Expanding the Potential Window through Synergistic Design and Oriented Heterostructure for Supercapacitor
Abstract
Metal telluride-based nanomaterials have recently gained attention as promising candidates for enhancing the performance of electrodes in energy storage devices. In this study, Co-Zr-Te@CuO electrode materials engineered through strategic approach are introduced, involving the deposition of a Co-Zr metal-organic framework (MOF) on CuO nanowires, followed by a tellurization. This composite material demonstrates an expanded potential window of 1.2 V, making it potential electrode material for supercapacitor applications. Electrochemical evaluations reveal that the Co-Zr-Te@CuO electrode exhibits 576 C g−1, 1.8 times higher than Co-Zr-MOF@CuO. Furthermore, density functional theory (DFT) calculations confirm enhancements in conductivity and explains the synergistic effects present within the heterostructure. Hybrid supercapacitor (HSC) device achieves a peak energy density of 69.4 Wh kg−1 at a power density of 1.4 kW kg−1. This evidence of Co-Zr-Te@CuO effective electrode performance demonstrates its potential and robust stability for real-world energy storage applications.
1 Introduction
Metal-organic frameworks (MOFs) with their highly porous crystalline structures have opened new avenues in the development of advanced materials.[1-3] MOFs are proclaimed for their potential in various applications due to their inherent chemical versatility and significant porosity.[4, 5] With the development of supercapacitor (SC) electrodes, MOFs are harnessed in a dual capacity: firstly as direct electrode materials and secondly as scaffolds for the synthesis of a variety of compounds, including porous carbons, metal sulfides, metal oxides, and metal phosphides, as well as composite materials.[5, 6] Nonetheless, MOFs direct incorporation into SC electrode frameworks often faces challenges, primarily those associated with their limited chemical stability and electrical conductivity, which restrain their broader application in supercapacitors.[7]
Researchers have directed efforts toward addressing the challenges associated with the low stability and conductivity of MOFs.[8] The development of composites made from MOF-derived metallic compounds and carbon, as well as transition metal chalcogenides like sulfur (S), selenium (Se), and tellurium (Te), has been pursued.[9] Tellurium demonstrates the much higher electrical conductivity (2 × 102 S m−1) compared to sulfur (5 × 10⁻¹⁶ S m−1) and selenium (1 × 10⁻⁴ S m−1).[9] When incorporated into MOFs, tellurium may enhance electrical conductivity and boost electrochemical activity, making the materials more effective for energy storage.[10, 11] The resulting telluride-based composites can demonstrate improved charge storage capacities and facilitate rapid, reversible redox reactions, essential for high-performance supercapacitors.[10] These advantages position telluride derivatives as promising candidates for the next generation of electrode materials, meeting the growing demands for efficient and durable energy storage.[10]
Notable findings have emerged concerning electrode materials based on metal telluride.[12, 13] Kshetri et al.[10] have developed a new type of supercapacitor electrode by synthesizing a porous cobalt telluride–carbon composite from a metal-organic frame integrated onto a nickel foam current collector. Shi et al.[12] fabricated bimetallic cobalt telluride from Co-MOF on carbon cloth for supercapacitor application. The electrode material showed a specific capacitance of 261.4 mAh g-1 at 1.0 A g-1 in 6 m KOH electrolyte. These materials are distinguished by their unique structures, high surface areas, and adjustable porosity, which are crucial for surmounting the challenges previously described. Specifically, MOFs that incorporate cobalt ions are notable for their precision-engineered structures and configurations, making them excellent precursors for developing cobalt-telluride-based electrode materials.[14] Additionally, incorporating zirconium into these cobalt-based MOFs can expand the potential window, thereby enhancing their electrochemical performance.[15]
However, most composites derived from MOFs are synthesized in powder form, presenting challenges in their integration into practical devices.[1, 16] Powders lack the mechanical strength and the directional electrical conductivity required for efficient electron transport in supercapacitors.[17] To address this, the development of structured electrodes where MOF-derived materials are supported on conductive substrates becomes imperative.[18, 19] Copper oxide (CuO), with its p-type semiconductive properties and high theoretical capacitance, emerges as an excellent candidate for this purpose.[15] The growth of CuO in the form of nanowires not only provides a high aspect ratio conducive to electron transport but also offers an oriented and stable backbone for the uniform deposition of MOF-derived materials. This hybrid configuration exploits the high surface area of MOFs while leveraging the conductive network of CuO nanowires, thus bridging the gap between the desirable electrochemical properties of MOFs and the practical requirements of supercapacitor electrodes.[20]
In our study, we synthesized Co-Zr-Te@CuO, a novel electrode material by layering a Co-Zr MOF framework on CuO nanowires and modifying it through tellurization, achieving a potential window of 1.2 V suitable for supercapacitors. This composite exhibits high specific capacity and rapid electron transport of the telluride with a robust CuO nanowire network for efficient charge transfer, significantly improving capacitance, energy density, and stability. Validated by density functional theory (DFT) calculations, our findings indicate that Co-Zr-Te@CuO not only surpasses typical MOF limitations but also sets new benchmarks for high-performance energy storage, advancing supercapacitor technology.
2 Results and Discussion
After the synthesis of CuO nanowires, it served as scaffolds for the formation of Co-Zr-MOF through a solvothermal route (Figure 1a). The SEM depiction of the CuO nanowires, Co-Zr-MOF@CuO, and Co-Zr-Te@CuO composites are displayed in Figure 1b–j. The CuO nanowires grew uniformly from the copper mesh substrate, shown in Figure 1b–d. This architecture could be advantageous for polarization processes, as the nanowires might enable easy transfer of charge to and from the copper base. Additionally, the delicate nanowire framework could also act as a foundation for the deposition of subsequent active substances. Figure 1e–g exhibits the Co-Zr-MOF@CuO composite diverges from the pure CuO nanowires, featuring an assembly of slender polyhedra and planar rods, as captured in the surface topology image. Gaps are obvious among these structures, and the polyhedral MOF is observed to be uniformly attached to the CuO nanowires.
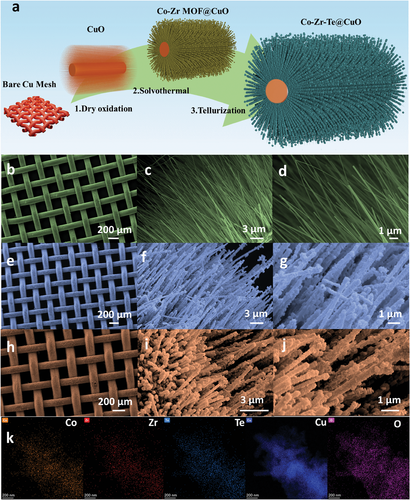
The synthesis of Co-Zr-MOF derived telluride on the CuO nanowires (Co-Zr-Te@CuO) resulted in a more defined surface texture, as shown in Figure 1h–j. A uniform layer of MOF derivative telluride with a distinct polyhedral orientation was observed to wrap each CuO nanowire. It is noteworthy that the Co-Zr telluride consistently appeared in an organized manner, particularly under higher magnification. Consequently, the Co-Zr-Te@CuO exhibited a neater, more sharply defined structure with polyhedrons, attributed to the successful growth of the MOF-derived telluride on the nanowires. Figure 1b,e,h display the aerial views of CuO@Cu, Co-Zr-MOF@CuO, and Co-Zr-Te@CuO, respectively. The low magnification SEM images show that the integrity of the Cu mesh remains intact throughout the procedure, from dry oxidation to the two solvothermal steps. This indicates that the Cu mesh serves as an excellent current collector, even under harsh conditions.
Elemental distribution within the Co-Zr-Te@CuO hybrid is depicted in Figure 1k. This mapping confirms the existence of zirconium, cobalt, carbon, copper, and oxygen elements, reinforcing the conclusion that the Co-Zr-Te@CuO composite was successfully synthesized, as also supported by TEM investigations.
Transmission electron microscopy (TEM) images were employed to verify the architectural details of the Co-Zr-Te@CuO composite, exhibited in Figure S1 (Supporting Information). The images confirmed the CuO nanowires were successfully integrated into the composite and the width of CuO nanowires is 70–80 nm (Figure S1a, Supporting Information). Figure S1b–d (Supporting Information) Co-Zr-Te@CuO are highlighted, where Co-Zr-Te particles, uniform in size and shape, were found to be evenly distributed along the CuO nanowire surface. The Co-Zr-Te@CuO overall width ranged from 200 to 300 nm. TEM results are well aligned with earlier found SEM results of uniform distributed Co-Zr-Te at CuO nanowires.
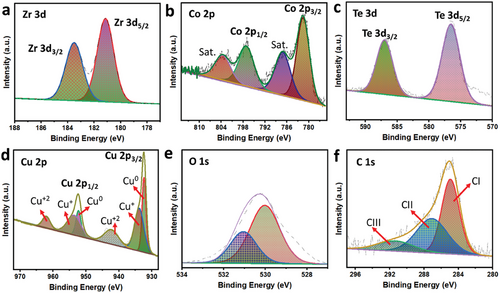
Figure S2 (Supporting Information) displays the XRD analysis, which confirms the crystalline structure of Co-Zr-Te @CuO. This figure reveals the presence of characteristic peaks corresponding to Cu from copper mesh (JCPDS No. 01-085-1326), CuO nanowire (JCPDS No. 01-080-1268), and M-Zr-Te (JCPDS No. 00-043-1142), all of which are well-indexed according to previous literature.[2] The presence of all three crystal phases in the XRD spectrum confirms the successful in situ integration of Co-Zr-Te on CuO nanowires. The XPS analysis was conducted to evaluate the valence states of the elements in Co-Zr-Te@CuO, shown in Figure 2. The Zr 3d core level spectrum exhibited spin-orbit doublets of Zr 3d3/2 and Zr 3d5/2 at approximately 184.2 and 181.5 eV, respectively, depicted in Figure 2a. Figure 2b shows the Co (2p) spectrum, a spin–orbital splitting was observed, with the 2p1/2 and 2p3/2 peaks appearing at binding energies of 797.5 and 780.91 eV, respectively, along with satellite peaks. Tellurium XPS peaks (Figure 2c) have two distinct peaks at 586.7 eV and 576.3 eV, associated with Te 3d3/2 and Te 3d5/2, suggesting the presence of a negative divalent state of tellurium. The Cu (2p) core level spectrum displayed characteristic peaks at binding energies of approximately 952.2 eV (Cu 2p1/2) and 932.4 eV (Cu 2p3/2), exhibited in Figure 2d. The deconvolution of Cu 2p1/2 and Cu 2p3/2 revealed peaks at 932.3 eV and 952.1 eV for Cu0, and peaks at 934.1 eV and 953.5 eV for Cu1+. The presence of divalent Cu was indicated by the satellite peaks. The presence of all three possible oxidation states of copper originates from the copper mesh used as the current collector, the CuO scaffold, and its interaction with Co-Zr-Te. The Gaussian fitting of the O 1s spectrum (Figure 2e) revealed the presence of three distinct peaks at binding energies of approximately 530.4 eV (OI), 530.7 eV (OII), and 533.0 eV (OIII). These peaks correspond to the metal─oxygen bond, surface oxygen, and adsorbed water on the surface, respectively. Figure 2f exhibits the XPS spectra of C 1s, a peak at 284.2 eV associated with sp carbon,[19] while peaks at 285.4 eV and 288.3 eV could be attributed to C─O and C═O, respectively. Overall, the XPS investigation confirmed the successful formation of Co-Zr-Te@CuO.
The electrochemical performance of the Co-Zr-Te@CuO and Co-Zr-MOF@CuO electrode materials was evaluated using cyclic voltammetry (CV), galvanostatic charge-discharge (GCD), and electrochemical impedance spectroscopy (EIS) in a three-electrode system with 3 m KOH as the electrolyte. The CV peaks of Co-Zr-Te@CuO and Co-Zr-MOF@CuO at 10 mV s−1 are shown in Figure 4a, with a potential operational window of −0.6–0.6 V versus Hg/HgO.
A noteworthy observation in the electrochemical analysis was the presence of a distinct peak at approximately −0.40 V for the Co-Zr-Te@CuO and −0.42 V for Co-Zr-MOF@CuO electrode. This peak broadening or positional shift could potentially be attributed to the reduction process of CuO transforming into its metallic state,[21] or it might reflect changes in electron transfer kinetics. The cyclic voltammetry (CV) profiles for both Co-Zr-MOF@CuO and Co-Zr-Te@CuO materials maintained a consistent shape across the range of applied potentials, indicative of their stable electrochemical behavior. The shift of anodic and cathodic peaks to new positions with an increase in scan rate, as presented in Figures S3,S4a (Supporting Information), suggests the occurrence of rapid and reversible redox processes, alongside minimal polarization effects.[2]
The GCD curves of both Co-Zr-MOF@CuO and Co-Zr-Te@CuO were measured at a current density of 10 A g−1 over a potential window of −0.5–0.6 V, shown in Figure 4b, it illustrates the comparison of the GCD curves, showing that Co-Zr-Te@CuO had a longer discharge time than Co-Zr-MOF@CuO. The CV and galvanostatic charge-discharge (GCD) results for the Co-Zr-Te@CuO composite revealed a blend of battery-like and capacitive characteristics at varying scan rates and current densities. The specific capacity of Co-Zr-Te@CuO was calculated to be 576 C g−1 (480 F g−1), which is 1.8x higher than that of Co-Zr-MOF@CuO (312 C g−1, 260 F g−1) at a higher current density of 4 A g−1 and detailed specific capacities from 4 to 20 A g−1 is shown in Figure 3c. The specific capacities of Co-Zr-MOF@CuO and Co-Zr-Te@CuO at various current densities, demonstrate the superior performance of Co-Zr-Te@CuO (Table S1, Supporting Information). Detailed CV and GCD curves at different scan rates for Co-Zr-MOF@CuO and Co-Zr-Te@CuO can be found in Figures S3 and S4 (Supporting Information), respectively.
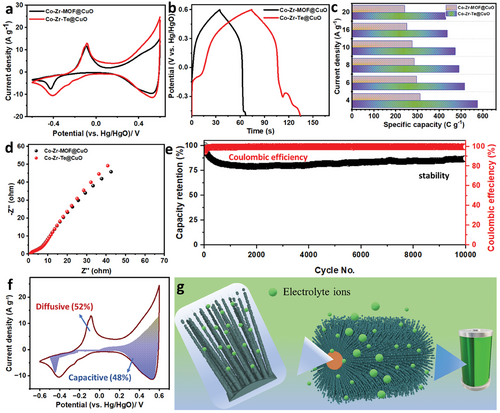
The equivalent series resistance (ESR) at a frequency ranging from 100 kHz to 0.01 Hz was visualized in Nyquist plots provided in Figure 3d. The electrode composed of Co-Zr-Te@CuO demonstrated a notably reduced ESR value than Co-Zr-MOF@CuO due to the high conductivity value of tellurium. This electrode also exhibited decreased resistances related to both charge transfer (relating to the electron movement at the electrode/electrolyte interface) and Warburg impedance (attributable to ion diffusion). Collectively, the Nyquist plot suggests that the hybrid electrode possesses relatively moderate conductivity when contrasted with the other specimens prepared. Further, Bode plot (Figure S5, Supporting Information) also facilitates the findings of Nyquist plot. The practicality of electrode materials is greatly influenced by their ability to maintain performance over many charge-discharge cycles. To assess this, the electrodes underwent a cycling test involving 10 000 galvanostatic charge–discharge (GCD) cycles at a current density of 10 A g−1 shown in Figure 3e. The results, depicted in Figure 3e, demonstrate that the electrodes preserved 99.3% Coulombic efficiency and maintained 88.7% of their capacity. The SEM images of Co-Zr-Te@CuO (Figure S6, Supporting Information) after 10 000 cycles in a cyclic stability test, demonstrate that the material has preserved its structural integrity, thereby exhibiting excellent stability.
The charge storage behavior of electrode materials was analyzed using a power law applied to the charge storage mechanism.[22] Analysis of the integrated area under the CV curves (shown in Figure 3f) provided insights into the diffusion-driven and capacitive contributions of the Co-Zr-Te@CuO electrode. It was observed that the capacitive behavior for Co-Zr-Te@CuO diminished from 48%, while the diffusion-controlled processes experienced an increase from 52% for the Co-Zr-Te@CuO. This significant shift underscores a predominance of relative diffusion-influenced redox reactions in Co-Zr-Te@CuO, which in turn contributes to the enhanced capacitance observed. The superior capacity of the Co-Zr-Te@CuO electrode can be linked to several key aspects and the energy storage mechanism for Co-Zr-Te@CuO electrode is illustrated in Figure 3g. Initially, the copper mesh facilitated the synthesis of a distinctive scaffold populated with nanowires, enhancing the integration with Co-Zr-MOF@CuO. The inherent porosity of MOF was retained in the transition to Co-Zr-Te@CuO, safeguarding the structural integrity. This transformation led to the formation of a synergistic heterostructure, seamlessly merging Co-Zr-Te and CuO into a cohesive unit. Finally, the conversion to telluride, while preserving the redox-active material and customizable cavities, conferred enhanced structural stability, promoting durability in charge cycles.
We constructed the Co-Zr-Te@CuO heterostructure by using a CuO with four layers and 48 atoms (Cu = 20 and O = 28). A small cluster of Zr6CoTe2 was introduced over the CuO slab to obtain the most stable configuration as shown in Figure 4. We carried out the spin-polarized atomistic simulations based on Density Functional Theory (DFT) to investigate the electronic properties of Co-Zr-Te@CuO heterostructure. Interestingly, the total and partial density of states analysis suggests that the heterostructure exhibits a significant metallic behavior with the valence band having a predominant contribution from Cu (d). Near the Fermi level, one can clearly see an overlap between Cu (d), O (p), and Zr (d) on either side of the valence and conduction bands (Figure 4a). This overlap represents the spatial interaction between the orbital wavefunctions, i.e., the exchange of charges between CuO and the Co-Zr-Te. The PDOS analysis clearly depicts that the Co-Zr-Te@CuO heterostructure has enhanced electronic properties as compared to CuO (Figure 4b), which exhibits a semiconducting character. It can be concluded that the presence of Co-Zr-Te in CuO turned the heterostructure into a metallic material with enhanced electronic conductivity.
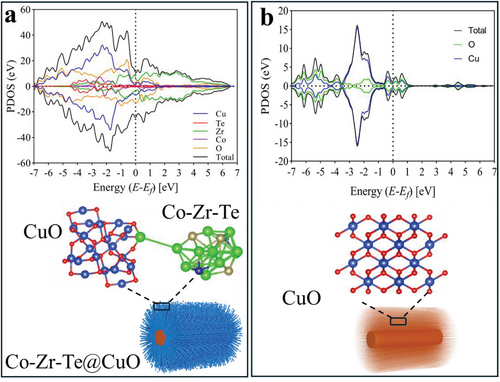
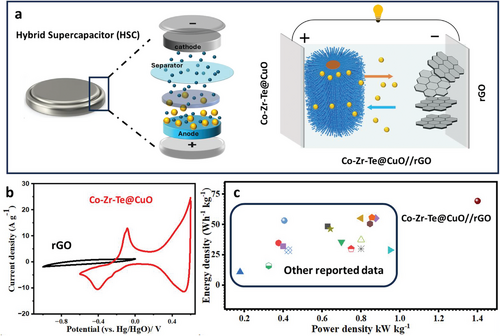
Figure 5 provides a visual representation of the newly developed hybrid supercapacitor (SC), featuring rGO and Co-Zr-Te@CuO as the electrode materials. rGO functions as the cathode, while Co-Zr-Te@CuO operates as the anode as shown in Figure 5a. Figure 5b exhibits the comparative CV analysis of the anode and cathode at a scan rate of 10 mV s−1. Considering the potential limits of electrodes, the working potential of HSC was found to be 1.6 V. The analysis of the CV at different scan rates revealed rectangular shapes, which suggests the Faradaic properties of the charge-storage mechanism for the HSC (Zr-Co-Te@CuO//rGO) device When the electrodes are charge-balanced and configured into a single device, they exhibit a consistent operational voltage of 1.6 V and detailed CV of HSC at various scan rate, shown in Figure S7a (Supporting Information). Following the establishment of the maximal voltage range (1.6 V), the galvanostatic charge–discharge (GCD) tests were conducted at varying current loads from 1 to 10 A g−1, illustrated in Figure S7b (Supporting Information). The Ragone plot, which compares energy and power densities with those reported in the literature,[23] is presented in Figure 5c and detailed in Table S2 of the Supporting Information. Based on the GCD profiles, the HSC device achieved a peak energy density of 69.4 Wh kg−1 at a power density of 1.4 kW kg−1. This evidence of Co-Zr-Te@CuO effective electrode performance demonstrates its potential and robust stability for real-world energy storage applications.
3 Conclusion
We developed the novel electrode material Co-Zr-Te@CuO by strategically depositing a Co-Zr metal-organic framework (MOF) on CuO nanowires and subsequently tellurizing it. This approach resulted in a composite that combines the rapid electron transport of telluride with the structured integrity of CuO nanowires, enhancing charge transfer pathways. The Co-Zr-Te@CuO electrode demonstrated a wider potential window of 1.2 V and a significant increase in specific capacitance to 576 C g−1, which is 1.8 times that of the precursor Co-Zr-MOF@CuO. Additionally, DFT calculations confirmed conductivity improvements and elucidated the synergetic effects within the heterostructure. In practical applications, this electrode achieved an energy density of 69.4 Wh kg−1 at a power density of 1.4 kW kg−1, showcasing its robustness and potential for high-performance energy storage applications. This advancement marks a significant step forward in the development of efficient and sustainable supercapacitor technology.
4 Experimental Section
CuO Nanowires Preparation
CuO nanowires were produced through a cost-efficient and precursor-free dry oxidation method, as elaborated in the prior publications.[2] The procedure involved placing a copper mesh with dimensions of 3 × 5 cm inside a muffle furnace, subsequently heating it to 450 °C over 5 h with a temperature increase of 5 °C per minute. Once cooled to ambient conditions, the mesh embedded with CuO nanowires was ready for use as electrode material.
Synthesis of Co-Zr-MOF@CuO
The Co-Zr-MOF@CuO was synthesized using a straightforward solvothermal process. Initially, 7.5 mmol of 2-methylimidazole (2-MIM) was dissolved in a 40 mL solvent composed of deionized water and ethanol (in a 30:5 mL ratio) to form solution A. Separately, a combined 1 mmol of cobalt nitrate hexahydrate and zirconium nitrate were dissolved in another 40 mL of the same solvent mixture and stirred for 20 min to yield solution B. Following this, solutions A and B were rapidly combined. The previously prepared CuO nanowire substrate was then immersed into this resultant solution, placed in a Teflon-lined stainless-steel autoclave, and heated at 60 °C for 10 h. After cooling down naturally to room temperature, the product was washed with deionized water and ethanol, then dried at 60 °C for 12 h.
Synthesis of Co-Zr-Te@CuO
The derivative Co-Zr-Te@CuO was obtained via a tellurization process. To commence synthesis, 0.1 mmol of tellurium powder was dissolved in a solution containing hydrazine hydrate and sodium borohydride, then mixed with 20 mL of deionized water. This solution was subjected to intense sonication for half an hour. The freshly prepared Co-Zr-MOF@CuO was introduced to this solution, transferred to a Teflon-lined stainless-steel autoclave, and baked at 140 °C for 12 h. Post-cooling, the copper mesh was rinsed with deionized water and ethanol, then dried in an oven and the resultant solution was named Co-Zr-Te@CuO electrode material.
Acknowledgements
This work was supported by the Hong Kong Research Grants Council (project number CityU 11201522).
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.