A Chemogenetic Toolkit for Inducible, Cell Type-Specific Actin Disassembly
Abstract
The actin cytoskeleton and its nanoscale organization are central to all eukaryotic cells—powering diverse cellular functions including morphology, motility, and cell division—and is dysregulated in multiple diseases. Historically studied largely with purified proteins or in isolated cells, tools to study cell type-specific roles of actin in multicellular contexts are greatly needed. DeActs are recently created, first-in-class genetic tools for perturbing actin nanostructures and dynamics in specific cell types across diverse eukaryotic model organisms. Here, ChiActs are introduced, the next generation of actin-perturbing genetic tools that can be rapidly activated in cells and optogenetically targeted to distinct subcellular locations using light. ChiActs are composed of split halves of DeAct-SpvB, whose potent actin disassembly-promoting activity is restored by chemical-induced dimerization or allosteric switching. It is shown that ChiActs function to rapidly induce actin disassembly in several model cell types and are able to perturb actin-dependent nano-assembly and cellular functions, including inhibiting lamellipodial protrusions and membrane ruffling, remodeling mitochondrial morphology, and reorganizing chromatin by locally constraining actin disassembly to specific subcellular compartments. ChiActs thus expand the toolbox of genetically-encoded tools for perturbing actin in living cells, unlocking studies of the many roles of actin nano-assembly and dynamics in complex multicellular systems.
1 Introduction
Conserved across eukaryotes, the polymerization and depolymerization of actin filaments at nanoscales is tightly regulated to define cellular shape and influences critical cellular functions such as cell motility, morphogenesis, and cell–cell adhesion.[1, 2] Until recently, inducible disassembly of actin filaments required pharmacological agents that lack cell-type specificity and produce off-target effects, providing limited utility for studying the roles of actin in complex biological contexts. Although tools exist for manipulating signaling pathways that indirectly affect actin dynamics,[3, 4] ideal tools for unambiguously assessing the role of actin in a cellular process of interest would directly perturb actin. The development of DeActs, a set of genetically-encodable tools that directly interact with actin monomers to block their ability to polymerize, achieves cell-autonomous control of actin filaments across a broad range of culture and in vivo experimental systems.[5] Since their introduction, DeActs have been used across eukaryotes to study the role of actin in diverse biological processes including neural development,[6-9] embryogenesis,[10, 11] cancer immunology,[12, 13] and plant morphogenesis.[14, 15]
The major limitation of the first generation of DeActs is that they are not rapidly controllable in space or time. Inducible promoters allow for induction of DeAct expression,[5] but the time scale of this (hours or longer) is likely too long to study rapid actin-based processes in cells. Ideally, we would have access to actin tools that could be rapidly activated in cells and that could be restricted or recruited to specific subcellular locations (e.g., to the plasma membrane or specific organelles). Here, we address these unmet needs by designing and optimizing ChiActs, a set of chemically-inducible tools, to rapidly induce actin disassembly in cells. By screening 88 different construct architectures, we optimized ChiActs for 1) potent, inducible actin filament disassembly in cells, and 2) low background filament disassembly in the absence of chemical ligand. ChiActs can induce remarkable actin filament disassembly in multiple mammalian cell types and can perturb actin-dependent cellular functions, such as the nucleocytoplasmic shuttling of the actin-regulated transcriptional coactivator, megakaryoblastic leukemia 1 or myocardin-related transcription factor A (MKL1/MRTFa).[16, 17] We further extend ChiActs for subcellularly-restricted actin depolymerization and demonstrate the applications of ChiActs for manipulating actin nano-assembly locally at the cell cortex, mitochondria, and nucleus to achieve tailored functions.
2 Results
2.1 Initial Screen for Chemogenetic Actin Disassembly Tools (ChiActs)
To develop genetically encoded tools for chemogenetic manipulation of actin disassembly (termed ChiAct; chi means “eat” in Mandarin), we set out to engineer chemically-inducible versions of the DeActs genetic tools we previously reported.[5] We started with a broad screen of 88 constructs encompassing two orthogonal DeAct constructs (DeAct-GS1 and DeAct-SpvB) and multiple strategies for chemically-induced activation (Figure S1, Supporting Information). First, we focused on the more potent version of these DeActs: DeAct-SpvB, the ADP-ribosyltransferase domain from Salmonella enterica that covalently modifies (ADP-ribosylates) actin monomers to prevent actin polymerization, leading to net disassembly of dynamic (treadmilling) actin filaments.[5, 18, 19] We selected four insertion sites for two protein engineering strategies (split or chemoswitch insertion) based on “split energy” calculated by the SPELL algorithm,[20] corresponding to positions P461/E462, T463/D464, S502/P503, and G534/E535 (Figure 1A). In our first design, we used the rapamycin-induced FRB/FKBP dimerization (CID) module[21] to control SpvB function. We envisioned that rapamycin-induced heterodimerization of FRB and FKBP would reconstitute the two split fragments of SpvB and restore its enzymatic function to catalyze ADP-ribosylation of actin monomers to prevent their polymerization into filaments, thereby permitting chemogenetic control of actin disassembly (Figure 1B). In a second complementary design, we used a single-component rapamycin-induced conformational switch, uniRapR,[22] to control SpvB. We hypothesized that a rapamycin-induced conformational change in uniRapR could allosterically modulate the enzymatic function of SpvB to conditionally perturb cellular actin filaments (Figure 1B).
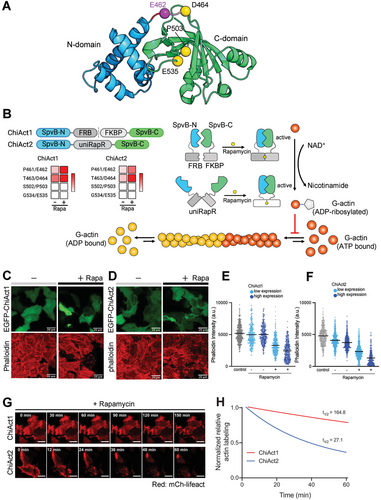
We first tested the actin-disassembly activities of engineered DeAct-SpvB variants by transfecting HeLa cells and then quantifying actin filament levels in fixed cells using rhodamine-conjugated phalloidin staining. We found that transient expression of SpvB with insertion of the FRB-T2A-FKBP module at both S502/P503 and G534/E535 did not affect actin filament levels regardless of the presence of rapamycin, indicating the complete abolishment of SpvB enzymatic activity in these constructs (Figure 1B). In contrast, insertion at both P461/E462 and T463/D464 led to reduced actin filament levels in the absence of rapamycin, indicating that SpvB enzymatic activity was still partially active upon protein splitting (Figure 1B). Moreover, rapamycin induced an additional reduction (or in some cells, complete disappearance) of actin labeling in cells expressing these constructs (Figure 1C,D), suggesting that rapamycin-induced FRB-FKBP dimerization successfully reconstituted the functional reassembly of split-SpvB proteins. Similarly, we found that insertion of uniRapR at both S502/P503 and G534/E535 completely abolished SpvB enzymatic activity, whereas insertion of uniRapR at both P461/E462 and T463/D464 partially caged the function of SpvB, which could be further uncaged in the presence of rapamycin (Figure 1B). In both engineering strategies (FRB-FKBP and uniRapR), the P461/E462 split site resulted in the highest inducible activity with the lowest background.
In addition to DeAct-SpvB, we tested an alternative construct architecture based on human gelsolin segment 1 (DeAct-GS1) that sequesters actin monomer to prevent actin polymerization.[5, 23, 24] Compared to DeAct-SpvB, we observed weaker rapamycin-induced actin disassembly in all split DeAct-GS1 constructs tested (Figures S2 and S3, Supporting Information). While these constructs may be useful in specific cases (e.g., inducing local actin disassembly at subcellular sites), we opted to focus our efforts on the more potent SpvB-based constructs.
Collectively, based on our screening results, we selected DeAct-SpvB with the P461/E462 insertion site for further optimization because of its rapamycin-inducible induction of actin disassembly and relatively low background enzymatic activity in the absence of rapamycin (Figure 1C,D).
2.2 Further Optimization of DeAct-SpvB to Reduce Background Activity
Despite showing promising rapamycin-inducible function, our initial variants of both split-SpvB and UniRapR-SpvB were “leaky” and caused reduced actin filament levels compared to wild-type (WT) cells in the absence of rapamycin. We next aimed to further develop these tools to minimize leakiness. First, we focused on optimizing split-SpvB (EGFP-SpvB(N)-FRB-T2A-FKBP-SpvB(C)). We observed a moderate decrease in actin labeling between untransfected cells and cells transiently expressing split-SpvB, even in the absence of rapamycin (Figure 1C). We reasoned that this background enzymatic activity of FRB-T2A-FKBP-inserted SpvB might be due to the incomplete cleavage of the T2A “self-cleaving” peptide in the FRB-T2A-FKBP chemogenetic module. To tackle this issue, we replaced T2A with an IRES (internal ribosome entry site) to ensure separate co-expression of two SpvB split fragments. Indeed, we observed no significant difference in actin labeling between untransfected cells and cells transiently expressing EGFP-SpvB(N)-FRB-IRES-FKBP-SpvB(C) in the absence of rapamycin, consistent with the complete separation of two SpvB split fragments and no “leakiness” (Figure 1E). Upon incubation with rapamycin for 3 h, we observed a substantial reduction or disappearance of actin labeling in EGFP-positive cells, with the extent of reduction correlating with expression levels (≈40% reduction in phalloidin staining for cells with low expression levels and ≈60% reduction for cells with high expression levels), indicating that rapamycin restored SpvB enzymatic activity to antagonize actin polymerization and that this effect is dose-dependent. We designated this optimized tool with low background activity and strong rapamycin inducibility (EGFP-SpvB(N)-FRB-IRES-FKBP-SpvB(C)) as “ChiAct1”.
Next, we further optimized SpvB-UniRapR for reduced background activity, by shortening the linker sequence to better cage its enzyme function. Indeed, we observed a moderate reduction of actin labeling without rapamycin treatment (≈10%), but substantial (≈60% for cells with low expression levels and ≈80% for cells with high expression levels) actin filament disassembly in response to 3 h of rapamycin treatment (Figure 1F). We designated this orthogonal tool with reduced background activity and strong rapamycin-dependent restoration of enzymatic activity (EGFP-SpvB-UniRapR) as “ChiAct2”.
Finally, we compared the efficacy and kinetics of ChiAct1 and ChiAct2 by time-lapse confocal imaging of actin dynamics in living cells, using the live-cell fluorescent actin reporter mCherry-Lifeact. ChiAct1 exhibited relatively slow kinetics of inducing actin disassembly, as reflected by mCherry (mCh)-Lifeact intensity in HeLa cells (t1/2 = 164.8 min; Figure 1G,H, Movie S1, Supporting Information). In contrast, the single-component ChiAct2 disrupted the actin filament more rapidly (t1/2 = 27.1 min; Movie S2, Supporting Information). Thus, there is a tradeoff between ChiAct1 and ChiAct2: ChiAct1 has negligible “leakiness” in the absence of rapamycin, but slower induction kinetics (likely due to its activation requiring the reassembly of two fully-separated peptides). In contrast, ChiAct2 has some degree of leakiness in the absence of rapamycin, but much faster kinetics of actin disassembly.
2.3 ChiActs Allow Inducible Actin Disassembly in Diverse Biological Systems
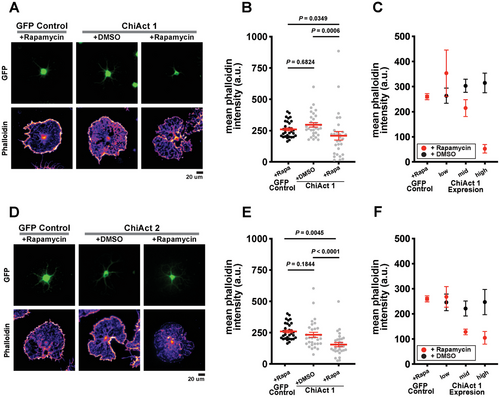
To assess how ChiActs function in primary cells, we next investigated the efficacy of ChiAct-inducible actin disassembly in primary rat oligodendrocytes. As oligodendrocytes differentiate, they extensively remodel their actin cytoskeletons and, ultimately, largely disassemble their actin to promote myelination in vivo or extensive cell spreading in culture.[25, 26] Since cultured oligodendrocytes do not require an intact actin filament to maintain their cell shape,[25, 26] they represent a robust cellular model system for prototyping tools that perturb actin assembly and disassembly.[5] In the absence of rapamycin, expression of either ChiAct1 (Figure 2A,C) or ChiAct2 (Figure 2D–F) in oligodendrocytes showed no significant difference in actin filament levels compared to cells expressing a control EGFP construct. After a two-hour incubation with rapamycin, we observed a significant reduction in actin filament levels in ChiAct1 (Figure 2A,B) and ChiAct2 expressing cells (Figure 2D,E). The rapamycin-induced decrease in actin filament levels required ChiAct expression, as rapamycin itself had no effect on actin in control (EGFP-expressing) oligodendrocytes (Figure S4, Supporting Information). We further quantified the effects of ChiAct expression by measuring phalloidin staining as a function of ChiAct1 and ChiAct2 expression levels. In comparison to EGFP-expressing controls, rapamycin-treated cells expressing either ChiActs exhibited a strong dose-dependent decrease in actin filament levels, with higher expression causing more severe actin disassembly (Figure 2C,F).
Next, to test the functional relevance of ChiAct-SpvB-induced actin disassembly, we focused on cellular events that are tightly regulated by cytoplasmic actin dynamics. MKL1 is a transcriptional co-activator of serum response factor,[27] whose transcriptional activity is directly regulated by its cytosol-to-nucleus shuttling in response to actin dynamics.[16, 17] When the cellular ratio of actin monomers to filaments is high, MKL1 is sequestered in the cytoplasm by forming a complex with actin monomers through its RPEL motifs. Upon cell activation and actin polymerization, MKL1 is liberated from actin monomers and translocates into the nucleus for subsequent gene activation[16, 17] (Figure 3A).
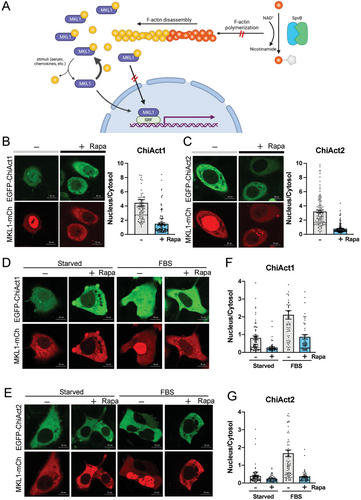
We observed substantial nuclear distribution of mCherry tagged MKL1 (MKL1-mCh) in HeLa cells coexpressing either EGFP-ChiAct1 or EGFP-ChiAct2 in the absence of rapamycin (Figure 3B,C), suggesting low cytoplasmic levels of actin monomers. However, rapamycin caused a much higher cytoplasmic retention of MKL1-mCh in both EGFP-ChiAct1 and EGFP-ChiAct2- expressing cells, consistent with an increase of polymerization-incompetent ADP-ribosylated actin monomers following restoration of SpvB function. We further confirmed this phenotype in cells coexpressing MKL1-mCh and EGFP-ChiAct1 or EGFP-ChiAct2 under different nutrient conditions. Serum (FBS) starvation led to cytoplasmic retention of MKL1-mCh and rapamycin treatment further promoted the retention, whereas FBS stimulation resulted in MKL1-mCh nuclear translocation that could be significantly blocked by pretreatment of rapamycin (Figure 3D,E). Interestingly, we noticed that nuclear retention of MKL1-mCh in cells coexpressing EGFP-ChiAct2 was moderately lower than that in cells coexpressing GFP-ChiAct1 in all four conditions (with or without rapamycin and/or FBS treatment after starvation), consistent with the incomplete caging and the faster kinetics of EGFP-ChiAct2 (Figure 3F,G). We confirmed that rapamycin alone did not affect the nucleocytoplasmic shuttling of MKL1, suggesting the effects observed in cells expressing ChiActs were due to ChiAct activation rather than off-target effects of rapamycin (e.g., on endogenous mTOR signaling; see Discussion) (Figure S5, Supporting Information). Together, these results show that ChiActs can be used to chemogenetically induce actin disassembly in diverse cell types and significantly perturb actin-dependent cellular functions.
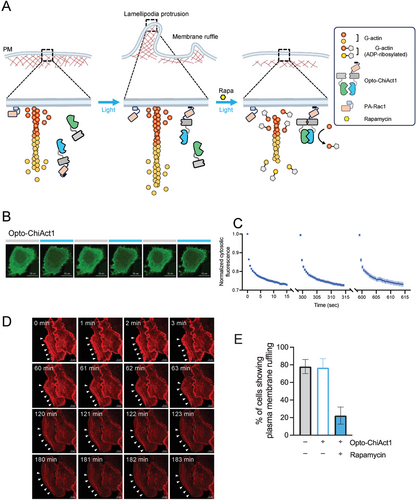
2.4 Adapting ChiActs for Optogenetic Control
To further extend the spatial resolution of ChiActs, we set out to tether ChiActs to different subcellular compartments, thereby confining ChiAct-induced actin disassembly without affecting actin levels at other organelles. OptoPB is an optogenetic tool composed of a polyphosphoinositide (PIP)-binding polybasic domain of Rit1 (Rit-PB) and a photoswitchable light-oxygen-voltage (LOV) domain.[28, 29] Upon blue light stimulation, the LOV domain undergoes a conformational change to uncage the Rit-PB domain to restore its binding to PIPs in the inner leaflet of the plasma membrane (PM), thereby enabling rapid and reversible control of protein translocation from the cytosol to the PM. By fusing OptoPB to ChiAct1 (EGFP-SpvB(N)-FRB-OptoPB-IRES-FKBP-SpvB(C), termed Opto-ChiAct1), we demonstrated the reversible PM targeting of Opto-ChiAct1 in a light-dependent manner (Figure 4A–C). We first validated that Opto-ChiAct1 exhibited similar kinetics as cytosolic ChiAct1 in promoting actin disassembly as reported by mCh-Lifeact in HeLa cells in dark conditions (Movie S3, Supporting Information). Next, we observed that Opto-ChiAct1 exerted a very mild effect on cellular actin dynamics under constant blue light stimulation over 2.5 h (Movie S4, Supporting Information). Activation of photoactivatable Rac1 (PA-Rac1) has been shown to induce actin-dependent lamellipodial protrusions and membrane ruffles around the cell edges[3, 30] (Movie S5, Supporting Information). We observed that PA-Rac1-induced lamellipodial protrusions and membrane ruffles were gradually attenuated by PM-targeted Opto-ChiAct1 in the presence of rapamycin under blue light stimulation, and the intact actin filaments were inwardly detached from the cell edges (Figure 4D,E, Movie S6, Supporting Information). The inhibition of lamellipodial protrusions and membrane ruffles was not due to rapamycin itself but required ChiAct expression (Movie S7, Supporting Information). Taken together, our results are consistent with PM-confined actin disassembly and accumulation of polymerization-incompetent ADP-ribosylated actin monomers by PM-targeted Opto-ChiAct1, which in turn lead to attenuation of PA-Rac1 induced lamellipodial protrusions and membrane ruffles as well as retraction of actin filaments from the cell edges.
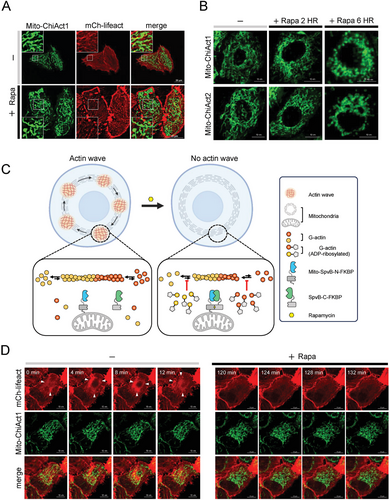
2.5 Organelle-Tethered ChiActs for Subcellular Control of Actin-Regulated Events
Mitochondria adapt to different cellular states and metabolic demands through the precise regulation of mitochondrial dynamics, including fission, fusion, and motility.[31-33] The actin network plays multiple distinct roles in these processes, yet its functions are still not well understood. By fusing a mitochondrial outer membrane localization tag NTOM20 to ChiAct1 (termed Mito-ChiAct1), we observed higher levels of fragmented actin filaments surrounding mitochondria in the presence of rapamycin (Figure 5A). With prolonged activation of Mito-ChiAct1 with rapamycin, mitochondrial fission dominated the fusion–fission dynamics and eventually mitochondrial fragmentation was prevalent in each cell, consistent with our findings of mitochondrion-confined actin depolymerization (Figure 5B). Similar to Opto-ChiAct1, Mito-ChiAct1 exhibited a much weaker effect on cellular actin disassembly as compared to cytosolic ChiAct1 (Movie S8, Supporting Information). Moreover, recent studies found that mitochondrial dynamics are regulated by cycling waves of transient actin polymerization/depolymerization that propagate through mitochondria subpopulations with a period of ≈15 min.[31-33] In the presence of rapamycin, Mito-ChiAct1 catalyzed ADP-ribosylation of actin monomers and depleted the polymerization-competent actin monomer pool surrounding mitochondria, thereby gradually inhibiting the presence of mitochondrial actin waves (Figure 5C,D, Movie S9, Supporting Information). As in earlier experiments, we confirmed that the inhibition of mitochondrial actin waves was not due to rapamycin itself, but required ChiAct expression (Movie S10, Supporting Information).
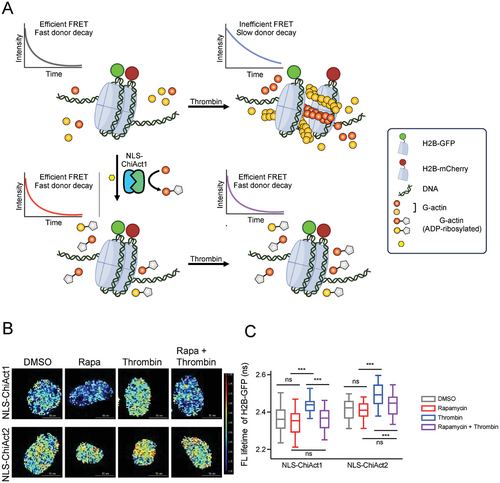
In addition to its many roles in the cytoplasm, accumulating evidence indicates that a dynamic pool of actin constantly shuttles between the cytoplasm and nucleus and has distinct roles in the nucleus. For example, nuclear actin monomers interact with chromatin-remodeling complexes and participate in transcription, and nuclear actin polymerization is involved in regulating spatiotemporal chromatin organization and the maintenance of nuclear architecture.[34-37] By fusing a nuclear localization signal NLS to both ChiAct1 and ChiAct2, we aimed to specifically perturb nuclear actin filament assembly and affect chromatin organization. Previous studies have documented that thrombin-induced GPCR activation and calcium influx promote nuclear actin filament assembly and trigger nuclear actin-dependent alterations in chromatin organization as reported by a fluorescence lifetime imaging microscopy (FLIM) enabled fluorescence energy transfer (FRET) assay between GFP- and mCherry-tagged histone H2B[34] (Figure 6A). Here we observed that chromatin became significantly more dynamic upon treatment of thrombin as indicated by a substantial increase in fluorescence lifetime of H2B-GFP, and this phenomenon was blocked by pretreatment of rapamycin (Figure 6B,C). Our results suggest that nuclear ChiActs inhibit thrombin-induced nuclear actin assembly and associated chromatin reorganization.
3 Discussion
The dynamic interplay between polymerization and depolymerization of actin filaments is essential for actin's many roles in intracellular trafficking, cell signaling, mechanical support, and structure scaffolding. Despite the rapidly expanding understanding of actin structure, organization, and its polymerization and depolymerization mechanisms, our understanding of its roles in specific organelles and cellular processes is largely confounded by a lack of tools for experimentally perturbing actin dynamics with desired spatial and temporal resolution. Traditional methods, such as pharmacological agents and genetic manipulation of actin-associated signaling pathways,[3, 5, 38-40] suffer from several drawbacks, including lack of cell-type specificity and off-target effects, limiting their utility in complex biological systems. These conventional approaches often lead to broad, uncontrolled perturbations of actin dynamics that do not allow for fine-tuned disassembly or assembly at specific cellular sites or in response to precise triggers.
Recent advances in optogenetics and photopharmacology have enabled the optical control of these pharmacological agents and genetically encoded tools. For instance, through functionalizing jasplakinolide or latrunculin with azobenzene photoswitches, optojasps and neo-optojasps were able to exert light-dependent disruption of actin dynamics by stabilizing actin protomer assemblies,[41, 42] while para N-benzyl azobenzene photoswitch was incorporated with latrunculin to achieve light-dependent sequestration of actin monomers.[43] In terms of genetically encoded tools, the photoreactive light oxygen voltage (LOV) domain was used to engineer photoactivatable Rac1 (PA-Rac1),[3] CRY2/CIBN light-gated dimerization system was used to develop optoGEF-RhoA,[40] and the LOV domain and its binding partner Zdk were used to devise Z-lock cofilin.[39] Nevertheless, none of these perfectly addresses the requirements of satisfying spatiotemporal resolution, cell-type specificity, and off-target effects.
The development of ChiActs introduces a significant advancement over existing tools for remote control of actin dynamics, offering the potential for more precise spatial and temporal control. ChiActs are genetically encoded tools that enable actin filament disassembly to be tightly controlled using chemical inducers or light for subcellular targeting, achieving both high specificity and minimal off-target effects. This is accomplished by harnessing chemical-induced dimerization or allosteric switching mechanisms to activate split fragments of DeAct-SpvB. The ability to target ChiActs to specific subcellular locations, such as mitochondria, the nucleus, or the plasma membrane, allows for the study of localized actin dynamics and its role in cellular functions with unprecedented precision. Moreover, since ChiActs directly target actin, and actin is highly conserved across eukaryotes, ChiActs have the additional potential advantage of being functional across model systems.
Compared to earlier generation tools like DeActs,[5] ChiActs address existing limitations by offering improved temporal control and subcellular precision. While DeActs provide a foundation for genetically encoded actin manipulation by blocking actin polymerization through interactions with actin monomers, they lack the ability to rapidly modulate actin dynamics or restrict disassembly to specific cellular regions. The induction of DeAct expression can take hours, limiting its application for studying rapid cellular processes. ChiActs, on the other hand, utilize a split-protein design combined with rapamycin-induced dimerization or allosteric switching to achieve actin disassembly within minutes, enabling real-time studies of actin-dependent processes. This rapid activation allows ChiActs to be used in dynamic cellular events, such as chromatin reorganization, mitochondrial fission, and lamellipodial protrusion, where precise timing and localization of actin remodeling are critical. Here we demonstrated that ChiActs were able to inhibit PA-Rac1-induced lamellipodial protrusions and membrane ruffling, to induce remodeling of mitochondrial morphology and abolish mitochondrial actin waves, and to affect chromatin organization as reported by FLIM-FRET between H2B histones within 2 h. Moreover, the engineering of ChiActs to minimize background activity in the absence of inducers ensures that actin disassembly is tightly regulated, providing more accurate experimental control and interpretation of results. Future efforts may also focus on re-engineering ChiActs to respond to Rapalog, thereby reducing the potential for “off-target effects” of rapamycin on endogenous mTOR signaling.
How could future versions of ChiActs continue to improve temporal control of induced actin disassembly? One potential strategy would be to use light-mediated dimerization to activate ChiActs instead of using rapamycin, as this would be immediate and not rely on the relatively slower kinetics of rapamycin crossing the cell membrane and binding to the ChiAct. Even with such optical control, since ChiActs target actin monomers, the speed of actin disassembly may be limited by the cellular rate of actin filament treadmilling, which could vary appreciably between cell types. To overcome this inherent barrier, future versions of ChiActs could instead directly induce the disassembly of existing actin filaments, for example by inducible actin filament severing. The framework developed here for optimizing and validating inducible actin disassembly tools could be repurposed for testing such next-generation ChiAct tools.
In summary, ChiActs significantly expand the molecular toolkit available for studying actin dynamics, allowing for targeted, rapid, and temporal modulation of actin structures in living cells. We anticipate these tools being useful in advancing our understanding of the multifaceted roles of actin nanostructures in cellular physiology and disease contexts.
4 Experimental Section
Plasmid DNA Constructs
Plasmids were constructed using the standard restriction enzyme cloning method, an adaptation of the QuikChange mutagenesis protocol, and the Gibson assembly method. KOD Hot Start DNA polymerase was purchased from EMD Millipore and used for PCR amplification. Oligonucleotides were synthesized by Sigma Aldrich. The T4 DNA ligase kit and NEBuilder HiFi DNA Assembly Master Mix were purchased from New England BioLabs. QuikChange Lightning Multi Site-Directed Mutagenesis Kit was obtained from Agilent Technologies. To construct EGFP-SpvB(N)-FRB-T2A-FKBP-SpvB(C), the FRB-T2A-FKBP gene fragment was synthesized as a gblock (Azenta Life Sciences) and then inserted into the pCMV-DeAct-SpvB (Addgene no. 89446) between candidate insertion sites (P461/E462, T463/D464, S502/P503, and G534/E535) by Gibson assembly. EGFP-SpvB-UniRapR, EGFP-SpvB-LOV2, and EGFP-SpvB(N)-iLiD-P2A-SspB-SpvB(C) were made by using the same strategy where UniRapR, LOV2, and iLiD-P2A-SspB were synthesized as gblock gene fragments and then inserted into the pCMV-DeAct-SpvB respectively by Gibson assembly. Similarly, gblock gene fragment encoding either FRB-T2A-FKBP, UniRapR, LOV2, or iLiD-P2A-SspB was inserted into pCMV-DeAct-GS1(Addgene no. 89445) between candidate insertion sites (G64/K65, P67/G68, T92/G93, G107/N108, G118/N119, Q151/G152, and S164/G165) to generate EGFP-GS1(N)-FRB-T2A-FKBP-GS1(C), EGFP-GS1-UniRapR, EGFP-GS1-LOV2, or EGFP-GS1(N)-iLiD-P2A-SspB-GS1(C). To construct EGFP-SpvB(N)-FRB-IRES-FKBP-SpvB(C) (designated as ChiAct1), T2A from EGFP-SpvB(N)-FRB-T2A-FKBP-SpvB(C) was replaced by IRES using an adaptation of the QuikChange mutagenesis protocol. EGFP-SpvB-UniRapR (designated as ChiAct2) variants with 0, 1, 2, 3-mer linkers were generated by PCR-based linker deletion. To construct Opto-ChiAct1, the cDNA seuqnce encoding OptoPB was PCR amplified from mCherry-OptoPB (addgene no. 113935) and inserted into EGFP-SpvB(N)-FRB-IRES-FKBP-SpvB(C) through Gibson assembly. To construct Mito-ChiACt1 and Mito-ChiAct2, cDNA encoding NTOM20 was PCR amplified from pTriEx-Ntom20-mVenus-Zdk2 (addgene no. 81011) and cloned into EGFP-SpvB(N)-FRB-T2A-FKBP-SpvB(C) or EGFP-SpvB-UniRapR between the NheI and AgeI restriction sites. To construct NLS-ChiAct1, the NLS sequence from SV40 (PKKKRKV) was sequentially inserted preceding and following EGFP-SpvB(N)-FRB-IRES-FKBP-SpvB(C) as NLS- EGFP-SpvB(N)-FRB-IRES-FKBP-SpvB(C)-NLS. To construct NLS-ChiAct2, SV40 NLS was inserted preceding EGFP-SpvB-UniRapR. To make mCh-Lifeact, Lifeact sequence (MGVADLIKKFESISKEE) was introduced in primers and cloned into mCherrry2-C1 (addgene no. 54563) by PCR. To construct MKL1-mCh, the cDNA sequence encoding mCherry2 was PCR amplified and cloned into p3xFLAG-MKL1 (addgene no. 11978) via Gibson assembly. H2B-GFP (addgene no. 11680) and H2B-mCherry (addgene no. 20972) were purchased from Addgene.
Cell Culture, Transient Transfection, and Drugging of HeLa Cells
HeLa cells were obtained from ATCC and cultured in Dulbecco's modified Eagle's media (DMEM, Sigma-Aldrich), supplemented with 10% fetal bovine serum (FBS) at 37 °C and 5% CO2. DNA transfections were carried out by using Lipofectamine 3000 reagent (Life Technologies) following the manufacturer's instructions. For live-cell or fixed-cell imaging experiments, cells were seeded in four-chamber 35-mm glass-bottom dishes (D35C4-20-1.5-N, Cellvis) at 40–60% confluency and then imaged after transfection for 24 h. Rapamycin (Fischer Cat No. NC9163747) was initially diluted to 10 mm in DMSO, then diluted again to 20 µm in fresh culture media and added to cells for 2 µm final concentration with a 10% volume change.
Confocal Imaging and Image Analysis of HeLa Cells
Confocal imaging was performed on a Nikon Ti2 inverted microscope equipped with a Yokogawa W1 spinning disk scan-head, a LUNV laser (405/488/561/640 nm), and a live-cell culture cage to maintain the temperature at 37 °C with 5% CO2. High-resolution imaging was captured using a 60× oil lens. Data analysis was performed using Nikon NIS-Elements imaging software, and the results were plotted using Prism 10.0.0 (GraphPad) software.
For screening and optimization of the ChiActs, HeLa cells were transfected with individual candidate EGFP-ChiAct plasmids for 24 h and incubated with 2 µm rapamycin for an additional 3 h at 5% CO2 and 37 °C. After drug treatments, cells were immediately fixed with 4% paraformaldehyde (PFA) for 15 min at room temperature (RT), rinsed three times with PBS, and permeabilized with 0.5% Triton X-100 in PBS for 5 min at RT, and then rinsed again with PBS. To stain for actin filaments, the cells were incubated with 100 nm of rhodamine phalloidin (Cytoskeleton Inc. Cat No. PHDR1) for 30 min at RT and washed three times with PBS. Images were acquired for both green (488 nm) and red (561 nm) fluorescence channels.
To monitor ChiActs-mediated actin filament disassembly in live cells, confocal time-lapse imaging was performed on HeLa cells cotransfected with either EGFP-ChiAct1 or EGFP-ChiAct2 and mCh-Lifeact. Images were acquired every 60 s over the course of 150 min, and the relative mCh-Lifeact intensity (expressed F/F0) was used as an indicator of actin filament levels over time. Rapamycin (2 µm final concentration) was added right before the first frame, and cells were kept at 5% CO2 and 37 °C.
To monitor nucleocytoplasmic shuttling of MKL1, confocal images of HeLa cells cotransfected with either EGFP-ChiAct1 or EGFP-ChiAct2 and MKL1-mCh were acquired after incubation with 2 µm rapamycin for 2 h at 5% CO2 and 37 °C. To start cell starvation, 24 h after transfection, the culture media was replaced with serum-free DMEM and incubated for an additional 24 h at 5% CO2 and 37 °C. To induce nuclear translocation of MKL1 in serum-starved cells, FBS was added back to the serum-free DMEM to achieve a final concentration of 10%. This was done either with or without pretreatment with 2 µm rapamycin for 2 h prior to FBS addition. The nucleocytoplasmic distribution of MKL1 was quantified by calculating the nucleus-to-cytosol (N/C) ratio of MKL1-mCh fluorescence intensities in individual cells.
To monitor light-inducible plasma membrane (PM) targeting of Opto-ChiAct1, confocal images of HeLa cells transfected with Opto-ChiAct1 were acquired every 0.5 s over the course of 15 s. The process was repeated three times with 5 min recovery interval between each session. The 488-nm laser was used for photostimulation of the LOV domain and excitation of EGFP (with 50% output and 0.1 s excitation), and the relative EGFP intensity (expressed F/F0) was used as an indicator of Opto-ChiAct1 PM targeting over time. To monitor Opto-ChiAct1 mediated inhibition of lamellipodial protrusions and membrane ruffles, confocal time-lapse imaging was performed on HeLa cells cotransfected with Opto-ChiAct1, PA-Rac1, and mCh-Lifeact every 20 s over the course of 270 min. An external blue LED light source (470 nm, 4 mW cm−2) was used for continuous photostimulation of Optp-ChiAct1. Rapamycin was added at 0 min, and cells were kept at 5% CO2 and 37 °C. Quantification of cells showing lamellipodial protrusions and membrane ruffles was made during 180–240 min.
To monitor Mito-ChiAct1 mediated actin filament fragmentation around mitochondria, confocal images of HeLa cells cotransfected with Mito-ChiAct1 and mCh-Lifeact were acquired with or without 2 h treatment of rapamycin. To monitor changes in mitochondrial morphology, confocal images of HeLa cells cotransfected with either Mito-ChiAct1 or Mito-ChiAct2 were acquired following rapamycin treatment for 0, 2, or 6 h under 5% CO2 at 37 °C. Additionally, to visualize Mito-ChiAt1 mediated inhibition of mitochondrial actin waves, confocal time-lapse imaging was performed on HeLa cells cotransfected with Mito-ChiAct1 and mCh-Lifeact every 1 min over the course of 180 min. Rapamycin was added at 30 min, and cells were kept under 5% CO2 at 37 °C.
Fluorescence Lifetime Imaging Microscopy Combined with Fluorescence Resonance Energy Transfer (FLIM-FRET)
HeLa cells were grown to ≈65% confluence on glass coverslips and transiently transfected overnight with indicated NLS-ChiActs and either H2B-GFP alone or with both H2B-GFP and H2B-mCherry using Lipofectamine according to manufacturer's instructions. Cells were pretreated with either 2 µm rapamycin or DMSO for 2 h and followed by 0.4 U ml−1 thrombin stimulation for 15 min before being fixed in 4% PFA. Cells were then imaged using a 60× Plan-Apo/1.4 NA oil immersion lens mounted on a Nikon Eclipse wide-field microscope and GFP fluorescence lifetime was measured using a FLIM unit (Lambert Instrument, Roden, The Netherlands). GFP was excited using a sinusoidally modulated 3-W 497-nm light-emitting diode (LED) at 40 MHz under epifluorescence. Fluorescein was used as a lifetime reference standard (≈4.1 ns). Three independent experiments were performed under each condition. Data from a total of 46–55 cells were pooled to calculate mean and S.E and statistical analysis was performed using one-way analysis of variance (ANOVA).
Animal and Ethics Statement
All rodent procedures were approved by Stanford University's Administrative Panel on Laboratory Animal Care (APLAC, IACUC Protocol 32260) and followed the National Institutes of Health guidelines. Rats were housed under standard 12:12 light–dark cycles at temperatures of 18–23 °C and 40–60% humidity with free access to food and water, and disposable bedding in plastic cages. Sprague–Dawley rats were ordered from Charles River Laboratories.
Purification and Culture of Primary Rat OPCs/Oligodendrocytes
Primary oligodendrocyte precursor cells (OPCs) were purified by immunopanning from P5-P7 Sprague Dawley rat brains as previously described.[25, 44] Rat pups were euthanized by rapid decapitation with no anaesthetic. OPCs were typically seeded at a density of 150 000–250 000 cells/10-cm dish and allowed to recover and proliferate for 4 days in culture before lifting cells via trypsinization and distributing for proliferation or differentiation assays. All plasticware for culturing oligodendrocyte precursors were coated with 0.01 mg ml−1 poly-D-lysine hydrobromide (PDL, Sigma P6407) resuspended in water. All glass coverslips for culturing oligodendrocyte precursors were coated with 0.01 mg ml−1 PDL, which was first resuspended at 100× in 150 mm boric acid pH 8.4 before diluting to 1× in water (PDL-borate).
To proliferate primary oligodendrocyte precursors, cells were cultured in serum-free defined media (DMEM-SATO base medium) supplemented with 4.2 µg ml−1 forskolin (Sigma-Aldrich, Cat#F6886), 10 ng ml−1 PDGF (Peprotech, Cat#100-13A), 10 ng ml−1 CNTF (Peprotech, Cat#450-02), and 1 ng ml−1 neurotrophin-3 (NT-3; Peprotech, Cat#450-03) at 37 °C with 10% CO2. To induce differentiation, cells were switched to DMEM-SATO base media containing 4.2 µg ml−1 forskolin (Sigma-Aldrich, Cat#F6886), 10 ng ml−1 CNTF (Peprotech, Cat#450-02), 40 ng ml−1 thyroid hormone (T3; Sigma-Aldrich, Cat#T6397) and (for mouse OPCs only) 1× NS21-MAX (R&D Systems AR008).
Transfection of OPCs
Oligodendrocyte precursor cells (OPCs) were dissociated with 0.05% trypsin, counted, and centrifuged (Sorvall Legend RT) for 10 min at 700 rpm into a cell pellet. The cells were resuspended with 20 ml of nucleofector solution (Lonza P3 Primary Cell 4D-Nucleofector V4XP-3032). 250 000 cells per transfection were aliquoted into tubes containing 0.4 µg of plasmid DNA. The cells were transfected in a Lonza 4D-Nucleofector X Unit (AAF-1003X) assembled with a 4D-Nucleofector Core Unit (AAF-1002B) with solution P3 and pulse code DC-218.
After transfection, the cells were incubated for 10 min at room temperature to recover, then resuspended in 80 µl antibiotics-free DMEM-SATO media. The cells were then diluted in 900 µl of antibiotic-free DMEM-SATO media with differentiation factors and 100 µl of that suspension was transferred to PDL-borate coated coverslips. After a ten-minute room temperature incubation, the wells were flooded with 400 µl of antibiotic-free DMEM-SATO media with differentiation factors added. To induce differentiation, DMEM-SATO base media was prepared with 4.2 µg ml−1 forskolin (Sigma-Aldrich, Cat#F6886), 10 ng ml−1 CNTF (Peprotech, Cat#450-02), and 40 ng ml−1 thyroid hormone (T3; Sigma-Aldrich, Cat#T6397).
Drugging of Cultured Cells
After four days in differentiation media, 2 µm rapamycin (Fischer Cat No. NC9163747) was added to differentiated oligodendrocytes for two hours at 10% CO2 and 37 °C. Rapamycin was initially diluted in DMSO to working a concentration, then diluted again to a 2× working concentration in fresh differentiation media and added to cells in a 50% media change.
Immunofluorescence of Oligodendrocytes
After drug treatments, cells were immediately fixed with 4% paraformaldehyde (PFA) for 15 min at room temperature (RT), rinsed three times with 1× PBS, and stored at 4C. The cells were permeabilized with 0.1% Triton X-100 in PBS for 3 min at RT and then blocked in 3% BSA for 20 min. To stain for actin filaments, the cells were incubated with 7 µl ml−1 of Alexa Fluor 594-Phalloidin (Invitrogen A12381) and CellMask blue (Fisher, H32720) for 15 min. The coverslips were washed three times with 1× PBS and mounted on Superfrost microscope slides (Fisher) with Fluormount-G (Southern Biotech, 0100–01). All coverslips were imaged by epifluorescence with a Zeiss Axio Observer Z1 microscope with a Plan-Apo 20×/0.8 NA objective. Images were acquired blinded with identical illumination and acquisition settings.
Data Analysis and Statistics
Fluorescence intensity and cell size were quantified with FIJI on blinded images. Data were analyzed and plotted using Excel and GraphPad Prism. Data was first assessed for normality with the Shapiro-Wilk's test and statistical significance was then determined with either a nonparametric one-way ANOVA and Dunn's multiple comparison tests, a one-way ANOVA and Tukey's multiple comparisons, or a Mann–Whitney t-test.
Acknowledgements
This work was supported by the Welch Foundation (BE-1913-20220331 to Y.Z.), the McKnight Endowment Fund for Neuroscience (JBZ), the National Multiple Sclerosis Society Harry Weaver Neuroscience Scholar Award (JBZ), the Beckman Young Investigator Award (JBZ), the Myra Reinhard Family Foundation (JBZ), and the Koret Family Foundation (JBZ), and the National Institutes of Health (R01GM144986, R21AI174606, and R21GM145063 to Y.Z.; R01NS119823 and R21NS131999 to J.B.Z.). Images were created by Biorender.com and Pymol.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
T.-H.L. and N.A. contributed equally to this work. Y.Z. and J.B.Z. conceived of the project. T.-H.L., N.A., Y.-T.L., and T.N., designed, performed, and analyzed all experiments. T.-H.L., N.A., Y.Z., and J.B.Z. wrote the manuscript. All authors reviewed and gave feedback on the manuscript.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.