Multifunctional Liposomes Targeting Amyloid-β Oligomers for Early Diagnosis and Therapy of Alzheimer's Disease
Abstract
Early detection and treatment are crucial for Alzheimer's disease (AD) management. Current diagnostic and therapeutic methods focus on late-stage amyloid fibrils and plaques, overlooking toxic soluble amyloid β oligomers (AβOs) accumulating early in AD. A multifunctional liposome-based platform is designed for early diagnosis and therapy of AD, leveraging a novel self-assembled cyclic d,l-α-peptide (CP-2) that selectively targets AβOs. Biocompatible CP-2 conjugated liposomes (CP-2-LPs) effectively disrupt Aβ aggregation and mitigate Aβ-mediated toxicity in human neuroblastoma cells. In transgenic Caenorhabditis elegans AD models, CP-2-LPs significantly outperformed free CP-2 by improving cognitive and behavioral functions, extending lifespan, and reducing toxic AβO levels. Intravenous injection of fluorescently labeled CP-2-LPs reveals effective blood-brain barrier penetration, with significantly higher brain fluorescence in transgenic mice than WT, enabling precise diagnosis. These findings underscore CP-2-LPs as a valuable tool for early detection and targeted therapy in AD.
1 Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder that represents the most common form of dementia. It is characterized by a gradual destruction of memory and impairment of cognitive functions, significantly impacting the daily lives of many individuals worldwide. At present, more than 50 million people are living with AD and related dementias, and this number is estimated to rise to 78 million by 2030 and 139 million by 2050.[1, 2] In the US, AD ranks as the third leading cause of death among people over 65 after heart disease and cancer.[1] The economic burden of AD and other dementias surpassed $600 billion in the US in 2021 with a projected substantial increase by 2050.[2]
Pathologically, AD is associated with extracellular accumulation of senile plaques composed of amyloid-β (Aβ) protein and intraneuronal tangles of aberrantly phosphorylated microtubule-associated tau protein.[3, 4] Recent studies showed that amyloid plaques do not strongly correlate with AD pathogenesis, and reducing Aβ plaques does not effectively slow disease progression.[5, 6] Instead, mounting evidence indicates that soluble low molecular weight Aβ oligomers (AβOs)[7-9] rather than insoluble amyloid plaques are the key pathogenic molecular assemblies that cause AD-related synaptic dysfunction and neuronal cell death in transgenic animal models[10, 11] as well as in AD patients.[12, 13] Starting to appear prior the tau aggregation[14, 15] and the onset of symptoms, targeting the toxic AβOs with FDA-approved monoclonal antibodies Aducanumab and Lecanemab has demonstrated promising results in recent clinical trials.[16, 17] However, controversy over efficacy, side effects such as micro-hemorrhages and brain swelling, and the high cost of treatment, combined with limited penetration through the blood-brain barrier (BBB), emphasize the urgent need for improved therapies and novel diagnostic tools to assess treatment efficacy.[18, 19]
In this context, BBB poses a formidable challenge for development of diagnostic and therapeutic agents for all central nervous system (CNS) disorders. The BBB serves as a highly selective and dynamic interface between the circulating blood and the CNS, which includes the brain and spinal cord. Its primary role is to regulate the influx and efflux of substances to maintain the brain's metabolic activity and neuronal function, and to protect the neurons from entry of toxins and pathogens. It is formed by specialized endothelial cells that are connected by tight junctions, which tightly seal the gaps between adjacent cells, restricting the diffusion of most substances, including ions and large molecules, through the paracellular pathway.[20, 21] Various modalities, such as nanoparticles, peptides, and monoclonal antibodies, have been explored to enhance BBB permeability to improve the delivery of therapeutic and diagnostic agents to the brain.[22]
Since AβOs play a crucial role in AD onset and progression, their early detection is critical for improving patient screening and accurately monitoring treatment response.[9, 14, 15, 23] However, detecting AβOs presents challenges due to their high instability and low concentration. Available FDA-approved PET imaging agents such as Amyvid™, Vizamyl™, Neuraceq™, and 11C-Pittsburgh B (11C-PIB) primarily target late-stage fibrils and insoluble plaques and do not effectively detect soluble AβOs.[23, 24] Probes specifically designed for detecting early AβOs in vivo are rare and rely mostly on fluorescence imaging techniques.[25-28] Recent PET and MRI imaging probes utilizing oligomer selective antibodies show promise in relatively aged transgenic mouse models with established Aβ pathology.[29]
Structural and functional similarities between different amyloidogenic proteins are responsible for their cross-interactions.[30-32] The cyclic d,l-α-peptide CP-2 (c-[Lys1-D-Leu2-Nle3-D-Trp4-His5-D-Ser6], Scheme 1, Figure 1) consisting of alternating d- and l-α-amino acids self-assembles into nanotubes that replicate the intermolecular hydrogen-bonding pattern of cross β-sheets and resemble many structural and functional features of amyloid proteins.[33, 34] CP-2 and its analogs interact with early soluble and low molecular weight oligomers of aggregation-prone polypeptides including Aβ, α-syn, and tau-derived hexapeptide AcPHF6, modulate aggregation, disassemble fibrils, and inhibit cell toxicity by an “off-pathway” mechanism.[35-37]
More specifically, in case of Aβ, a range of biophysical and biochemical studies, including kinetic thioflavin T (ThT), immunochemical dot-blot, photoinduced cross-linking of unmodified proteins (PICUP) combined with SDS/PAGE, suggest that CP-2 and its analogs inhibit Aβ aggregation by interacting with and stabilizing small Aβ oligomers (1-3 mers) and influencing the secondary β-sheet conformation. NMR spectroscopy studies further indicate that CP-2 primarily interacts with the central hydrophobic and aromatic region within residues 16-23 (KLVFF) and the hydrophobic C-terminus, which play a pivotal role in self-assembly of Aβ.[35, 38] In longitudinal PET imaging of pre-symptomatic transgenic mouse model brains, 64Cu-radiolabeled analogs of CP-2 enable unprecedented detection of early AβOs.[38] Effectively crossing the BBB, CP-2, and its aza-analogs reduce AβO levels, extend the lifespan of transgenic Caenorhabditis (C.) elegans, and ameliorate memory and behavioral deficits in nematode and murine AD models.[38, 39]
Nature often uses polyvalent presentation of ligands and receptors on cell surface to augment binding affinity and biological activity.[40, 41] Conjugated polymers, dendrimers, nanoparticles, and self-assembled architectures have been utilized to generate multivalent systems for enhanced biological activity.[41-43] The multivalency effect has also been applied to enhance the antiamyloidogenic activity of small molecules and biologics targeting Aβ by conjugating them to different carriers.[44-47] As the dynamic levels of AβOs in the brain correlate with AD progression,[29] development of a reliable and safe system for their selective detection and inhibition, ideally before the onset of the symptoms, is of utmost scientific and clinical importance. In this study, multivalency effect was utilized to augment the interaction of CP-2 with early Aβ species. Toward this goal, a multifunctional liposome-based platform capable of targeting toxic AβOs was designed and tested for early diagnosis and treatment of AD. Clinically used liposomes were conjugated with BBB permeable and AβO specific CP-2 and labeled with Cy5 as a near-IR (NIR) fluorescent probe. The conjugated LPs were fully characterized and evaluated in vitro and in transgenic C. elegans and mouse models of AD as potential diagnostic and therapeutic agents.
2 Results and Discussion
2.1 Preparation and Characterization of Multifunctional Liposomes
Cyclic d,l-α-peptide, CP-2 was synthesized using the solid-phase peptide synthesis method,[35] purified to homogeneity by RP-HPLC, and characterized by mass spectroscopy (Figure S1, Supporting Information). Scheme 1 outlines the preparation of multifunctional liposomes conjugated to the fluorescent molecule Cy5 and oligomer-specific CP-2 (Cy5-CP-2-LPs). Liposomes were prepared from 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-PEG-carboxylic acid (DSPE-PEG2k-COOH) at a molar ratio of 55:40:5 by extrusion through a polycarbonate membrane with a pore size of 100 nm.[48, 49] Carboxylic acid groups on the liposome surface were activated with EDC/NHS and reacted with CP-2 to generate liposome-conjugated CP-2 (CP-2-LPs). For preparation of multifunctional liposomes containing both CP-2 and Cy5 NIR fluorescent probe, the same liposome composition was used but a small amount of DSPE-PEG2k-NH2 was added to introduce free amino groups on the liposome surface. The generated liposomes were first reacted with Cy5-NHS and then the free carboxylic acids were activated with NHS/EDC and coupled with CP-2. The conjugation efficiency of CP-2 and Cy5 with the liposome was ≈67% and 63%, respectively.
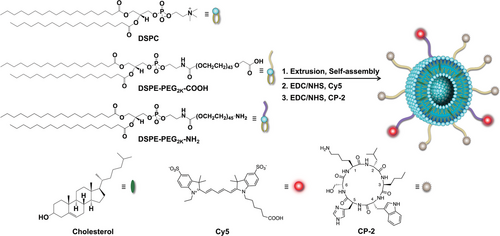
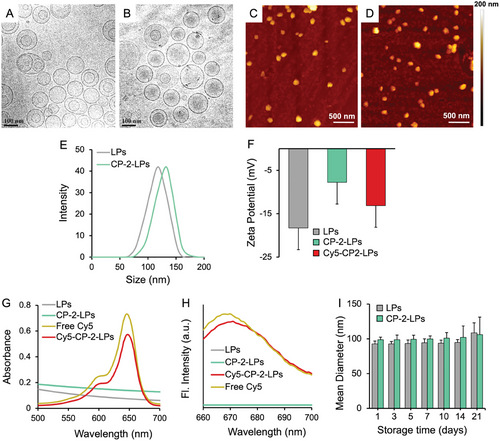
Cryo-EM, AFM, and DLS were used to analyze morphology, hydrodynamic size, and ζ potential of the particles. Cryo-EM studies confirmed the presence of well-dispersed spherical liposomes with average size of 109 ± 3 nm and 113 ± 5 nm for the blank liposomes and CP-2-LPs, respectively (Figure 1A,B; Figure S2, Supporting Information). AFM images (Figure 1C,D) further confirmed the spherical morphology of both the blank liposomes and CP-2-LPs with average size of 114 ± 3 and 115 ± 2 nm, respectively. Consistent with the Cryo-EM and AFM studies, DLS measurements demonstrated an average hydrodynamic size of 118 ± 4 and 123 ± 2 nm for the naked liposomes and CP-2-LPs with a PDI of 0.14 and 0.17, respectively (Figure 1E). Zeta potential measurements were performed to estimate the charges of the particles. The blank liposomes had a ζ potential of -18.3 ± 1.1 mV, indicating a negative charge due to the presence of free –COOH groups from DSPE-PEG2k-COOH (Figure 1F). Upon conjugation of carboxylic acids with CP-2 to generate CP-2-LPs, the ζ potential increased to -7.6 ± 0.64 mV. The ζ potential of Cy5-CP-2-LPs was more negative (-13.5 ± 0.73 mV) confirming successful incorporation of the negatively charged Cy5. Conjugation of Cy5 to the particles was further validated with visible and fluorescence spectroscopy, which showed similar absorption and fluorescence spectra for free Cy5 and Cy5-CP-2-LPs (Figure 1G,H).
DLS was used to assess the stability of the liposomes over a period of ≈1 month (Figure 1I). Up to 14 days of incubation at 4–8 °C, no significant change in particle size was apparent indicating a high stability with no evidence of particle fusion or aggregation. However, minor changes in particle size observed after 21 days of storage suggest some instability in prolonged incubation.
2.2 CP-2 Conjugated Liposomes Inhibit the Aggregation of Aβ
ThT fluorescence assay was used to monitor Aβ aggregation in the absence and presence of the liposomes. ThT binds specifically to the cross-β-sheets of amyloid fibrils to generate distinctive fluorescence signals.[50, 51] Fibrillization of Aβ40 follows a sigmoidal amyloid kinetic with a lag phase (≈20 h), an elongation phase, and a plateau phase as expected (Figure 2A). CP-2-LPs dose dependently reduced the aggregation of Aβ40 even at the lowest concentration tested. Aggregation of Aβ was decreased by 76% and almost completely with Aβ:CP-2-LPs ratios of 1:1 and 1:5, respectively. In control experiments CP-2 reduced the aggregation of Aβ40 only by 35 and 60% at the same ratios. The enhanced efficacy of CP-2-LPs in inhibiting Aβ aggregation, compared to CP-2, is likely attributed from the multivalency effect,[40] which has been also induced in other systems.[44-47, 52] The ThT results also demonstrated that the blank liposomes had no significant effect on Aβ aggregation. Notably, the role of lipid membranes and synthetic liposomes in amyloid formation has been a subject of debate.[53, 54] While there are reports indicating that aggregation of Aβ decreases in a dose-dependent manner with an increase in the negative charge of the membrane,[55] other studies demonstrate that zwitterionic lipid membranes significantly catalyse the rate of amyloid formation.[56] This debatable effect is largely ascribed to the complexity of the membranes concerning factors such as charge, hydrophobicity, and the presence of cholesterol. Thus, the lack of an impact of blank liposomes on Aβ aggregation in this study is likely attributable to differences in lipid composition and surface characteristics, including phase behavior.[57]
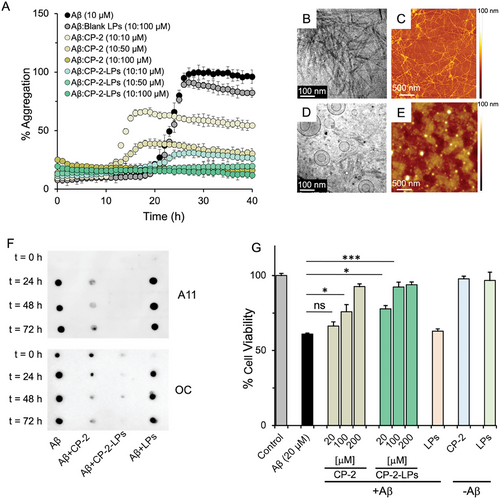
Cryo-EM and AFM were used to examine the morphology of Aβ in absence or presence of CP-2-LPs (Figure 2B–E). Aβ forms long fibrillar structures after 72 h of incubation. Consistent with the ThT results, significantly fewer fibrils were formed when Aβ was treated with a fivefold excess of CP-2-LPs.
The time course effect of CP-2-LPs on Aβ oligomer and fibril formation was assessed by dot blot assays employing conformation-specific antibodies A11 and OC, which recognize soluble oligomers and fibrils, respectively.[32] The reactivity of Aβ to both antibodies increased steadily over time, indicating conversion of monomeric Aβ to oligomers and fibrils (Figure 2F). Soluble toxic oligomers and fibril content both decreased more effectively upon incubation of Aβ40 with fivefold excess of CP-2-LPs compared with a similar excess of CP-2. In control experiments blank liposomes had no effect on the generation of Aβ oligomers or fibrils.
AβOs are believed to play a crucial role in the pathogenesis of AD.[58] The potential of CP-2-conjugated liposomes to modulate Aβ-induced neurotoxicity was examined using SH-SY5Y human neuroblastoma and neuronal-like rat pheochromocytoma PC12 cells. Initially, the toxicity profile of CP-2-LPs was assessed by exposing SH-SY5Y cells to increasing concentrations of CP-2-LPs and evaluating the cells’ metabolic activity through the well-established MTT assay.[59] Unconjugated CP-2 was used as the control. Consistent with previous studies demonstrating the non-toxic nature of naked LPs and CP-2,[38, 60] the MTT results indicated no significant reduction in cell viability, even at the highest concentration of CP-2-LPs tested (200 µM). This suggests that neither CP-2 nor CP-2-LPs exert toxicity on the cells (Figure 2G; Figure S3, Supporting Information).
Next, immunoblotting studies unveiled that Aβ requires an incubation period of at least 24 h to generate AβOs and fibrils (Figure 2F). Accordingly, Aβ40 was aged for 24 h with and without increasing concentrations of CP-2-LPs and CP-2 and incubated with the cells for an additional 48 h. Cell viability was then assessed by the MTT assay. Incubation of SH-SY5Y and PC12 cells with aged Aβ40 decreased cell viability to ≈60%. However, at a 1:5 Aβ:CP-2 equivalent ratio, CP-2, and CP-2-LPs increased cell viability to 75% and 92%, respectively. Notably, only CP-2-LPs demonstrated significant neuroprotecting activity (78%) at a 1:1 ratio. Similar results were obtained when PC12 cells were incubated with Aβ40 and CP-2-LPs (Figure S4, Supporting Information)
2.3 CP-2-LPs Rescue Aβ42-Induced Dysfunction in C. Elegans by Reducing AβO Levels
Therapeutic effect of CP-2-LPs was evaluated in two in vivo C. elegans models expressing human Aβ42. Transgenic C. elegans models are widely utilized to study AD and other age-related neurodegenerative diseases due to their age-dependent and human-like physiological changes observed at the tissue, cellular, and molecular levels.[61, 62] The C. elegans strain CL2006 expresses constitutively human Aβ42 in the body-wall muscles, aggregation of which leads to progressive paralysis and premature death.[63] The untreated CL2006 worms display an average mean lifespan of 12.8 days, while the control WT CL802 worms had a significantly longer lifespan of 14.6 days. Treatment of CL2006 worms with 50 µm of CP-2 increased the mean lifespan to 13.9 days (p <0.05). In contrast, treatment with an equivalent amount of CP-2-LPs extended the lifespan to that of WT worms (14.6 days; p <0.001) and mitigated the adverse effects of over-expressed Aβ (Figure 3A,B). These findings indicate that CP-2-LPs are more effective in prolonging the lifespan compared to CP-2 alone. Notably, neither CP-2-LP nor CP-2 had a significant effect on the longevity of WT CL802 worms.
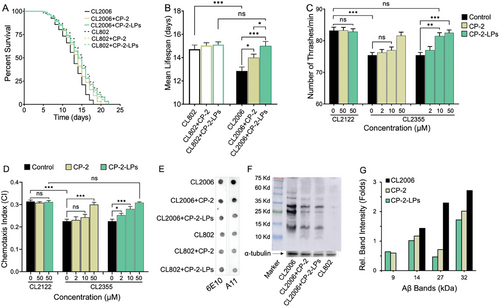
The CL2355 transgenic C. elegans expressing pan-neuronal human Aβ1–42 exhibits difficulties in learning, chemotaxis, and thrashing when temperature is up-shifted to 23–25 °C.[64] At elevated temperature (25 °C), CL2355 worms exhibit a reduced bending rate of ≈75 bends per minute compared to ≈82 bends per minute in WT CL2122 animals due to Aβ-induced toxicity (Figure 3C). Feeding the CL2355 mutants with increasing concentrations of CP-2 and CP-2-LPs increased dose-dependently the motility of CL2355 mutants to the level of untreated WT worms (Figure 3C). Notably, while CP-2 was not effective at a low concentration (10 µm), CP-2-LPs showed significant improvement at this dose.
Chemotaxis behavior in C. elegans is essential for seeking food sources, avoiding toxic substances, finding mates, and laying eggs. The chemotaxis index (CI) is a measure of the fraction of animals that reach the location of attractant using their chemosensory detection system.[65] In transgenic CL2355 worms, which express pan-neuronal Aβ42, chemotaxis is impaired due to Aβ-induced neurotoxicity. The CI of the WT CL2122 and CL2355 animals is 0.31 ± 0.01 and 0.22 ± 0.01 (p <0.001), respectively, indicating chemotactic dysfunction in the transgenic animals. Feeding the CL2355 mutants with CP-2-LPs and CP-2 dose dependently increased the CI. At 50 µm CP-2-LPs increased the CI to the WT level and neutralized the damaging effect of aggregated Aβ (Figure 3D). Notably, CP-2-LPs demonstrated significant protective effects on the chemotaxis behavior of CL2355 even at low micromolar concentrations whereas CP-2 failed to induce a similar protective activity. The CI levels of WT CL2122 worms were not affected by treatment with CP-2 or CP-2-LPs. Collectively, the C. elegans studies indicate that CP-2-LPs more effectively restore the impaired behavioral profiles of the transgenic worms compared to CP-2, likely due to the multivalency effect.
Immunochemical dot-blot and western blot (WB) experiments were conducted to investigate the effect of CP-2-LPs and CP-2 on Aβ species extracted from treated worms. Immunoblot analysis of equal amounts of extracted proteins revealed that CP-2-LPs more effectively reduced overall Aβ levels, with more pronounced effects on A11-reactive oligomers than CP-2 (Figure 3E), suggesting that CP-2-LPs produced a superior effect against the toxic oligomers. WB analysis of extracted proteins showed elevated levels of trimers (≈14 kDa), hexamers (≈27 kDa), and heptamers (≈32 kDa) in the transgenic CL2006 animals (Figure 3F). Trimers and hexamers of Aβ were proposed as building blocks for toxic aggregates, including Aβ-derived diffusible ligands, Aβ dodecamers, and globulomers, all associated with AD pathology.[66-69] Both CP-2-LPs and CP-2 reduced the amounts of trimers, tetramers, and hexamers in transgenic CL2006 worms while concomitantly increasing the levels of soluble Aβ dimers (≈8.5 kDa; Figure 3F,G). Stabilization of dimers by CP-2 was demonstrated in photoinduced cross-linking of unmodified proteins (PICUP) experiments to inhibit Aβ aggregation and toxicity.[35]
Notably, in a similar study, a series of amphiphilic cationic N-substituted oligo-pyrrolamides capable of forming vesicular structures were strategically designed to exhibit antiamyloidogenic activity against Aβ.[70] Among the synthesized oligo-pyrrolamides, the peptidomimetic ADH-353 generated functional vesicles, displaying effective inhibition of Aβ fibrillation, disruption of the formation of neurotoxic Aβ oligomers, and rescue of cells from Aβ-mediated cytotoxicity. It is noteworthy that ADH-353 vesicles inhibit Aβ aggregation and toxicity through a different mechanism compared to CP-2-LPs. While CP-2-LPs are negatively charged particles that target toxic Aβ oligomers by modulating the levels of Aβ dimers to hexamers, ADH-353 generates positively charged vesicles that reduce the amount of Aβ oligomers by trapping the negatively charged monomeric Aβ42 on their surfaces.[70]
2.4 Biodistribution of Liposomes in WT Mice
Both CP-2 and its aza-Gly analog have demonstrated beneficial effect in ameliorating memory and behavioral deficits after their injection to a murine AD model.[38] Additionally, confocal cell microscopy and in vivo brain positron emission tomography (PET) imaging studies revealed that CP-2 and its aza-Gly analog maintain their permeability through cell membrane and the BBB even after conjugation to NBD (4-chloro-7-nitro-1,2,3-benzoxadiazole) and NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid), used respectively as a fluorescent molecule and a chelating agent for radioactive 64Cu.[36, 38] Consistent with these results, in vivo fluorescence imaging of WT female C57BL/6J mice intravenously injected with Cy5-CP-2-LPs detected high fluorescence signals in their brains (Figure S5A, Supporting Information), indicating successful penetration of CP-2-LPs through the BBB. Subsequently, the animals were perfused with saline prior to dissection of tissues to examine the uptake of fluorescent CP-2-LPs. Intense fluorescence was observed in the liver, heart, spleen, kidney, and lungs 4 h post injection (Figure S5B, Supporting Information). Figure S5C (Supporting Information) presents the average fluorescence intensities of different organs at 4 and 24 h post Cy5-CP-2-LPs injection. The fluorescence intensity signals gradually decreased in most tissues after 24 h of injection, indicating efficient clearance of the liposomes upon injection. Liposome fusion and their internalization through endocytosis/phagocytosis followed by degradation within cells, exocytosis, or lysosomal pathways, along with potential interaction with interstitial fluid and cerebrospinal fluid, are likely responsible for the clearance and drainage of the liposomes.[71] Notably, detectable fluorescence was also observed in the brains of WT mice 4 h after injection but the signal decreased after 24 h, suggesting that Cy5-CP-2-LPs penetrate the BBB in WT mice. Enhanced brain permeability was also reported for other nanoparticles when conjugated with cell-penetrating peptides.[47]
2.5 Fluorescence Imaging of Aβ Pathology in Transgenic Mice
The capability of Cy5-CP-2-LPs in accumulating and detecting Aβ species was tested in 5xFAD transgenic mice. These mice develop rapidly severe amyloid pathology due to the presence of the Swedish (K670N/M671L), Florida (I716V), and London (V717I) mutations in Amyloid Precursor Protein (APP) and the M146L and L286V mutations in Presenuilin-1 genes. At an age of ≈1.5 months, these mice begin to accumulate intraneuronal Aβ42 aggregates in the cortex, hippocampus, and thalamus followed by extracellular amyloid deposition at an age of ≈2 months, which progressively increases with age. Initial memory impairment is observed at an age of 4 to 5 months.[72]
In vivo fluorescence imaging of ≈5-month-old female 5xFAD mice brains at 4 h post-injection of Cy5-CP-2-LPs revealed significantly higher (p <0.05) fluorescence compared to WT mice (Figure 4A,B). Remarkably, while the fluorescence decreased over time in WT brains, it increased over 24 h and remained stable for up to 72 h post-injection in 5xFAD brains, enabling clear visualization of AD brains with minimal background signal (Figure 4A,B). Collectively, the imaging studies demonstrate the effective penetration of Cy5-CP-2-LPs through the BBB in both WT and 5xFAD mice. However, the clearance of Cy5-CP-2-LPs from the brains of 5xFAD mice was significantly slower compared to WT mice (p <0.05), leading to their accumulation in the brain. Although the precise mechanism behind the prolonged retention of Cy5-CP-2-LPs in the brain of 5xFAD mice is still under investigation, it may be attributed to the compromised BBB in this AD model[73] or, more likely, to the binding of Cy5-CP-2-LPs to Aβ species in the brains of 5xFAD mice, enabling the effective visualization of amyloid species in the young transgenic 5xFAD mice brains.
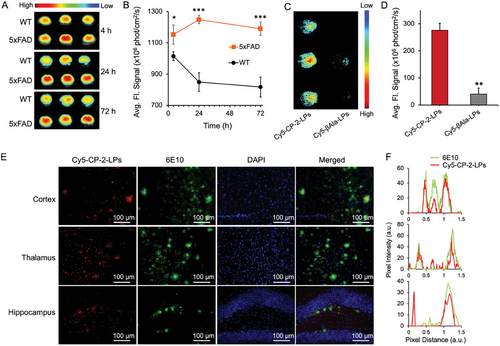
2.6 Role of CP-2 on BBB Permeability of Cy5-CP-2-LPs
Fluorescence imaging of perfused WT and 5xFAD mice revealed the effective permeation of the BBB by Cy5-CP-2-LPs, leading to their accumulation in the brains of 5xFAD mice. Given the increased accumulation observed in the brains of 5xFAD mice, a condition that may be associated with a compromised BBB in the AD model,[73] and considering the limited inherent ability of naked liposomes to traverse the BBB,[74] the specific role of CP-2 in facilitating BBB penetration was investigated. To discern the specific contribution of CP-2 to BBB penetration, Cy5-liposomes were conjugated to β-alanine ethyl ester to generate Cy5-β-Ala-LPs. In contrast to the successful BBB penetration of Cy5-CP-2-LPs in AD mice, Cy5-β-Ala-LPs failed to permeate the BBB (Figure 4C,D). This observation indicates that the penetration of Cy5-CP-2-LPs in AD mice is not solely due to the compromised BBB in AD but is mainly attributed to the ability of CP-2 to enhance the BBB permeability of the liposomes.
2.7 Postmortem Immunofluorescence Analysis of Brain Tissue
After fluorescence imaging, perfused brains from Cy5-CP-2-LPs treated mice were sectioned and stained with sequence-specific anti-Aβ 6E10 to assess the localization of Aβ species and Cy5-CP-2-LPs. Confocal fluorescence microscopy from the hippocampus, cortex, and thalamus regions demonstrated that the 6E10 antibody colocalized with Cy5-CP-2-LPs, indicating that the latter bind specifically to Aβ species (Figure 4E,F). Consistent with these results, recent immunofluorescence studies utilizing the A11 antibody and PET imaging using 64Cu-labeled CP-2 showed high levels of AβOs in the thalamus of young pre-symptomatic 5xFAD mice, followed by the cortex and hippocampus.[38] Collectively, the in vivo experiments indicate that CP-2-LPs can efficiently penetrate the BBB and bind early Aβ species present in the brains of young AD mice.
3 Conclusion
Early diagnosis of AD is crucial for optimizing therapy and preventing irreversible brain damage. In this study, we successfully developed a multifunctional liposome-based platform conjugated to the cyclic d,l-α-peptide CP-2 to enable BBB permeation and targeting of AβOs. CP-2-LPs were thoroughly characterized in terms of size, charge, shape, and stability using DLS, cryo-EM, and AFM. In ThT fluorescence studies and in vitro immunochemical assays, physiologically stable CP-2-LPs demonstrated promising safety profile, inhibited Aβ oligomerization and fibrillation, and reduced Aβ-induced toxicity in neuronal-like rat pheochromocytoma PC12 and human neuroblastoma SH-SY5Y cells significantly better than CP-2. Using C. elegans models over-expressing human Aβ42, CP-2-LPs exhibited superior therapeutic effects compared to CP-2. They effectively restored normal lifespan and improved memory, cognition, and other behavioral deficits by more efficiently reducing the concentration of toxic AβOs, including Aβ hexamers. The higher antiamyloidogenic activity of CP-2-LPs compared to that of CP-2 originates most likely from multivalent presentation of CP-2 on the surface of the liposomes.[44-46] Fluorescence imaging studies on young 5xFAD transgenic mice injected with Cy5-CP-2-LPs effectively diagnosed early-stage AD by detecting the binding of Cy5-CP-2-LPs to Aβ species in the thalamus, cortex, and hippocampus. In control experiments, Cy5-β-Ala-LPs did not permeate the BBB and failed to accumulate in the brains of AD mice, indicating that permeation through BBB is specifically mediated by CP-2. Toward early AD diagnosis and treatment, CP-2-LPs emerge as a promising theranostic platform for non-invasive early diagnosis and intervention by delaying the progression of cognitive deficits and memory impairments. Notably, these liposomes not only cross the BBB due to the cell-penetrating properties of CP-2 but also hold the potential to encapsulate additional therapeutic agents for effective brain drug delivery. Further research and clinical investigations are crucial to fully explore the transformative potential of CP-2-LPs in early AD diagnosis and intervention.
4 Experimental Section
Aβ40 was purchased from Hanhong Scientific (Shanghai, China) and stored as a lyophilized powder at –20 °C. CP-2 was prepared using a solid-phase approach as previously described.[35] Lipid derivatives including 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-PEG-carboxylic acid (DSPE-PEG2k-COOH) and 1,2-istearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)] (DSPE-PEG2k-NH2) were purchased from Nanosoft Polymers (NC, USA), while 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and cholesterol were acquired from TCI Chemicals. Sulfo-Cy5 was purchased from AAPPTec (Louisville, KY, USA). All other chemicals and reagents were of analytical grade. A11 and OC antibodies were generously provided by Dr. Rakez Kayed (Department of Neurology, University of Texas Medical Branch)[32], 6E10 antibody was obtained from Covance, and secondary antibodies were purchased from Merck.
Preparation of Naked Liposomes
Liposomes were prepared using a thin-film hydration method.[48, 49] In brief, DSPC, cholesterol, and DSPE-PEG2k-COOH at a molar ratio of 55:40:5 were dissolved in chloroform-methanol (4:1, v/v) and stirred continuously for 1 h. The solvents were then evaporated using a stream of N2 gas, and the remaining lipid components were dried under high vacuum to form a thin lipid film. The film was hydrated in PBS (100 mm, pH 7.4) at 65 °C, and the resulting suspension was sonicated for 2 min in a water bath followed by vigorous mixing with a vortex. The lipid suspension was extruded through a polycarbonate membrane with a pore size of 100 nm for 20 cycles using a mini-extruder (Avanti Polar Lipids, Inc.). After extrusion, the suspension was dialyzed against PBS (100 mm, pH 7.4) for 24 h (×3, 8 h each), using a membrane with MWCO of 6000–8000 to obtain blank liposomes.
Preparation of CP-2 Conjugated Liposomes (CP-2-LPs)
CP-2 was coupled through its free amine group to the carboxyl groups presented on the surface of the liposomes using 1-ethyl-3-(3- (dimethylamino)propyl)carbodiimide hydrochloride (EDC.HCl) and N-hydroxysuccinimide (NHS).[48] In brief, carboxyl groups of the liposomes were first activated using an excess of EDC and NHS (DSPE-PEG2k-COOH:EDC:NHS, 1:10:10 molar ratio) for 10 min at room temperature (RT) under gentle shaking. CP-2 was added to the reaction mixture at a 1:1.5 molar ratio of DSPE-PEG2k-COOH:CP-2 and the reaction was allowed to proceed for an additional 12 h. Excess of unreacted reagents was removed by dialysis against PBS (100 mm, pH 7.4) using a membrane with a molecular cutoff filter of 6–8kDa. The dialyzed solution containing CP-2-LPs was collected, and the concentration of unreacted CP-2 was determined at 280 nm using the extinction coefficient of the tryptophan residue.
Preparation of Fluorescently Labeled CP-2-LPs
Multifunctional liposomes were prepared from DSPC, cholesterol, DSPE-PEG2k-COOH, and DSPE-PEG2k-NH2 at a molar ratio of 55:40:4.5:0.5, as described above. Fluorescent labeling was achieved by covalent conjugation of Cy5 with the free amine groups in the liposomes. In brief, the carboxylic groups of Cy5 were initially activated with EDC/NHS for 1 h in double-distilled water (DDW). The activated NHS-Cy5 was then added to the liposome solution at a 1:1.5 DSPE-PEG-NH2:Cy5 molar ratio and the mixture was stirred gently overnight. The free carboxylic acids of the liposomes were then activated with EDC/NHS and conjugated to CP-2, as described above. Unbound Cy5 and CP-2 were removed from the solution by dialysis in PBS (×3, 8 h each) with a 6–8 kDa cutoff filter.
Conjugation efficiency of CP-2 and Cy5 to the liposomes
Accordingly, the conjugation efficiency of CP-2 and Cy5 with the liposome was 67% and 63%, respectively.
Characterization—Cryogenic Transmission Electron Microscopy (Cryo-EM)
The morphology and size of blank liposomes and CP-2-LPs were analyzed with a Tecnai G2 cryo-EM (FEI Tecnai G2, Hillsboro, Oregon) operating at an acceleration voltage of 120 kV. For imaging, samples (3 µL) were loaded onto lacey grids, blotted, and rapidly frozen by plunging into liquid ethane at −180 °C. Grids with frozen samples were stored in liquid nitrogen. Images were digitally recorded with a multiScan camera (Gatan794) at various magnifications.
Characterization—Atomic Force Microscopy (AFM)
Samples (5 µL) were deposited on a silicon wafer substrate. After drying overnight at RT, images were acquired using a Bio FastScan scanning probe microscope (Bruker AXS) in tapping mode using a silicon probe with a spring constant of 18 N/m and a cantilever with a resonance frequency of ≈1400 kHz.
Characterization—Particle Size, Stability, and Zeta Potential Measurements
The average size, polydispersity index (PDI), and ζ-potential of the liposomes were determined in 10 mm PBS (pH 7.4) at RT using a ZetaSizer 3000 HS dynamic light scattering (DLS) instrument (Malvern, UK). The stability of the liposomes in PBS stored at 4–8 °C was monitored over a period of one month by assessing the hydrodynamic size.
Characterization—Thioflavin T (ThT) Aggregation Assay
Anti-amyloidogenic activity was assessed by a kinetic ThT assay, as described.[35] Monomerized solutions of Aβ40 (33 µm, 60 µL) in PBS (50 mm, pH 7.2) were incubated in a 96-well flat-bottom black plate with increasing concentrations of CP-2 and CP-2-LPs (20 µL of samples containing 5% DMSO). A solution of ThT (30 µm, 120 µL) in PBS was added to each well resulting in final concentrations of 10 µm for Aβ40 and 10, 50, and 100 µm for CP-2 or an equivalent amount of CP-2-LPs in 0.5% DMSO. The plate was covered with a transparent polyolefin film and placed in a microplate reader (Synergy H1, BioTek, USA). Fluorescence intensity of amyloid-bound ThT samples was monitored hourly at 37°C over a period of 72 h using excitation and emission wavelengths of 430 and 492 nm, respectively. Before each reading, the plate was shaken for 2 min. The experiments were repeated three times.
Characterization—Antibody (A11 and OC) Dot-Blot Assay
Monomerized solutions of Aβ40 (30 µm) in PBS (50 mm, pH 7.4) were incubated at 37 °C for different durations with or without 150 µm CP-2 or equivalent amount of CP-2-LPs in PBS (50 mm, pH 7.4), and stored at −80 °C until analysis. Samples (2 µL) were spotted onto nitrocellulose membranes (0.2 µm, Whatman) and dried at RT. The membranes were blocked for 1 h with 5% nonfat milk solution in Tris-buffered saline (TBS, 10 mm) containing 0.1% Tween-20 (TBST) and washed three times (10 min each) with TBST and incubated at 4 °C overnight with oligomer specific antibody A11 (at 1:5000 dilution), anti-amyloid fibril antibody OC (1:5000 dilution) and anti-Aβ antibody 6E10 (1:1000 dilution) in 0.5% nonfat milk in TBST. The membranes were washed again thrice with TBST (10 min each) and incubated for another hour at RT with either horseradish peroxidase (HRP) conjugated anti-rabbit IgG (1:5000 dilution) for A11 and OC or HRP-conjugated anti-mouse IgG (1:10000 dilution) for 6E10 in 0.5% nonfat milk solution in TBST. The blots were washed thrice with TBST and developed using the enhanced chemiluminescence (ECL) reagent kit (Bio-rad, Israel). The chemiluminescence was measured using an ImageQuant LAS 4000 system.
Characterization—Cell Culture Studies
Neuronal-like rat pheochromocytoma PC12 cell line was cultured in low-glucose Dulbecco's modified eagle medium (DMEM) supplemented with horse serum (10%), fetal bovine serum (FBS; 5%), L-glutamine, penicillin, and streptomycin at 37 °C in a 5% CO2 incubator. Human neuroblastoma SH-SY5Y cells were grown in the same medium except that 10% FBS was used. The effect of CP-2-LPs and CP-2 on Aβ-induced toxicity was determined as described before.[39] In brief, Aβ40 (200 µm) was aged for 48 h without or with increasing concentrations of CP-2-LPs or CP-2 in PBS containing 5% DMSO in a total volume of 50 µL. On the day of the experiment, the medium was replaced by fresh medium (90 µL) and the aged samples (10 µL) were diluted by a factor of 10 in the medium. Cells treated with 0.5% DMSO served as control (100% cell viability). After 24 h incubation, cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Experiments were performed in quintuplicate and repeated three times.
Characterization—C. elegans Strains and Maintenance
Transgenic C. elegans strains CL2355 [dvIs50, pCL45 (snb-1::Abeta 1-42::3' UTR(long) + mtl-2::GFP] and CL2006 [dvIs2, pCL12(unc-54/human Abeta peptide 1-42 minigene) + rol-6(su1006)] were used along with respective control strains CL2122 [dvIs15, (pPD30.38) unc-54(vector) + (pCL26) mtl-2::GFP}] and CL802 [smg-1(cc546) I; rol-6(su1006) II]. The C. elegans strains and Escherichia coli strain OP50 were obtained from the Caenorhabditis Genetics Center (CGC; University of Minnesota, Minneapolis, MN, USA). Animals were cultured at 16 °C on solid nematode growth medium (NGM) plates. For all assays, age-synchronized nematodes were prepared by hypochlorite bleaching (0.1 m KOH, 1% NaClO).[77] Synchronized eggs were hatched overnight (L1) in M9 medium and cultured on fresh NGM plates.
Characterization—Lifespan Assay
The survival and lifespan of transgenic CL2006 and WT CL802 C. elegans were monitored as described previously.[63] Synchronized eggs of transgenic CL2006 and WT CL802 animals were cultured on 60 mm NGM plates at 16°C. Each treatment group consisted of 100 synchronized L1 worms, which were seeded onto the plates at a density of 25 worms per plate. The worms were fed with heat inactivated OP50 solution containing the vehicle (5% DMSO), CP-2 (50 µm) or an equivalent amount of CP-2-LPs. Animals were transferred to new plates every alternate second day and their status was recorded daily. Worms with internal hatching were removed from the plates and excluded from lifespan calculations. The experiment was concluded when all worms were scored as deceased or censored. The statistical analysis of the lifespan data was performed using the Kaplan–Meier survival analysis method.
Characterization—Thrashing Assay
The thrashing assay was performed as described before with minor modifications.[38, 39] Synchronized L1 worms of transgenic CL2355 and control CL2122 strains (n = 20) were cultured on NGM plates at 16 °C and fed for 36 h with heat-dead OP50 solution mixed without or with increasing concentrations of CP-2 (0–50 µm) or equivalent amount of CP-2-LPs. Animals were incubated at 23 °C for 36 h and individually transferred to a 12-well plate containing 1 mL of M9 buffer. After a 2 min settling period, the number of thrashes for each animal was counted for 1 min. Body thrash was recorded as bending the worm's body from one side to the other and back to the original position. Each experiment was repeated three times.
Characterization—Chemotaxis Assay
The chemotaxis assay was performed following a previously described protocol with some modifications.[64] In brief, synchronized transgenic C. elegans strains CL2355 and the WT control strain CL2122 were cultured on NGM plates at 16 °C and fed with heat-dead OP50 solution containing the vehicle (5% DMSO), CP-2 (0–50 µm) or an equivalent amount of CP-2-LPs for 36 h. The animals were incubated at 23 °C for an additional 36 h, collected, and washed three times with M9 buffer. To perform the assay, odorants were prepared by spotting sodium azide (1 µL, 0.25 m, Sigma–Aldrich) and benzaldehyde (0.1%, 1 µL, Sigma–Aldrich) in absolute ethanol as “attractant” spots on opposite corners of a 100 mm agar plate. Absolute ethanol (1 µL) and sodium azide (1µL) were spotted on the two other corners as controls. A suspension of worms (5 µL, ≈30 worms) was immediately applied to the center of each agar plate. The plates were incubated at 23 °C for 1 h, and the number of worms in each quadrant was scored. The chemotaxis index (CI) was calculated by dividing the difference between the number of worms in both attractant and both control quadrants by the total number of scored worms.
Characterization—Immunoblot Assay
The effect of each treatment on the oligomeric state of Aβ42 in worms was investigated as described.[] Treated worms were collected, washed with M9 buffer, rapidly frozen in liquid nitrogen, and stored at –80 °C until analysis. Protein samples were extracted using a 2× volume of lysis buffer solution (2% SDS, 10% glycerol, 4% β-mercaptoethanol and protease inhibitor cocktail in 62 mm Tris-HCl, pH 6.8) and boiled at 101 °C for 5 min. The protein content was quantified using the Bradford reagent (Bio-Rad) or by measuring the absorbance at 280 nm using a NanoDrop spectrophotometer. Equal amounts of protein samples were spotted in duplicate on a nitrocellulose membrane (0.2 µm) and blocked with 5% BSA in PBS buffer (pH 7.5) containing 0.1% (v/v) Tween 20 (PBST). The blots were incubated overnight at 5 °C with either 6E10 monoclonal (1:1000 dilution) or A11 (1:5000) antibody in PBST with 5% BSA. After washing four times with PBST, the blots were incubated at RT with HRP-conjugated anti-mouse IgG (1:10000 in 0.5% BSA in PBST) or anti-rabbit IgG (1:5000 in 0.5% BSA in PBST) for 1 h. The membranes were washed five times with PBST and developed with an ECL reagent kit (Bio-Rad).
The Aβ species in treated worms were also identified by Western blot analysis. Equal amounts of protein lysate were heated at 101 °C for 3 min with sample buffer containing 5% β-mercaptoethanol, loaded on a 15% Tricine-SDS-PAGE gel, and run at 100 V for 1 h. The protein bands were transferred to a nitrocellulose membrane using transfer buffer containing 10% methanol at 75 V for 1 h. The blots were blocked with 5% BSA in TBS containing 0.01% (v/v) Tween 20 for 1 h at RT, incubated with the 6E10 antibody, and developed as above. Identical blots were reacted with an anti-α-tubulin antibody and developed. The mean density of Aβ reactive bands was analyzed using ImageJ (National Institutes of Health, USA).
Characterization—Mice
Transgenic 5xFAD mice were bred with non-transgenic background C57BL/6J (Jackson Laboratory) and maintained at the Bar-Ilan University animal facility. The mice were kept in a controlled environment with a 12/12 h light/dark cycle and provided with ad libitum access to food and water. Housing, breeding, and all animal experiments were conducted in compliance with the Bar-Ilan University Animal Care and Use Committee.
Characterization—Biodistribution Study in Wild-Type Mice
Five-month-old wild-type female C57BL/6J mice (n = 6, each group) were injected intravenously through the tail vein with 0.1 mL of Cy5-labeled CP-2-LPs (Cy5-CP-2-LPs) in PBS containing 3.8 µm of Cy5 and 28 mm of total lipids. Mice treated with vehicle were used as control. At different time intervals post-injection, the mice were euthanized and perfused intracardially with PBS (100 mm, pH 7.4). The major organs including brain, kidney, heart, liver, spleen, and blood were collected and imaged using a Maestro II fluorescence imaging system (Cri, Inc., MA, USA). For quantitative analysis, the average fluorescence signal (measured in ×106photons×cm-2×s-1) was determined for each specific area.
Characterization—BBB Permeability and AβO Targeting Studies
Five- to six-month-old female transgenic 5xFAD mice and WT C57BL/6J mice (n = 6, each group) were injected intravenously with 0.1mL of Cy5-CP-2-LPs in PBS under isoflurane anesthesia. At different time points (4, 24, and 72 h) post injections, the mice were euthanized and perfused with 4% PFA solution. Brains were collected and imaged using the Maestro II fluorescence imaging system, as described previously. Immunofluorescent staining was performed on PFA fixed 40 µm thick sections from 5xFAD mouse brains. Free-floating sections were washed with 0.1 M PBS (3×10 min) and blocked with 2% BSA for 1 h. The sections were incubated with the primary 6E10 mouse monoclonal antibody (1: 1000) in 2% BSA in 0.1 m PBS with 0.3% Triton X (PBST) overnight at 4°C. Sections were washed (3×10 min with 0.1 M PBS) and incubated with the secondary antibody Alexa Fluor 488 goat anti-mouse IgG antibody (1:1000, ThermoFisher) in 2% BSA in PBST for 1 h at RT. Sections were stained with DAPI (4',6-diamidino-2-phenylindole dihydrochloride; 1:500, MP Biomedicals) and washed for 10 min in 0.1% PBST. The stained sections were mounted on super frost glass slides, embedded with ImmunoMount (Sigma-Aldrich), cover-slipped and stored in dark for microscopic imaging.
Statistical Analysis
Data were analyzed by one-way ANOVA followed by post-hoc analysis where appropriate or by two-tailed unpaired Student's t-test as indicated in the respective figure legends.
Acknowledgements
The authors thank Dr. Anat Haviv-Chesner for her support in conducting C. elegans studies, Dr. Michal Richman for assistance with biophysical studies, and Dr. Ronen Yehuda for his contributions to fluorescence imaging. Funding from the Israel Science Foundation (grant No. 2926/21) is gratefully acknowledged. Careful proofreading of the manuscript by Dr. Yuval Elias is appreciated. S.S. acknowledges the Planning and Budgeting Committee, Israel for the postdoctoral fellowship award.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




