Bone histology of dinocephalians (Therapsida, Dinocephalia): palaeobiological and palaeoecological inferences
Abstract
We investigate the bone histology of multiple skeletal elements of dinocephalian taxa from the middle Permian Tapinocephalus Assemblage Zone of the Karoo Basin of South Africa. The results show that the cortex is predominantly composed of fibrolamellar bone, suggesting rapid osteogenesis for these basal non-mammalian therapsids. However, in a few skeletal elements, growth marks interrupt the deposition of the fibrolamellar bone tissue, indicating periodic arrests in growth. Ontogenetic differences are observed among the specimens studied: the majority have fibrolamellar bone deposited up to the peripheral margin of the bone wall, indicating continuous fast growth right up to the time of death, while a few specimens have avascular lamellar bone tissue with multiple, closely spaced rest lines, indicating maturity and a slowing down of growth prior to death. Several taxon-specific histological variations in terms of orientation of vascular canals and primary osteons, incidence of growth marks, and extent of secondary medullary reconstruction suggest slight differences in growth trajectories between the different taxa. The skeletal elements of the herbivorous taxa (Keratocephalus, Moschops and Struthiocephalus) and the omnivorous Jonkeria, previously studied, are characterized by a thick cortex, and extensively developed medullary spongiosa, suggestive of semi-aquatic habits. In contrast, the femoral histology of the carnivorous Anteosaurus suggests that it was more terrestrial.
Dinocephalians were a group of large-bodied basal therapsids that radiated during the Guadalupian epoch of the Permian Period (Boonstra 1963a, b; Rubidge 1991; Nicolas & Rubidge 2010; Day et al. 2015). The group includes carnivores (e.g. Anteosauridae), herbivores (e.g. Tapinocephalidae) and omnivores (e.g. Titanosuchidae), with the tapinocephalids being the most abundant in the fossil record (Boonstra 1963a, b; Barghusen 1975; Nicolas & Rubidge 2010). The dinocephalians are characterized by their thick walled, ornamented skulls with interdigitating upper and lower incisors (Rubidge & Sidor 2001; Kemp 2005, 2012). Moschops and Tapinocephalus had the heaviest and thickest skulls of all the dinocephalians (Kemp 2012).
Dinocephalians experienced their first phase of diversification in the Eodicynodon Assemblage Zone of the Beaufort Group of the Karoo Supergroup of South Africa (Boonstra 1969; Rubidge 1991; Govender et al. 2002), and were abundant in the early parts of the overlying Tapinocephalus Assemblage Zone (TAZ, sensu Smith & Keyser 1995; Kemp 2012; Day & Rubidge 2020; Day & Smith 2020). The TAZ has the highest tetrapod diversity of all the Karoo biozones, with dinocephalians representing more than 30% of the total tetrapod fauna (Chinsamy-Turan 2012; Kruger et al. 2018). However, by the end of the TAZ they completely disappear from the fossil record leaving no known descendants (Boonstra 1971; Kemp 1982, 2012). Their disappearance near the top of the TAZ marks the base of the Pristerognathus Assemblage Zone (Day et al. 2015).
Dinocephalians are also known from the middle Permian rocks of Russia (Ivachnenko 1995, 2000), Zimbabwe (Lepper et al. 2000; Munyikwa 2001), Brazil (Langer 2000; Cisneros et al. 2012), Tanzania (Simon et al. 2010) and China (Li & Cheng 1995; Cheng & Ji 1996; Li et al. 1996; Cheng & Li 1997; Liu 2013), but their remains are best represented in the Abrahamskraal Formation of the Beaufort Group, Karoo Supergroup, South Africa (King 1988; Rubidge 2005; Day et al. 2015). Four dinocephalian families (Anteosauridae, Titanosuchidae, Styracocephalidae and Tapinocephalidae) are known from the Tapinocephalus Assemblage Zone of the Beaufort Group (Rubidge et al. 2019), of which the carnivorous Anteosauridae is considered the most basal while the herbivorous Tapinocephalidae are the most derived (Kammerer 2011; Smith et al. 2012; Kruger et al. 2018), with Tapinocaninus considered to be the most basal (Rubidge 1991; Smith et al. 2012).
Over the years, dinocephalians have been considered to have occupied different ecological niches: terrestrial (e.g. Anteosaurus; Kammerer 2011) and littoral and/or semi-aquatic (e.g. Moschops; Gregory 1926; Boonstra 1955, 1962; King 1988). However, anteosaurs have also been considered riparian, obligate fish-eaters or even amphibious (Boonstra 1955, 1962; Olson 1962; King 1988; Ivakhnenko 2001, 2003, 2008). More recently, Kammerer (2011) supported a fully terrestrial lifestyle for them given that they possessed heavy skulls equipped with large canines and incisors (Sennikov 1996; Van Valkenburgh & Jenkins 2002). Gregory (1926) listed numerous postcranial skeletal features of Moschops that point towards a semi-aquatic adaptation (e.g. slight regional differentiation of the presacral vertebrae, the relatively small size of the neural spines and the immense size of the thorax, as well as the thick cartilaginous epiphyses on the carpals, tarsals and long bones). He further suggested that Moschops had extensive fore and aft reach with wide limbs, useful for propelling the animal through water, while their massive forehead provided an advantage during diving in water. Boonstra (1956) suggested that the cumbersome body plan of herbivorous dinocephalians with their poor locomotor apparatus, feeble lower jaw and massive cranium, would allow them to feed on soft marshy vegetation rather than tough land vegetation. von Huene (1931) suggested a hippo-like lifestyle for Keratocephalus (King 1988). However, Benoit et al. (2017) suggested that the snout and lowered head posture of tapinocephalids may have facilitated ground-level browsing (Barghusen 1975), contradicting previous proposed semi-aquatic adaptation for the group (King 1988). Hence, based on the peculiar cranial features and bulky body plan of dinocephalians, varied interpretations of the lifestyle adaptations and palaeoecology have been proposed (Gregory 1926; King 1988; Kammerer 2011; Benoit et al. 2017).
Bone microanatomical studies have demonstrated that tetrapods change their bone architecture (microanatomy) and internal tissue structure (histology) according to their lifestyle adaptations (e.g. Wall 1983; Chinsamy 1991; Ray et al. 2005; Gray et al. 2007; Hayashi et al. 2013; Houssaye et al. 2016; Canoville et al. 2016; Canoville & Chinsamy 2017), which suggests that bone density and cortical thickness is correlated with mode of life (Wall 1983). In addition, the limb bone histology of various vertebrates provides direct assessment of various aspects of tetrapod palaeobiology (e.g. ontogeny, growth patterns and sexual maturity; de Ricqlès 1969, 1972; Francillon-Vieillot et al. 1990; Chinsamy 1997; Horner et al. 1999, 2000; Chinsamy-Turan 2005, 2012; Klein & Sander 2008; Chinsamy et al. 2013, 2019, 2020; Woodward et al. 2014, 2015, 2020; Huttenlocker & Botha-Brink 2014; Botha-Brink et al. 2018; Botha 2020; Cullen et al. 2020; Huttenlocker & Shelton 2020). A comprehensive histological and microanatomical assessment of dinocephalian taxa has been lacking (Chinsamy-Turan 2012) ever since the early work of Armand de Ricqlès (1972). Until recently the only histological work on Titanosuchus has been the identification of osteomyelitis in a femur of Jonkeria parva (Shelton et al. 2019). However, even more recent bone histology research has been conducted on Jonkeria (Bhat et al. in press), and this has suggested that they were fast-growing animals adapted to a semi-aquatic lifestyle like the modern graviportal Hippopotamus, given that both share thick cortices, extensive secondary reconstruction and extensively developed medullary spongiosa. Here, we apply histological and microanatomical approaches to several dinocephalian taxa from the Tapinocephalus Assemblage Zone of the Karoo Basin of South Africa and include different skeletal elements to assess inter-elemental and intraspecies-level histological variations in the dinocephalians. Our study also aims to shed light on the lifestyle adaptations evident from the bone microanatomy and histology.
Material and method
The specimens studied here were excavated from the middle Permian Tapinocephalus Assemblage Zone of the South African Karoo Basin (Boonstra 1969). For our analysis, several skeletal elements, (femora, humeri, radii, ulna, fibula, and ribs) were examined to assess both inter-elemental and intraspecies-level histological variability. These skeletal elements are positively identified at the species or genus levels (Table 1; references therein). We followed Kammerer (2011) for the identification of the specimen SAM-PK-K291, which was referred to as the proximal end of a femur. All specimens were obtained from Iziko South African Museums, Cape Town and Evolutionary Studies Institute (formerly the Bernard Price Institute) at the University of the Witwatersrand, Johannesburg, South Africa. Permission to section the fossils was obtained from the South African Heritage Resources Agency (SAHRA; permits 2076, 2131, and 3752-4658). Upon completion of this study, the resulting thin sections will be returned to the institutions from which the fossils were obtained.
| Specimen number | Skeletal element | Taxon | Locality | Section type | |
|---|---|---|---|---|---|
| SAM-PK-K291* | Femur | Anteosaurus magnificus | Die Krans, Beaufort West, Tapinocephalus Assemblage Zone, Beaufort Group, Karoo Supergroup, South Africa | Transverse | |
| BP/1/5591a | Femur | Anteosaurus sp. | Rheboksfontein Tapinocephalus Assemblage Zone, Beaufort Group, Karoo Supergroup, South Africa | Transverse | |
| SAM-PK-8946 | Ulna | Keratocephalus moloch | Mynhardtskraal, Tapinocephalus Assemblage Zone, Beaufort Group, Karoo Supergroup, South Africa | Transverse core | |
| BP/1/6987 | Proximal humerus | Moschops sp. | Rooifontein, Abrahamskraal Formation, Tapinocephalus Assemblage Zone, Beaufort Group, Karoo Supergroup, South Africa | Transverse core | |
| BP/1/6986 | Ribs (fused) | Transverse | |||
| SAM-PK-11939 | a | Femur head | Struthiocephalus whaitsi | Dikbome, Laingsburg, Tapinocephalus Assemblage Zone, Beaufort Group, Karoo Supergroup, South Africa | Transverse core |
| b | Humerus | Transverse core | |||
- All material was recovered from the Tapinocephalus Assemblage Zone (Beaufort Group, Karoo Supergroup), South Africa.
- BP, Evolutionary Sciences Institute (previously Bernard Price Institute), University of the Witwatersrand, Johannesburg, South Africa; SAM, Iziko South African Museums, Cape Town, South Africa.
- * We followed Kammerer (2011) for the identification of specimen SAM-PK-K291, which was referred to as the proximal end of the femur.
Given that vertebrates display a wide range of histological characteristics (e.g. Horner et al. 1999, 2000; Ray et al. 2004; Ray & Chinsamy 2004; Chinsamy-Turan 2005, 2012), as well as bone deposition rates (e.g. Amprino 1947; de Margerie et al. 2002, 2004; Starck & Chinsamy 2002), multi-element studies of individuals provide a better assessment of growth patterns, lifestyle habits and various aspects of the life history (Botha & Chinsamy 2004, 2005; Chinsamy-Turan 2005, 2012; Ray et al. 2009). The destructive nature of histological analyses and the scarcity of complete specimens prohibited the sectioning of a large number of bones; however, a reasonable sample for the study was obtained by selecting incomplete skeletal elements from (as far as possible) diagnostic dinocephalian species. Transverse sections were prepared from the midshaft level whenever possible because this is the region of the bone that undergoes the least amount of secondary remodelling (Enlow 1963; Chinsamy 1995; Chinsamy-Turan 2005), and limb bones were preferentially selected because they represent the best track record of growth (Francillon-Vieillot et al. 1990; Chinsamy-Turan 2005; Bhat et al. 2019). Both stylopodial (e.g. femur, humerus) and zeugopodial (radius, fibula) bones were sampled because they are considered to show different ecological signals and respond differently to any change in the habitat (de Buffrénil & Schoevaert 1989; de Margerie et al. 2005; Canoville & Laurin 2010; Quemeneur et al. 2013). Due to their general abundance in the museum collections we also sectioned non-weight-bearing bones (e.g. the proximal ends of rib shafts) to investigate whether, as in sauropod dinosaurs (Stein & Sander 2009; Waskow & Sander 2014), they exhibit a better growth mark record than weight-bearing long bones. To date, this study represents the most comprehensive bone histology investigation of dinocephalians in terms of taxonomic diversity and the number of skeletal elements sectioned (Table 1). In the text, different elements of the same individual have the same specimen numbers but are differentiated by the suffixes of a/b/c (Table 1).
Thin sections of long bones were petrographically prepared using cutting and grinding techniques following Chinsamy & Raath (1992). Considering the technical challenge of sectioning large dinocephalian skeletal elements, limb bones were sampled by the hydraulic coring method using a drill with a diamond encrusted coring bit or cut using a Dremel precision tool, following the standard procedures outlined in Stein & Sander (2009). Core drilling was carried out preferentially in an undiagnostic area that leads to the least damage to the skeletal anatomy. After the 1 cm cores were obtained, the holes were infilled with plaster to preserve the overall morphology of the skeletal element. Depending on the fossil bones, the cores were either embedded in an epoxy resin (EpoxAcast 690 and/or Struers Epofix; Chinsamy & Raath 1992; Chinsamy-Turan 2005) or subjected to direct cutting/slicing along the preferred direction. Note that the coring, sectioning, embedding and thin sectioning, as well as microscopy thereafter were performed in the thin sectioning laboratory of the Palaeobiology Research Group at the Department of Biological Sciences, University of Cape Town. The embedded bones were mounted on frosted glass slides and thin sectioned using a Struers Accutom-50 and thereafter ground and polished using carborundum (silicon carbide) discs of various grit sizes (400–1200 μm). This was followed by a final polish on a lap wheel with a velvet cloth using an aluminium oxide (Al₂O₃) solution. The final thickness of the thin section was 50–45 μm, which proved to be optimum for our analyses. Several sections from a single bone were prepared. All the prepared bones were studied and photographed using a digital compact Nikon DS-Fi1 camera mounted on a Carl Zeiss Axio Lab A1 polarizing microscope. The histological nomenclature follows Francillon-Vieillot et al. (1990) and Chinsamy-Turan (2005, 2012).
Results
Although, in a few specimens, diagenesis substantially altered the original orientation of the crystallites and obscured their histological features, in general the bone histology of the specimens is well preserved (Figs 1-7). In a few sections (Figs 4, 6, 7) the outermost margins of the bones are missing due to weathering, and some sections developed cracks during thin section preparation.
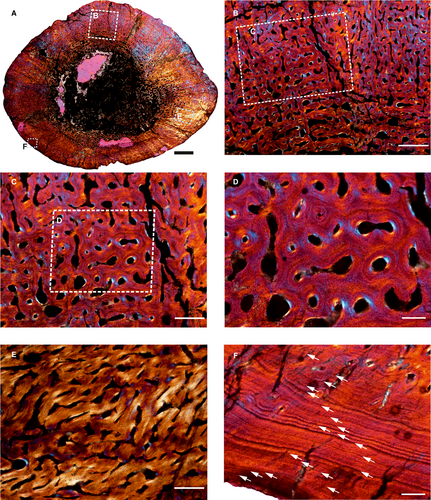


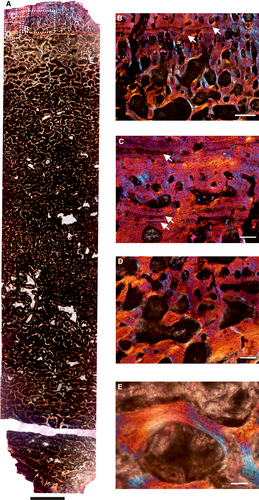
Histological description of Anteosaurus
The femur (SAM-PK-K291) belongs to A. magnificus (Table 1; reference therein). It has a distinct, thick outer cortex and medullary region partially infilled by thick trabeculae (Fig. 1A). The cancellous bone is unevenly distributed inside the medulla and it progressively grades into cortical bone. The predominant bone tissue of the outer cortex is highly vascularized, uninterrupted fibrolamellar bone tissue (Fig. 1B, C) with a woven matrix as defined by fibre orientation, and globular and randomly distributed osteocyte lacunae (Fig. 1D). In the cross-section, fibrolamellar bone deposition continues up to the periphery, however, there is a thin layer of poorly vascularized lamellar bone tissue with multiple closely spaced rest lines (Fig. 1F), with isolated simple vascular channels. The perimedullary region shows extensive secondary reconstruction that leads to a number of large erosion cavities that give it a cancellous texture (Fig. 1A). It should be noted that the amount of cancellous bone varies in the cross-section. Despite extensive secondary reconstruction in the inner cortex, islands of primary bone tissue are present within the core of trabeculae. Secondary osteons are absent in the compacta. Circumferentially oriented longitudinal canals are predominant, but a large number of radial and reticular canals are present in the compacta (Fig. 1B–D).
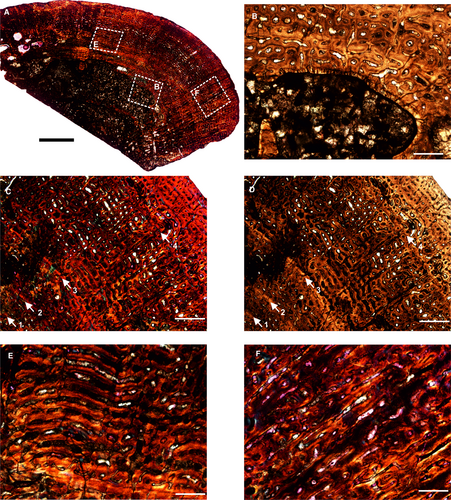
Another femur (BP/1/5591) belonging to the genus Anteosaurus displays an open medullary cavity with thick struts of trabeculae extending into the medullary cavity (Fig. 2A). The medullary cavity is irregular and encircled by a thin layer of endosteal lamellar bone tissue (Fig. 2B). Similar to SAM-PK-K291, the outer cortex consists of highly vascularized fibrolamellar bone tissue with numerous primary osteons (Fig. 2C, D). The vascular channels are mostly consistent in size and density throughout the compacta. Vascular canals are mostly circumferential in orientation with a laminar (Fig. 2E) and reticular arrangement (Fig. 2C, D), with the former restricted to the inner cortex whereas the latter dominates the middle cortex and peripheral regions (Fig. 2A, E). However, radially oriented canals are present mostly in areas of muscle attachment such as the trochanter (Fig. 2F) and penetrate deep into the cortex, reaching the medullary cavity (Fig. 2A). Some radial canals occur locally in the compacta. Four widely spaced growth marks are present in the compacta (Fig. 2C, D), and it should be noted that there is no associated change in degree or pattern of vascularization prior to or after the growth marks (Fig. 2C, D).
Histological description of Keratocephalus moloch
The core sample of the ulna of K. moloch shows a laminar fibrolamellar bone tissue surrounding a medullary region that is infilled by bony trabeculae (Fig. 3A–C). The vascular canals near the medullary cavity are highly remodelled (Fig. 3A). The resorption between these canals is extensive and results in the large erosional spaces (Fig. 3D, E). On the ventral side, the cross-section is crushed (Fig. 3A), showing a mixture of circumferential and longitudinal vascular canals towards the inner region (Fig. 3G). The inner cortex has large erosional spaces (Fig. 3A) with islands of broken trabeculae with large erosion spaces (Fig. 3F), and some of them reach cancellous proportions towards the inner cortex (Fig. 3A). Lamellar bone tissue encircles these erosional spaces (Fig. 3D, E). Growth marks were not visible in the compacta. The periosteal surface is broken on the ventral side where the skeletal element was damaged, whereas it is smooth dorsally.
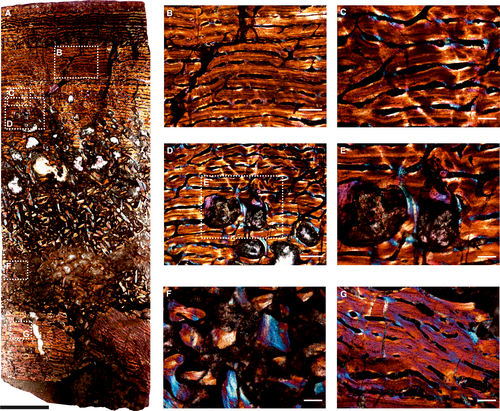
Histological description of Moschops
The core (Fig. 4A) of the humerus (BP/1/6987) shows the inner medullary cavity infilled with bony trabeculae and an outer layer of highly vascularized fibrolamellar bone that extends to the periosteal surface (Fig. 4B–E). Due to intense remodelling in the inner cortex, the transition between the medullary region and cortical bone is progressive. The inner cortex, formed during early ontogeny, consists of reticular fibrolamellar bone tissue (Fig. 4F, G). The subperiosteal and peripheral cortex consists of laminar fibrolamellar bone (Fig. 4A–E). The outer vascular canals are distinctly narrower than the inner vascular canals as the spacing between consecutive vascular canals decreases towards the periphery (Fig. 4B–E). However, the overall orientation of the vascular canals is circumferential, with the major axis parallel to the outer cortical margin (Fig. 4B–D). The inner cortex has large secondarily formed erosional spaces, and few primary osteons are preserved in the bony trabeculae (Fig. 4F, G). The open spaces in the bone tissue of the medullary region are mostly infilled with mineral sediment and most reach cancellous dimensions (Fig. 4A, F, G). These resorption cavities show varying degrees of centripetal deposition of lamellated bone (Fig. 4E–G), and some islands of primary bone consist of woven matrix towards the inner region (Fig. 4G).
The two ribs fused together are composed entirely of well-vascularized fibrolamellar bone (Fig. 5). Osteocyte lacunae are abundant and are arranged in a disorderly manner typical of fibrolamellar tissue (Fig. 5B). Circumferential vascular canals are predominant in a laminar arrangement (Fig. 5). The open spaces in the bone tissue are frequently infilled with mineral sediment (Fig. 5B, D). Growth marks are absent.
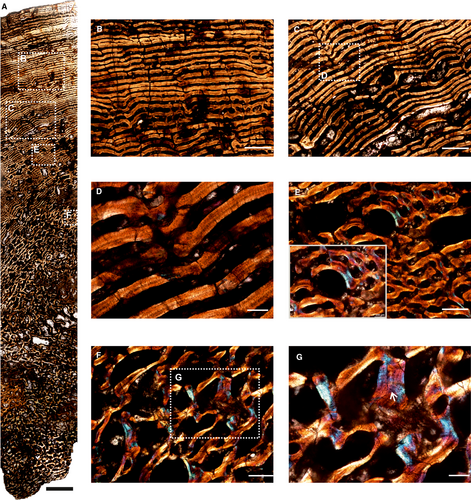
Histological description of Struthiocephalus whaitsi
The core of the femur (SAM-PK-11939a) has a distinct, thick cortex and a medullary region occupied by cancellous bone tissue (Fig. 6A). The outer cortex is highly vascularized and consists of fibrolamellar bone tissue (Fig. 6B) and numerous primary osteons within the woven bone matrix (Fig. 6C, D). Globular osteocyte lacunae are densely distributed in the matrix throughout the cortex. The inner cortex contains circumferential fibrolamellar bone (Fig. 6A) whereas the outer peripheral cortex has laminar bone tissue towards the dorsal and ventral margin of the skeletal element (Fig. 6B). The vascularization pattern is quite variable in the section given that the core was obtained near the femoral head. A laminar vascular pattern is seen in the middle of the section (Fig. 6A, E), which continues towards the ventral margin (Fig. 6A, H). In addition, in localized areas in the middle cortex few localized radial and longitudinal canals occur (Fig. 6C, D). The medullary region is highly remodelled (Fig. 6A) and many erosional cavities are visible (Fig. 6F, G). Some islands of primary bone tissue are preserved in the middle and inner cortex between resorption cavities and consist of a woven bone matrix (Fig. 6F).
The core sample of the humerus (SAM-PK-11939b; Fig. 7) shows an extremely thin cortex with an extensive amount of cancellous bone that extends from the middle cortex and completely infills the medullary cavity. Due to intense remodelling and endosteal deposition in the inner cortex (Fig. 7A–D), the transition between the medullary region and cortical bone is progressive (Fig. 7B). The dorsal and ventral periosteal bone margins are irregular and internal cracks developed during thin section preparation (Fig. 7A). The outer rim of cortical bone preserved is entirely composed of fibrolamellar bone tissue with longitudinal primary osteons (Fig. 7B). The medullary region is highly cancellous in nature with large erosional cavities (Fig. 7A), with many of them showing lamellar deposits along their endosteal surfaces (Fig. 7E). Three growth marks are present (Fig. 7C), two of which are closely spaced in the inner cortex, representing a double line of arrested growth (LAG) and the third is present toward the peripheral cortex.
Discussion
In this study, a histological and microanatomical approach is applied to a range of dinocephalian taxa and a variety of skeletal elements to assess inter-elemental and intraspecies-level histology and to shed light on their lifestyle adaptations. Interestingly, irrespective of their taxonomic identity, bone histology of all of the long bones except for the Anteosaurus femur (BP/1/5591; Fig. 2A) shows a distinct, thick outer cortex and completely infilled medullary region (Figs 1, 3-7). The predominant bone tissue of the cortex is fibrolamellar bone tissue, indicating that the early stages of growth were rapid and resulted in the deposition of the more rapidly formed fibrolamellar bone tissue (Amprino 1947; Francillon-Vieillot et al. 1990; de Margerie et al. 2002, 2004; Starck & Chinsamy 2002; Chinsamy-Turan 2005). In most of the limb bones, fibrolamellar bone deposition continues up to the periosteal periphery, indicating continuous fast growth up until the time of death (Botha & Chinsamy 2004; de Margerie et al. 2004; Bhat et al. 2019). In a few skeletal elements (Figs 2, 7), growth marks or LAGs (sensu Francillon-Vieillot et al. 1990; Castanet et al. 1993; Chinsamy et al. 1995) are present within the fibrolamellar bone tissue. LAGS or growth marks are hyper-mineralized lines interrupting the appositional growth (i.e. periosteal deposition of bone; Castanet et al. 1993; Chinsamy & Dodson 1995; Chinsamy-Turan 2005, 2012). They generally have a smooth and distinct appearance that follows the contour of the periosteal surface (Chinsamy-Turan 2005, 2012). Most often, LAGs form individually but sometimes occur in groups and are a common attribute of most vertebrates, irrespective of their size, tissue type, or phylogenetic affinities (Castanet et al. 1993). They result in a distinctive stratification of the cortical bone into a series of growth rings that are indicative of periodic arrests in bone deposition and growth (Chinsamy-Turan 2005, 2012). The number of LAGs varies across skeletal elements; however, some limb bones lack any LAGs. The absence or presence of growth marks or LAGs indicates a variable response to prevailing environmental conditions (Köhler et al. 2012; Chinsamy & Warburton 2020). However, in one specimen (Fig. 1F), the peripheral cortex constitutes avascular lamellar bone tissue with multiple, closely spaced rest lines, indicating that the growth has significantly slowed down at the time of death (Francillon-Vieillot et al. 1990; Castanet et al. 1993; Chinsamy et al. 1995; Starck & Chinsamy 2002; Chinsamy-Turan 2005; Erickson 2005). These rest lines are related to the attainment of skeletal maturity (Horner et al. 2000; Chinsamy-Turan 2005; Erickson 2005; Cerda et al. 2017). However, it should be noted that differences in histology and microstructure are to be expected because the skeletal elements were not sectioned in standardized places along the shaft, and furthermore, different animals and individuals were studied.
Interestingly, in all of the bones, it appears that the original primary compacta is progressively eroded away by secondary reconstruction to form cancellous bone that extends into and completely fills the medullary cavity. It should be noted that the amount of cancellous bone varies across skeletal elements and also in a single cross-section. Despite extensive resorption and secondary reconstruction in the medullary regions, primary bone tissues are still visible, especially in the thicker trabecular areas. Although the interstitial matrix in the trabeculae is woven in nature, erosional spaces are lined to some degree with lamellar bone tissue. Overall, the cortical vascularity varies across individuals and within the same diaphyseal sections. Radial canals are distributed densely in areas of muscle attachment whereas there are very few in the rest of the compacta. All the skeletal elements of dinocephalians are well vascularized with large, interconnected vascular channels resulting in the formation of a laminar pattern with an overall concentric arrangement, whereas in others the channels vary from reticular to plexiform.
Inter-elemental and intraspecies-level histological variation
Bone histology of the multiple skeletal elements of dinocephalians varied substantially in terms of degree of vascularization, tissue organization and amount of cancellous bone and appeared to be related to growth dynamics, palaeoecology and taxonomic differences. In a study on humeral microanatomy of terrestrial, semi-aquatic, and aquatic carnivorans, Nakajima & Endo (2013) reported that the degree of cancellousness around the ossification centres of the bone (i.e. the amount of resorption in periosteal compact bone) was the lowest in the semi-aquatic and the highest in terrestrial animals. Given that the degree of cancellous bone varies around ossification centres in animals of different modes of life (Nakajima & Endo 2013), it was also noted that the tissue type and vascularization pattern vary between species of different ecological and dietary niches (Nakajima et al. 2014). In the current study, histological variations in terms of degree of vascularization, tissue organization and amount of cancellous bone are visible in the bones studied. Furthermore, these variations were noted in the same diaphyseal section and between skeletal elements of the same or different taxa. In addition, there are distinctive differences in the vascular orientation of the tissue between an earlier formed bone and a later formed bone.
It appears that bone of carnivorous Anteosaurus had the most histological variations in terms of change in tissue type and growth strategy through ontogeny. In addition to differences in the amount of trabecular bone in the medullary region, the femur BP/1/5591 (Fig. 2A) has highly vascularized fibrolamellar bone in the peripheral region (Fig. 2C, D), suggesting that osteogenesis had not yet slowed down in this individual, whereas the femur SAM-PK-K291 (Fig. 1A) belonging to Anteosaurus magnificus has numerous peripheral rest lines (Enlow 1962; Fig. 1F). Although the early growth in BP/1/5591 (Fig. 2C, D) was periodic, as suggested by the presence of multiple growth marks in the fibrolamellar bone tissue, in SAM-PK-K291 (Fig. 1A) it is uninterrupted and continuous. The femur BP/1/5591 (Fig. 2A) belongs to a separate Anteosaurus species (Table 1). It is interesting to note that the diameter of the femur BP/1/5591 of Anteosaurus sp. is higher than the femur (SAM-PK-K291) of Anteosaurus magnificus, which had already attained skeletal maturity, as shown by the presence of peripheral rest lines in the outer cortex of the femur (Chinsamy-Turan 2005; Nacarino-Meneses et al. 2016; Calderón et al. 2019).
All the bones of the herbivorous dinocephalians (Keratocephalus, Moschops, Struthiocephalus) lack growth marks in the compacta, except for the humerus of Struthiocephalus, suggesting uninterrupted, rapid growth for the skeletal elements until this stage of their lives. The absence of LAGs and peripheral rest lines suggests that these elements belong to sub-adult individuals (e.g. Ray et al. 2005). In contrast, the femur of Struthiocephalus whaitsi shows changes in the organization of the vascular canals in different parts of the compacta, suggesting different rates of bone deposition in the fibrolamellar bone complex (de Margerie et al. 2004). Extensive remodelling of the original primary bone in the humerus of S. whaitsi leads to a completely remodelled inner and subperiosteal cortex. The LAGs in the peripheral margin (Fig. 6) and the decrease in vascularization indicate that the rate of bone deposition had slowed down substantially (Chinsamy-Turan 2005). It may be noted that the core of the femur (SAM-PK-11939a) was obtained from the proximal end of the bone and it is likely that the histological features of the humerus and femur may vary.
Histological variations and palaeobiology
In the current study, carnivorous (e.g. Anteosaurus) and herbivorous (e.g. Keratocephalus, Moschops, Struthiocephalus) dinocephalians were studied to assess for histological differences. To broaden the comparison, the histological information of the omnivorous Jonkeria (Bhat et al. in press) was included for comparative purposes. Overall, differences were seen in the multi-element comparison of dinocephalians with the skeletal elements of Anteosaurus, which showed changes in its growth strategy through ontogeny. Although the overall growth was fast, multiple growth marks were present in the femur (BP/1/5591) of Anteosaurus. The presence of multiple growth marks indicates a periodic or flexible growth strategy and directly suggests that this individual grew in an interrupted manner (Starck & Chinsamy 2002; Chinsamy-Turan 2005). Although the earlier growth marks may have been erased as a result of secondary reconstruction (Fig. 2A), we assume that it is unlikely that this individual was older than 4 years at the time of death, given that traces of growth marks are visible in the perimedullary region. Another femur (SAM-PK-K291) showed uninterrupted fibrolamellar bone. However, a few closely spaced peripheral rest lines are present, indicating that the growth has significantly slowed down prior to death (Francillon-Vieillot et al. 1990; Castanet et al. 1993; Chinsamy et al. 1995; Starck & Chinsamy 2002; Chinsamy-Turan 2005; Erickson 2005). In addition, the medullary regions of all of the bones except the femur (BP/1/5591) are secondarily infilled by bony trabeculae.
In contrast, the herbivorous dinocephalians (Keratocephalus and Moschops) had highly vascularized cortices without any interruptions in growth. However, the humerus of Struthiocephalus showed several LAGs in the outermost cortex, indicating a periodic ceasing of bone deposition. The femur of Moschops and the ulna of Keratocephalus have laminar fibrolamellar bone in the outer cortex surrounding a medullary cavity infilled with spongy bone. Both these animals are thought to be adapted to a semi-aquatic (hippo-like) lifestyle (von Huene 1931) and to have fed on fairly resistant food such as rhizomes or stems (Gregory 1926; King 1988). Similar histological features were noted in the femur of the herbivorous Struthiocephalus that is thought to have lived in marshy/littoral areas and fed on soft vegetation, possibly under water (Boonstra 1965; King 1988). The absence of LAGs in the femur of Struthiocephalus is similar to that in the femur of Moschops and the ulna of Keratocephalus, suggesting continuous and rapid deposition of bone tissue. The fused ribs of Moschops lack LAGs, which may indicate that they are from a young individual, or that, contrary to the observations made in sauropod dinosaurs (Stein & Sander 2009; Waskow & Sander 2014), ribs do not preserve a good track record of growth in this taxon. Further research on more ribs of different taxa through ontogeny is warranted to verify these deductions. The skeletal elements of the omnivorous Jonkeria had mixed histological features (Shelton et al. 2019; Bhat et al. in press), similar to both the herbivorous and carnivorous dinocephalians in the current study. Shelton (2015) reported different bone histology in herbivorous and carnivorous late Carboniferous and early Permian pelycosaur-grade synapsids. He showed that the carnivores grew much faster than the herbivores, which appeared to have secondarily reduced their growth rates despite having evolved to large sizes. In contrast, contemporaneous graviportal terrestrial pareiasaurs (Canoville et al. 2014) had a relatively rapid growth early in ontogeny but later slowed down (Canoville & Chinsamy 2017), with the primary bone matrix reported as parallel-fibred to lamellar. We note a predominance of fibrolamellar bone as the primary bone tissue in the compacta, suggesting rapid osteogenesis (Amprino 1947; Francillon-Vieillot et al. 1990; de Margerie et al. 2002, 2004; Starck & Chinsamy 2002; Chinsamy-Turan 2005) and an overall fast rate of bone deposition and growth for dinocephalians. Moreover, Haversian substitution with several generations of secondary osteons was prevalent in pareiasaurs (Canoville & Chinsamy 2017) but it was not present in our sample. However, histological variations were observed in the skeletal elements of the studied taxa, suggesting slight differences in growth trajectories.
Lifestyle adaptations
Cross-sectional geometry (cortical thickness or relative bone wall thickness) and bone microanatomy, as well as osteohistological characteristics have been used to deduce the lifestyle adaptations of a variety of tetrapods (Wall 1983; Chinsamy 1997; de Ricqlès & de Buffrénil 2001; Germain & Laurin 2005; Gray et al. 2007; Canoville & Laurin 2009, 2010; Houssaye 2009; Laurin et al. 2011; Hayashi et al. 2013; Nakajima et al. 2014; Houssaye et al. 2016; Canoville & Chinsamy 2017; Montoya-Sanhueza & Chinsamy 2018; Botha 2020). The bone of semi-aquatic and aquatic animals often shows an increase in bone compactness to reduce buoyancy in water (Chinsamy 1991; Gray et al. 2007; Kriloff et al. 2008; Hayashi et al. 2013), which often results in the loss of the medullary cavity and therefore increased bone wall thickness (Wall 1983). However, it should be noted that burrowing animals generally have thick bone walls with medullary spongiosa (Bramble 1982; Ultsch & Anderson 1988; Lips 1991; Magwene 1993; Botha & Chinsamy 2004, 2005; Ray & Chinsamy 2004; Chinsamy & Abdala 2008; Meier et al. 2013; Lyson et al. 2016; Montoya-Sanhueza & Chinsamy 2017; Legendre & Botha-Brink 2018; Bhat et al. 2019) as compared with terrestrial animals, which have lower bone wall thickness (relative bone wall thickness <30%; sensu Wall 1983) and open medullary regions. In such circumstances, it is essential to consider additional morphological adaptations for burrowing as well as other anatomical features that might aid in deducing their lifestyle habits (Nasterlack et al. 2012; Houssaye et al. 2016; Montoya-Sanhueza & Chinsamy 2017).
Tetrapods showing semi-aquatic adaptation achieve high bone density and compactness by losing medullary cavity through the extensive infilling of inner cavities by bony trabeculae (de Ricqlès & de Buffrénil 2001; Ray et al. 2005). The development of internal medullary spongiosa in the bones augments bone density to maximize the efficacy of buoyancy in water (Wall 1983; Taylor 1994; Gray et al. 2007), and flexural rigidity to increase the strength and toughness of the bone (Carter & Spengler 1978; Rogers & LaBarbera 1993). In the current study, all the long bones of herbivorous dinocephalians show extensive development of medullary spongiosa as compared with the carnivorous Anteosaurus. It was also noted that the resorption cavities in the long bones of Anteosaurus are extensively developed with wide open spaces between trabeculae. Similar features were noted in the skeletal elements of Jonkeria, which is an omnivore (Shelton et al. 2019; Bhat et al. in press). The medullary regions of the long bones of Jonkeria are completely infilled by spongiosa as compared with Anteosaurus. In contrast, the herbivorous animals in our sample (Keratocephalus, Moschops and Struthiocephalus) have medullary regions virtually filled with cancellous bone tissue.
Interestingly, the dicynodont Lystrosaurus murrayi has compact bone with spongy bone tissues and, based on this type of bone histology, Ray et al. (2005) proposed a semi-aquatic/aquatic lifestyle for Lystrosaurus, contradicting earlier work supporting a fully terrestrial lifestyle (King 1991; King & Cluver 1991). However, anatomical features such as a widened knee and flared scapula blade present in Lystrosaurus point towards a digging function. Botha-Brink & Angielczyk (2010), and later Botha-Brink et al. (2016), hypothesized that the rapid growth observed in Triassic Lystrosaurus is the result of a high degree of vascularization. They further suggested that the success of this taxon may also be attributed to similar life history adaptations including digging behaviour. However, more recently, Botha (2020) proposed a fully terrestrial mode of life for Lystrosaurus, using the same histological characteristic features noted by Ray et al. (2005). Botha (2020) further suggested biomechanical constraints as the cause of the extensive development of medullary spongiosa in Lystrosaurus to support the large body weight. Aquatic tetrapods have been shown to have compact bone walls with the medullary regions infilled with spongy bone tissue (Houssaye et al. 2016), which is considered to counterbalance buoyancy in water (Chinsamy 1991; Gray et al. 2007; Hayashi et al. 2013; Houssaye et al. 2016). In contrast, terrestrial graviportal animals such as elephants (Houssaye et al. 2016), giraffes (Smith 2020) and bison (Sander & Andrassy 2006; Canoville et al. 2016; Houssaye et al. 2016) do not develop medullary cavities with cancellous bone (Ray et al. 2010; Houssaye et al. 2016) as a biomechanical adaption for their bulk, contra Botha (2020). However, as in the current sample, the ribs of the known aquatic reptile, Claudiosaurus (de Buffrénil & Mazin 1989) show a complete infilling of the medullary region by cancellous bone and are considered pachyostotic as an adaptation for a semi-aquatic lifestyle (de Ricqlès & de Buffrénil 2001). The contemporaneous middle Permian pareiasaurs, which were similarly large bodied tetrapods, had similar histological characteristics to dinocephalians (i.e. spongy inner medullary regions, although not as extensive as those seen in dinocephalians; Canoville & Chinsamy 2017). Canoville et al. (2014) performed isotopic analysis of the teeth of dinocephalians, pareiasaurs and therocephalians and suggested that these large graviportal animals inhabited different ecological niches during the middle and late Permian times, with pareiasaurs and therocephalians sharing a terrestrial habitat. In addition, their data also suggest that both dinocephalians and pareiasaurs were terrestrial and that oxygen isotope differences reflect physiology and higher water turnover rates for dinocephalians.
On the basis of our histological findings, we cautiously propose that the histology of the herbivorous dinocephalians (e.g. Keratocephalus, Moschops and Struthiocephalus) and the previously described omnivorous Jonkeria (Bhat et al. in press) are indicative of a semi-aquatic habitat, while the apex predator Anteosaurus has histological features indicative of a more terrestrial lifestyle. It is, however, probable that Anteosaurus may have occasionally inhabited ephemeral water ponds, like the stereospondyl Lydekkerina (Canoville & Chinsamy 2015). Given our small sample size, we suggest that future more comprehensive sampling should be undertaken to verify our conclusions.
Conclusion
- Histological assessment of multiple skeletal elements of different dinocephalian taxa shows that their bone cortex was predominantly composed of fibrolamellar bone, suggesting rapid osteogenesis and an overall fast rate of bone deposition and growth. However, a few skeletal elements have growth marks or LAGs in the fibrolamellar bone tissue, indicating periodic cessation of growth.
- In most of the limb bones, fibrolamellar bone deposition continues up to the periphery, indicating continuous fast growth at the time of death. However, in a few specimens the peripheral cortex consists of avascular lamellar bone tissue containing multiple, closely spaced rest lines, indicating that the growth has significantly slowed down at the time of death and suggesting that these specimens were from mature individuals.
- Several taxon-specific histological variations in terms of the orientation of the vascular canals, incidence of annuli and growth marks, and the extent of secondary reconstruction were noted in the skeletal elements of the studied taxa, which reflects slight differences in growth trajectories, as well as individual variation as a result of ontogeny.
- Intensive distribution of radially organized vascular canals was observed in areas of muscle attachment.
- In herbivorous dinocephalians, large, interconnected vascular channels result in the formation of laminar vascular canals with an overall concentric arrangement; these canals are tightly stacked together, giving a compact appearance to the overall thickness of the cortex.
- The skeletal elements of herbivorous (Keratocephalus, Moschops and Struthiocephalus) and previously described omnivorous (Jonkeria) dinocephalians (Bhat et al. in press) are characterized by a thick cortex and extensively developed medullary spongiosa, which suggest a semi-aquatic lifestyle for these animals.
- The present results suggest that Anteosaurus may have been more terrestrial than its herbivorous/omnivorous relatives, but we note that further sampling is warranted.
Acknowledgements
We are grateful to Iziko South African Museums, Cape Town and Evolutionary Studies Institute (formerly the Bernard Price Institute) at the University of the Witwatersrand, Johannesburg, South Africa for providing access to the specimens used in the current study. Permits 2076, 2131 and 3752-4658 (for histological analyses) were provided by the South African Heritage Resources Agency (SAHRA). The constructive comments and helpful suggestions by K. Angielczyk (Editor) and two anonymous reviewers are gratefully acknowledged. The DST-NRF Centre of Excellence in Palaeosciences (CoE in Palaeosciences) is acknowledged for postdoctoral support for MSB (grant numbers COE2018-12POST, COE2019-PD09 and COE2020-PD09). The Claude Leon Foundation is acknowledged for financial support of CDS (grant number 98813). AC is supported by the NRF African Origins Platform (grant number 117716). Opinions expressed and conclusions arrived at are those of the authors and are not necessarily to be attributed to the CoE.
Author contributions
AC developed the initial concept for the research. AC, CDS and MSB devised and planned the research strategy. CDS performed the original histological sectioning. MSB further polished the histological sections, performed data collection and analysis, and wrote the first draft of the manuscript. AC assisted with the sample selection and supervised the histological work and reviewed the drafts of the manuscript. CDS read and contributed to the final draft.




