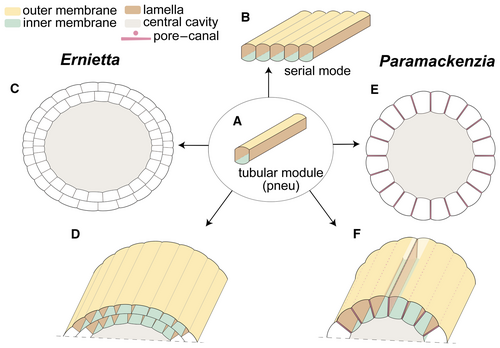
Through ontogenetic growth in serial mode, the body volume was increased by inflating and adding new modules transversely to the body axis (Seilacher 2007) (Fig. 7B). As a result, a flat quilted architecture was formed, as best exemplified by the iconic Dickinsonia (Seilacher 1989). A further complex quilted serial mode architecture consists of at least two vanes, with a seam between them, to form a flat or boat-shaped body architecture, such as in Pteridinium (Grazhdankin & Seilacher 2002). Two (or more) quilted vanes can also join and grow upward to form a cylindrical body as in Ernietta, with a zigzag seam in the basal part (Seilacher et al. 2003).
The tubular modules of mackenziids are serially arranged to form an elongate, cylindrical body shape, similar to the architecture of the erect erniettomorphs, both of which consist of axial tubular modules (Fig. 7C–F). However, the body wall of Ernietta is constituted by two-layer modules (Elliott et al. 2016). Individual Ernietta specimens appear to have had a stable number of modules through life (Ivantsov et al. 2016), with a higher number of modules than either Paramackenzia or Mackenzia. In contrast, mackenziids differ from Ernietta in having a differentiated stalk and/or holdfast at the proximal end and in lacking a seam along the base where modules join together. Nothing is known about a possible canal system in Ernietta (Elliott et al. 2016; Ivantsov et al. 2016).
Irrespective of these differences, our findings support the idea that Mackenzia is an ‘Ediacaran survivor’ in the Cambrian (Conway Morris 1993), and furthermore, they show that the body plan of mackenziids has links to the Ediacaran modular body plans composed of hydrostatic pneus. Previously, the bag-shaped body of Mackenzia had been compared with Ediacaran Platypholinia, Inaria and Protechiurus (Conway Morris 1993), of which Protechiurus is most notable. A recent study has argued that Protechiurus may be a peridermal theca, attributable to conulariids and the medusozoan cnidarians (Ivantsov et al. 2019). Remarkable similarities also deserve to be noted with Vaveliksia vana, a radially symmetric organism that possesses an attachment disc and a capsule-like body with longitudinal irregular bulging lobes, which may be derived from tubular modules but appear too irregular in comparison to mackenziids (Ivantsov et al. 2004). In summary, mackenziids are distinct from typical Ediacaran taxa in some respects, but the modular, hydrostatic pneu skeleton does affiliate it with some members of the modular Ediacaran macro-organisms.
Mackenziid biological affinity
Mackenzia was originally interpreted as a holothurian based on its cylindrical body, bearing longitudinal bands and supposedly an apical circlet of plates (Walcott 1911). This assignment was soon challenged (Clark 1912), and an affinity to the actinian family Edwardsiidae, which includes the model organism Nematostella, was put forward (Clark 1913). This interpretation assumed that the longitudinal strands in Mackenzia could be mesenteries within the columnar body of a polypoid organism, and subsequently gained traction (Croneis & McCormack 1932; Madsen 1957). The latest and most comprehensive study on Mackenzia carefully analysed c. 60 specimens (Conway Morris 1993). It also supported a placement within the cnidarians (Actiniaria) based on similar arguments to those previously made for a mesentery-lined columnar body. The claims have met criticism, however, due to the lack of distinct tentacles (Hou et al. 2005; Lech 2009) and the incongruence between the preserved characters and those predicted/expected by the experimental decay (McMahon et al. 2017). There are flaws, however, in the idea of directly relating decay stages to fossils, because it ignores the importance of the relative preservation potential of tissues, which acts independently of the rate of decay (Parry et al. 2018). Meanwhile, other decay experiments have placed Mackenzia among actinarians (Gibson et al. 2018), and a lack of tentacles has already been noted to occur occasionally in some extant anthozoan species (Conway Morris 1993).
The new anatomical information here provided by Paramackenzia indicates that the body plan of mackenziids is inconsistent with the mesentery-lined body plan of anthozoan and other cnidarians (Fig. 5E, F). The septal lamellae (i.e. dark/reflective strands) have a different topological origin and interconnection. Although, apically, the mesentery of anthozoans may look similar in cross-section due to the presence of the actinopharynx, its functional morphology and overall extent preclude further comparison.
The pore system of Paramackenzia could be indicative of a trophic mode, analogous to sponges, in which water is pumped through several smaller ostia and exhaled through a larger apical osculum. Sponges, however, do not have a pneu architecture, nor do they utilize hydrostatic skeletal support. Instead, they have spicules and/or hypercalcified supporting skeletons. Mackenziids lack any type of spicules or a hypercalcified body plan, as observed in, for example, archaeocyathids, stromatoporoids and hypercalcified demosponges.
Some Cambrian sponge taxa are noteworthy for having a more regular axial symmetry exhibiting tetraradiality (Botting et al. 2014), such as Takakkawia, which has a sub-conical body consisting of skeletal elements (modified spicules) arranged into four (or eight) longitudinal columns (Botting 2012). This arrangement is reminiscent of the canal-bearing septal lamellae observed in our specimens of Paramackenzia, but the nature of these tissues is different and hence difficult to convincingly homologize. Cylindrical aspiculate sponges also appeared in the Cambrian, bearing distinct fibrous elements to form two-layer body walls; these fibres are capable of being preserved in exceptional fossils, such as vauxiids (e.g. Wei et al. 2021). More compellingly, we also note some similarities between mackenziids and the extinct Cambrian archaeocyathids, which are characterized by a conical cup consisting of porous inner and outer walls that are connected by longitudinal septa and other partitions (Rowland 2001; Debrenne et al. 2015a). The body architecture of archaeocyathids may offer the closest anatomical comparison with the mackenziids. However, the non-mineralized, hydrostatic skeleton in mackenziids, along with the presumed contractibility of the body (Conway Morris 1993), differ from the hypercalcified archaeocyathid skeleton (Debrenne et al. 2015a). In addition, the mackenziid pore–canal system is present only in the septal lamellae between tubular modules, while the inhalant pores of archaeocyathids occur across outer and/or inner walls and intermediate partitions (septa and others) (Debrenne et al. 2015b). We therefore argue that the unique body plan of mackenziids precludes further comparison and direct affiliation to either living or fossil sponges, although we cannot exclude the possibility (Botting et al. 2014) that ancestral sponges had the ability to form more regular symmetries and that it was inherited by members of the eumetazoan stem lineage.
The suspension feeding and osmotrophic body plan present in sponges and as hypothesized for Ediacaran rangeomorph taxa are noteworthy, especially against the background of body plan evolution at the transition between the node separating sponges and eumetazoans. Feeding on dissolved organic carbon and bacteria may have been a plesiomorphic strategy prior to the acquisition of digestive epithelia and a functional gut (Sperling et al. 2007). Sponges have been recovered as a paraphyletic grade in some molecular phylogenetic studies (e.g. Borchiellini et al. 2001; Sperling et al. 2009; Erwin et al. 2011), which would render this feeding strategy ancestral to all metazoans. Although sponge monophyly is also frequently recovered (see Wörheide et al. 2012 and references therein), this feeding strategy would still be optimized as ancestral due to choanoflagellates being the metazoan sister group (Carr et al. 2008). The common metazoan ancestor would in that case be optimized as a multicellular organism that is capable of differentiating cells into distinct functional tissues and feeding through choanocytes.
Rangeomorphs appear to have been adapted for such a suspension feeding mode of life, which would require well-developed interconnection between adjacent cells in order to form hydrostatic soft-bodied modules, and meet the approximate conditions of true epithelia as seen in eumetazoans. However, rangeomorphs lack evidence of differentiation into distinct digestive and protective epithelia. Such a scenario would optimize rangeomorphs as being below eumetazoans and placozoans on a node above sponges. It has been argued that the body plan of rangeomorphs was adapted for exposing surface area to seawater for feeding, for instance, by means of osmotrophy (Laflamme et al. 2009), or inside the pneu modules through pores (Butterfield 2020). It has also been proposed that these organisms could instead have relied on bacterial chemosymbiosis (McIlroy et al. 2021).
Cautiously, it could thus be reasonable to argue that mackenziids bridge the functional style of feeding observed in sponges and rangeomorphs, although it is currently premature to speculate if there is a phylogenetic implication of this hypothesis and whether mackenziids evolved their unique combinations of traits partly through convergence.
In summary, combining the new morphological information from Chengjiang and Burgess Shale fossils, mackenziids can be shown to lack triploblastic attributes such as bilateral symmetry, a through gut (with a mouth and an anus) and complex internal organs. Although the gross morphology of mackenziids is reminiscent of the diploblastic form of cnidarians and ctenophores, they lack particular traits seen in living cnidarians or ctenophores (e.g. the internal cavity does not seem to have functioned as a gastrovascular cavity), or the early fossil taxa affiliated to these phyla (e.g. Van Iten et al. 2014; Zhao et al. 2019). Mackenziids possess quasi-radial symmetry along an axial body and more robust epithelia (probably with a basal lamina and cell adhesion molecules to allow for building up hydrostatic pressure). It is thus proposed here that mackenziids could occupy a position on the eumetazoan stem or total group. If mackenziids lack a digestive true epithelium, they could represent an essentially ‘blastic’ evolutionary grade (in the embryological sense) that would optimize them as being below placozoans and eumetazoans, but above sponges.
Conclusion
- The elongate, cylindrical body of mackenziids is composed of simple, axially arranged tubular modules that formed a hydrostatic skeleton. A regular pore–canal system is developed between adjacent modules (septal lamellae) in Paramackenzia.
- The tubular modules resemble the hydrostatic pneu construction present in a number of Ediacaran macro-organisms, such as the erniettomorphs and possibly some less well-understood bag-shaped taxa (Protechiurus, Vaveliksia).
- The existence of a regular pore–canal system is unique, but may have gone unreported from some Ediacaran taxa due to the mode of preservation, which precludes the observation of such high fidelity structures.
- It appears that the mackenziids are functionally and structurally a mix of the sponge body plan and that of the modular Ediacaran macro-organism. Whether this has phylogenetic implications is premature.
- Mackenziids have no direct affiliation to the crown, or total, group of either the Porifera (sponges) or the coelenterate phyla (cnidarians and ctenophores).
Acknowledgements
We thank Luke A. Parry from the University of Oxford for his insightful discussion during preparation of the manuscript, along with Alexander G. Liu, Sally Thomas and an anonymous referee for their constructive comments and thoughtful suggestions. Mengying Yin is thanked for creating the reconstruction in Figure 6. This work was supported by the National Natural Science Foundation of China (42072019, 42062001), the Department of Education of Yunnan Province (2019Y0017) and the Strategic Priority Research Program of Chinese Academy of Sciences (XDB26000000). YZ was funded by graduate grants from the China Scholarship Council (201907030012) to work for an extended period at the University of Bristol for his PhD.
Author contributions
X-GH, P-YC, Y-JL and FW collected the specimens. YZ, JV and P-YC designed the study and interpreted the fossil specimens. YZ and P-YC prepared and photographed the specimens. YZ, under the supervision of JV, created the reconstruction and all of the figures. All authors discussed the data. YZ, JV and P-YC wrote the manuscript with input from the other authors.
Open Research
Data archiving statement
This published work and the nomenclatural acts it contains, have been registered in ZooBank: http://zoobank.org/References/E8BBF44C-DA7A-4FD6-BFE0-20C666115E26




