Multigenerational Exposures of Daphnia Magna to Pristine and Aged Silver Nanoparticles: Epigenetic Changes and Phenotypical Ageing Related Effects
Abstract
Engineered nanoparticles (NPs) undergo physical, chemical, and biological transformation after environmental release, resulting in different properties of the “aged” versus “pristine” forms. While many studies have investigated the ecotoxicological effects of silver (Ag) NPs, the majority focus on “pristine” Ag NPs in simple exposure media, rather than investigating realistic environmental exposure scenarios with transformed NPs. Here, the effects of “pristine” and “aged” Ag NPs are systematically evaluated with different surface coatings on Daphnia magna over four generations, comparing continuous exposure versus parental only exposure to assess recovery potential for three generations. Biological endpoints including survival, growth and reproduction and genetic effects associated with Ag NP exposure are investigated. Parental exposure to “pristine” Ag NPs has an inhibitory effect on reproduction, inducing expression of antioxidant stress related genes and reducing survival. Pristine Ag NPs also induce morphological changes including tail losses and lipid accumulation associated with aging phenotypes in the heart, abdomen, and abdominal claw. These effects are epigenetic remaining two generations post-maternal exposure (F2 and F3). Exposure to identical Ag NPs (same concentrations) aged for 6 months in environmentally realistic water containing natural organic matter shows considerably reduced toxicological effects in continuously exposed generations and to the recovery generations.
1 Introduction
The nanotechnology revolution has enabled the manipulation of materials at the nanoscale in order to exploit novel optical, thermal, and photocatalytic properties[1] along with many other potential effects brought about by the increased surface area and advanced functionality of engineered nanomaterials. Silver nanoparticles (Ag NPs) are one of the most commonly used materials in consumer applications, particularly in the healthcare industries,[2] which take advantage of the antibacterial and antimicrobial properties of Ag NPs.[3] Although these technological advances are important, the resulting environmental repercussions need to be addressed in parallel with their exploitation, since Ag NPs are readily released from nanoenhanced products through washing, weathering (including run-off), and general usage. Ag NPs are likely to be incorporated into sewage effluent, which may be used on agricultural land, and are potentially released into surface waters.[4]
To address the possible environmental impacts of Ag NPs, multiple studies have focused on determining the physicochemical changes undergone by Ag NPs during and after their use,[5] assessing stability,[6, 7] fate/transformations,[8] and bioavailability.[9] Despite this understanding of the environmental transformations undergone by Ag NPs, acute toxicity assessments of Ag NPs with various test organisms[10-13] have mainly determined the effects of only the “pristine” Ag NPs. Given that many of these transformations occur rapidly, i.e., on the timescales of the toxicity assessments, the resulting datasets are often conflicting, and true dose-response information is difficult to obtain. These environmental transformations, or in effect “ageing” processes, alter the Ag NP physicochemical traits and impact their behavior and toxicity in aquatic environments.
The main toxicity mechanism of Ag NPs has been debated extensively with some studies proposing its toxicity is due to the release of Ag+ via dissolution, and the inhibition of sodium and/or potassium ion transporter networks by Ag+.14] Other research suggests Ag NP toxicity is mediated by oxidative stress leading to mitochondrial damage, lipid damage, and cellular apoptosis[15, 16] the extent of which can be tuned by NP surface coating and media composition. Surface coatings may act as a stabilizing mechanism by limiting the dissolution process of the core material.[16] Environmental transformations such as sulfidation may also decrease the rate of dissolution in anaerobic environments and thus reduce Ag NP toxicity.[17] Using environmentally transformed Ag NPs, which are “aged” under realistic exposure conditions, in ecotoxicity assessments will result in data which is more predictive of realistic exposure scenarios, and therefore more appropriate for regulatory and risk assessment requirements.[18]
One of the most common and sensitive species used for chemical and NP toxicity screening are the microcrustacean Daphnia magna.[19] Daphnia reproduce parthenogenetically facilitating their use as a central toxicological model and indicator species for water quality. They are an optimal genomic model well suited for gene regulation studies to monitor stress and adaptive changes to their environments.[20] When “stressed,” daphnids can develop different phenotypes and switch from clonal to sexual reproduction.[21] Consequently, genetic processes may be altered when daphnids are under chronic stress, which can be easily monitored by identification of epigenetic (heritable from one generation to the next) changes in subsequent generations. These changes are due to modifications of the histone proteins of chromatin and DNA methylation, which results in altered gene expression.[22, 23] To understand the mechanisms responsible for the toxicity of Ag NPs, it is necessary to determine the molecular changes that occur as a result of the NPs making contact with tissues within the organism. Gene expression provides a molecular level of understanding of how the NPs interact in vivo,[24] and how Ag NPs with different properties, i.e., surface chemistries (Ag0 vs Ag2S NPs), surface coatings (Polyvinylpyrrolidone (PVP) vs uncoated), and “aged” or “pristine” NPs, may differentially impact the daphnids. Molecular indicators may provide essential information regarding what pathways are affected and the mode of action of various Ag NPs. The mechanistic information can then be imposed on standardized toxicological tests and risk assessments.
Currently, no studies exist, that report on chronic and mutigenerational effects in daphnids exposed to both “pristine” and long term “aged” Ag NPs in environmentally realistic water conditions. We have argued previously that the presence of biological macromolecules is essential for NP ecotoxicity assessment to allow formation of the eco-corona and reduce the surface energy of the NPs as would occur instantaneously in the environment.[25-27] The present study investigates the effects of chronic (from 24 h old to 24–30 days) parental (F0) exposures, to both pristine and 6-month aged uncoated Ag, PVP Ag and Ag2S NPs in a standard Daphnia culture medium (HH Combo) and in a synthetic European lowland water (Class V from[28]) containing natural organic matter (NOM). The subsequent three generations (F1-3) were split (from F1 onward) into two groups–half were continuously exposed for multiple generations (F1–F3exp) and half were removed from the maternal exposure and grown in NP-free medium for 3 generations (F1–F3rec) to identify the potential recovery scenario, as shown schematically in Figure S13 in the Supporting Information. The use of two media, a salt-only medium in which Daphnia growth is optimized and a more environmentally realistic representative synthetic water containing NOM) aims to highlight the influence of NOM on the physico-chemistry of “pristine” and “aged” NPs, and the resulting effect on ecotoxicity. Parental (F0) generations of daphnids were exposed for a minimum of 25 days (until their fifth broods). Life history traits were assessed in all four generations including NP effects on longevity, growth, reproductive effects and changes in the expression of key genes related to metal toxicity and oxidative stress were assessed. The results explore whether the initial exposure to pristine Ag NPs resulting in morphological, phenotypic and/or epigenetic changes, and whether these effects are diminished by particle ageing and/or utilizing environmentally realistic medium.
2 Results
2.1 Nanoparticle Characterization
Both pristine and aged Ag NPs used in this study were characterized using transmission electron microscopy (TEM) and dynamic light scattering (DLS) in the standard Daphnia culture media (HH Combo) and in synthetic Class V water. The results are presented in Table 1 and Figure S1 (Supporting Information). Ageing in the HH combo medium and Class V water had differential effects on the Ag NPs, showing increased sizes for all aged Ag NMs in comparison to their pristine forms.
| Identifier | Pristine TEM individual particle size [nm] | Aged in HH combo TEM individual size [nm] | Aged in Class V TEM individual size [nm]a) | Pristine DLS particle size [nm] | Aged in HH combo DLS size [nm]a) | Aged in Class V river water DLS size [nm]a) | Surface coating b) |
|---|---|---|---|---|---|---|---|
| Ag uncoated | 61 ± 36 | 36 ± 16 | 95 ± 111 | 120 ± 30.5 | 7363 ± 1054 | 1423 ± 545 | Bare |
| Ag PVP | 18 ± 11 | 38 ± 19 | 105 ± 102 | 260 ± 180 | 129 ± 22 | 129 ± 141 | PVP10 |
| Ag2S | 44 ± 14 | 39 ± 15 | 45 ± 15 | 299 ± 6 | 145 ± 2 | 171 ± 22 | PVP10 |
- a) Aged in medium for 6 months
- b) According to the manufacturer.
2.2 Survival
The pristine Ag NPs in the HH combo medium significantly affected mortality throughout the exposed generations, with the extent of the impact being surface coating dependent. The F0 parent generations (Figure 1A) had a total survival of 100% (uncoated Ag NPs), 53% (PVP Ag NPs), 80% (Ag2S), and 88% (bulk Ag) over the exposure duration of 25 days. However, sensitivity was exhibited in the following generations exposed to the pristine uncoated Ag NPs, with only 25% (F1exp), 3% (F2exp), and 27% (F3exp) survival at day 25. By contrast, the recovery generations (whereby the 3rd broods from the maternal exposure were further cultured in Ag NP free HH combo medium) had 90% (F1rec), 77% (F2rec), and 100% (F3rec) survival. More severe effects were observed in daphnids exposed to pristine PVP Ag NPs where the F3exp generation did not survive 24 h post birth (Figure 1A). Chemically ageing each of the Ag NPs (for 6 months prior to exposure) in the HH combo medium increased the survivorship (Figure 1B) for all successive generations (F0-F3) of both the continuously exposed and recovery generations. The survival of the F0 parent generations were 100% (aged uncoated Ag NPs), 97% (aged PVP Ag NPs), and 90% (aged Ag2S NPs). The survival of the F1-3 generations in both continuously exposed and recovery populations were all >93% for daphnids exposed to aged uncoated Ag NPs and aged PVP Ag NPs, and were >97% for the aged Ag2S (Figure 1) in the HH combo medium.
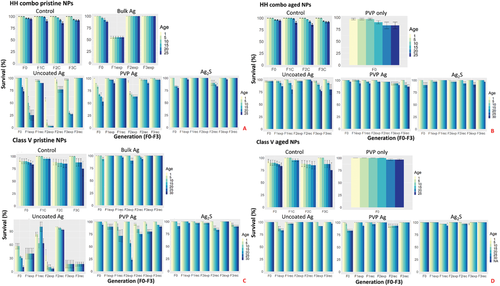
To identify the effects of medium composition on Ag NP toxicity, exposures (pristine/aged) were further undertaken using a Class V river water standard to mimic realistic environmental exposure conditions (Figure 1C,D). Here also, the most sensitive populations were daphnids exposed to the pristine uncoated Ag NPs with survival rates of 10% (F0), 40% (F1exp), 7% (F2exp), and 17% (F3exp). The recovery generations had survival of 63% (F1rec), 93% (F2rec), and 17% (F3rec), showing differences in the ability to recover between the two water conditions. Exposure to the pristine PVP Ag NPs was more tolerated by the Daphnia cultured in Class V water, with a total survival of 94% (F0), 90% (F1exp), 71% (F1rec), 27% (F2exp), 79% (F2rec), 96% (F3exp), and 80% (F3rec) (Figure 1C), showing surface coating specific differences as well as medium-related differences. The chemically aged Ag NPs in Class V river water had significantly less effect on the longevity when compared to all previous conditions (Figure 1D). For the daphnids exposed to the aged uncoated and PVP Ag NPs, no mortality was observed in the F0 generations over the 25 days, and those that remained in continuous exposure had a total population survival of over 83% in the F1-3exp generations after 25 days. Recovery was observed in the daphnids removed from exposure to the aged uncoated and PVP Ag NPs, with ≥93% survival in the F2rec and F3rec generations in the Class V water.
Overall, daphnids exposed to the pristine uncoated Ag NPs in HH combo medium had the highest mortality per generation. Aged Ag2S NPs in the Class V river water induced the least mortality overall. The results provide initial evidence to support the hypothesis that environmental conditions combined with Ag NP ageing (and surface coating/functionalization) plays an important role in reducing the toxicological effects, which therefore are likely to be over-estimated from traditional pristine NP studies in salt-only model media.
2.3 Reproduction
Under temperature-controlled conditions at 20 °C, juvenile Daphnia will normally pass through 6 different instar stages, before they are able to produce eggs, which typically first occurs between 5–10 days post birth. Embryos hatch from the eggs after 1 day and are held in the brood chamber for ≈2/3 days before they are released as neonates. Thereafter, daphnids reproduce asexually producing a clutch of parthenogenetic eggs after every adult moult (every 3/4 days).[29] An example of a typical moult is shown in Figure S2 (Supporting Information). The control populations released their first broods over days 11 and 12 (Figure S2, Supporting Information) and had produced their fifth broods between days 24–26 in both the HH combo and Class V water exposures. The average number of offspring per control daphnid was around 6 neonates per brood (Tables S1–4, Supporting Information) leading to cumulative totals of 27–30 offspring over the 5 broods, which was comparable with other studies using the same strain of Daphnia.[30]
Delays in the brood timings compared to the control populations for the F0 generation exposure to pristine uncoated Ag NPs were observed in HH combo medium. The first broods from the F0 generation exposed to the pristine uncoated Ag NPs were released on day 13 with each daphnid producing on average 7 neonates. Thereafter, the broods continued to be produced 3–4 days later than the controls. The reproductive delays are associated with a lack of maturity in the exposed daphnids as evidenced by the stunted growth resulting from Ag NP exposure, as observed in Figure 3A and Figure S3B (Supporting Information). On day 18 (second brood), resting eggs were observed in some of the F0 daphnids after exposure to pristine uncoated Ag NPs in HH combo medium (Figure 4C), highlighting induced stress responses. When environmental conditions deteriorate and stress levels elevate, Daphnia are known to release haploid resting eggs,[31] consisting of two eggs encased in a robust ephippium structure carried on the back of the female, that can survive dormant for many years under difficult conditions.[29, 32] Environmental stressors that influence resting egg production include chemical stress, alterations in photoperiod, predators, environmental conditions, temperature and maternal food intake. The condition of the mothers has a significant influence on the frequency of resting egg production, and influences the phenotypic response of their subsequent neonates to adapt to their environmental conditions.[33, 34] Identified morphological deformations, especially deformation of the carapace morphology, production of males and ephippia (or dormant haploid egg), as well as changes in the eggs' color and eggs abortion was observed in Daphnia chronically exposed to lead as an environmental pollutant. It should be noted that the effects observed by[34] were different to ours with the incidence of bubbling in the abdominal carapace, whereas we observed shortening and losses of tails. This may be due to NP specific exposure in the present study compared to lead solution exposure.[34] The incidence of resting egg production in the current study further evidences the pristine Ag NP mediated maternal stress.
Interestingly, the third broods of the F0 generations (pristine uncoated Ag) in HH combo medium were released on day 22 (4 days later than controls) and contained 46% males (Figure S3B, Supporting Information). Male production is directly linked to environmental stress.[35] Reproduction in the F1exp generation had an increased average of 20 neonates per daphnid in the third brood, but failed to produce fourth and fifth broods for the remainder of the exposure (Figure S3 and Table S1, Supporting Information). Increased neonate numbers for the F2exp populations were also observed (Figure 2A), combined with earlier releases of the first brood on day 9 compared to day 11 in the controls. Males were present in the broods of both the F2 and F3 exposed and recovery generations (Figure S3B, Supporting Information), highlighting maternal stress from the initial parental exposure. The recovery generations were comparable with the controls in terms of brood releases. The reproductive success of the F0 populations exposed to the pristine PVP Ag and Ag2S NPs in HH combo medium was less affected than daphnids exposed to the pristine uncoated Ag NPs (Table S1, Supporting Information).
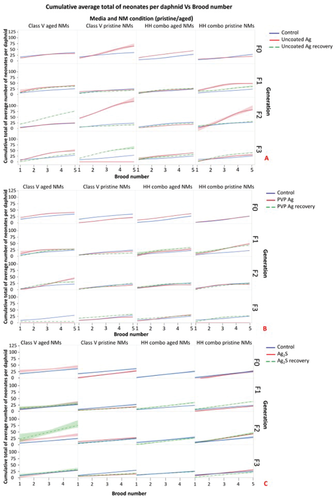
The aged Ag NPs exerted fewer toxic effects on D. magna reproduction. The exposed generations (F0–F3) had brood timings which were within ±2 days of the controls (Table S2 and Figure S3, Supporting Information). Less toxic consequences to the reproductive cycle were also observed for both the pristine and aged uncoated Ag NP exposures in the Class V (Figure 2C,D and Table S4, Supporting Information) water confirming the role of NOM to mitigate Ag NP toxicity, by reducing the releases of Ag+ (Table 2) likely as a result of formation on an eco-corona around the NPs.
| Exposure condition and generation | Pristine Ag NP exposure (HH combo) | Aged Ag NP exposure (HH combo) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Most Freq. Size [nm] | Mean NP Sizein solution [nm] | NP. Conc.In solution [particles per mL] | Diss. Conc.Remaining in solution [µg L−1] | Starting concentration [µg L−1] | Measured Ag Daphnia uptake [µg per daphnid] | BCE | Most Freq.Size [nm] | Mean NP Sizein solution [nm] | NP Conc.In solution [particles per mL] | Diss. Conc.Remaining in solution [µg L−1] | Starting concentration [µg L−1] | Measured Ag Daphnia uptake [µg per daphnid] | BCE | |
| Uncoated Ag | 32 ± 6 | 43 ± 17 | 5000 ± 180 | 0.62 ± 0.03 | 20 | 0.39 ± 0.09 | 0.63 | 31 ± 3 | 54 ± 15 | 735 ± 235 | 0.24 ± 0.00 | 20 | 0.7 ± 0.10 | 2.92 |
| F0 | ||||||||||||||
| F1 | 34 ± 5 | 49 ± 15 | 4500 ± 130 | 0.64 ± 0.03 | 20 | 0.46 ± 0.00 | 0.72 | 41 ± 2 | 63 ± 8 | 715 ± 200 | 0.7 ± 0.01 | 20 | 0.78 ± 0.09 | 1.11 |
| F2 | 29 ±4 | 38 ± 19 | 9000 ± 720 | 0.6 ± 0.00 | 20 | 0.35 ± 0.01 | 0.58 | 37 ± 1 | 37 ± 15 | 835 ± 230 | 0.28 ± 0.00 | 20 | 0.77 ± 0.02 | 2.75 |
| F3 | 33 ±4 | 47 ± 21 | 3500 ± 230 | 0.68 ± 0.00 | 20 | 0.71 ± 0.02 | 1.04 | 41 ± 3 | 57 ± 12 | 358 ± 90 | 0.69 ± 0.02 | 20 | 0.66 ± 0.05 | 0.96 |
| PVP Ag | 15 ±5 | 19 ± 7 | 16 500 ± 1500 | 0.2 ± 0.001 | 20 | 0.67 ± 0.00 | 3.35 | 11 ± 1 | 23 ± 9 | 22 000 ± 980 | 1.11 ± 0.00 | 20 | 0.49 ± 0.5 | 0.44 |
| F0 | ||||||||||||||
| F1 | 16 ±2 | 23 ± 5 | 7500 ± 560 | 0.18 ± 0.01 | 20 | 0.92 ± 0.01 | 5.11 | 16 ± 3 | 21 ± 6 | 6500 ± 600 | 0.06 ± 0.00 | 20 | 0.32 ± 0.02 | 5.33 |
| F2 | 13 ±2 | 17 ± 5 | 15 000 ± 1380 | 0.19 ± 0.00 | 20 | 0.65 ± 0.00 | 0.03 | 17 ± 2 | 43 ± 17 | 32 500 ± 4210 | 1.13 ± 0.01 | 20 | 0.21 ± 0.02 | 0.19 |
| F3 | 15 ± 1 | 18 ± 4 | 34 900 ± 2840 | 0.22 ± 0.05 | 20 | 0.59 ± 0.06 | 2.68 | 16 ± 1 | 21 ± 6 | 3600 ± 1990 | 0.06 ± 0.02 | 20 | 0.29 ± 0.06 | 4.83 |
| Ag2S | 26 ± 2 | 33 ± 13 | 160 100 ± 2110 | 0.36 ± 0.02 | 100 | 0.72 ± 0.01 | 2.00 | 25 ± 2 | 32 ± 7 | 43 000 ± 8620 | 0.06 ± 0.00 | 100 | 0.17 ± 0.03 | 2.83 |
| F0 | ||||||||||||||
| F1 | 27 ± 3 | 33 ± 15 | 126 000 ± 3860 | 0.36 ± 0.01 | 100 | 0.67 ± 0.09 | 1.86 | 24 ± 3 | 30 ± 6 | 4200 ± 4340 | 0.06 ± 0.01 | 100 | 0.2 ± 0.02 | 3.33 |
| F2 | 26 ± 2 | 32 ± 14 | 15 050 ± 2930 | 0.35 ± 0.01 | 100 | 0.67 ± 0.04 | 1.91 | 24 ± 2 | 30 ± 5 | 4500 ± 3480 | 0.06 ± 0.02 | 100 | 0.15± 0.02 | 2.50 |
| F3 | 23 ± 1 | 29 ± 16 | 207 000 ± 3990 | 0.18 ± 0.02 | 100 | 0.83 ± 0.00 | 4.61 | 24 ±3 | 30 ± 7 | 44 500 ± 5540 | 0.06 ± 0.01 | 100 | 0.24 ± 0.03 | 4.00 |
| Pristine Ag NP exposure (Class V water exposures) | Aged Ag NP exposure (Class V water exposures) | |||||||||||||
| Uncoated Ag | 30 ± 2 | 41 ± 4 | 6250 ± 950 | 0.47 ± 0.01 | 20 | 0.55 ± 0.01 | 1.17 | 35 ± 13 | 40 ± 7 | 59 400 ± 22 220 | 0.09 ± 0.00 | 20 | 0.34 ± 0.09 | 3.78 |
| F0 | ||||||||||||||
| F1 | 32 ± 3 | 42 ± 5 | 6600 ± 1010 | 0.45 ± 0.01 | 20 | 0.47 ± 0.00 | 1.04 | 43 ± 8 | 50 ± 9 | 57 900 ±20 990 | 0.11 ± 0.00 | 20 | 0.27 ± 0.07 | 2.45 |
| F2 | 30 ± 4 | 41 ± 5 | 5900 ± 640 | 0.39 ± 0.02 | 20 | 0.49 ± 0.00 | 1.26 | 45 ± 8 | 54 ± 8 | 60 550 ± 24 820 | 0.19 ± 0.01 | 20 | 0.43 ±0.04 | 2.26 |
| F3 | 33 ± 4 | 41 ± 7 | 7350 ± 860 | 0.5 ± 0.03 | 20 | 0.34 ± 0.01 | 0.68 | 42 ± 5 | 49 ± 3 | 51 900 ± 19 220 | 0.23 ± 0.03 | 20 | 0.55 ± 0.04 | 2.39 |
| PVP Ag | 24 ± 2 | 31 ± 6 | 452 150 ± 17 600 | 0.05 ± 0.00 | 20 | 0.69 ± 0.01 | 13.80 | 21 ± 5 | 30 ± 4 | 105 750 ± 27 850 | 0.06 ± 0.00 | 20 | 0.26 ± 0.02 | 4.33 |
| F0 | ||||||||||||||
| F1 | 25 ± 4 | 31 ± 8 | 47 700 ± 4330 | 0.06 ± 0.00 | 20 | 0.74 ± 0.0.3 | 12.33 | 21 ± 3 | 30 ± 3 | 123 900 ± 19 330 | 0.07 ± 0.00 | 20 | 0.29 ± 0.03 | 4.14 |
| F2 | 25 ± 3 | 32 ± 7 | 39 400 ± 3980 | 0.06 ± 0.00 | 20 | 0.55 ± 0.03 | 9.17 | 24 ± 2 | 31 ± 5 | 94 950 ± 9800 | 0.06 ± 0.00 | 20 | 0.3 ± 0.00 | 5.00 |
| F3 | 23 ± 3 | 30 ± 9 | 41 400 ±2960 | 0.06 ± 0.00 | 20 | 0.97 ± 0.04 | 16.17 | 24 ± 2 | 31 ± 5 | 92 090 ± 8950 | 0.06 ± 0.00 | 20 | 0.25 ± 0.00 | 4.17 |
| Ag2S | 47 ± 3 | 50 ± 10 | 277 000 ± 13 440 | 1.27 ± 0.02 | 100 | 1.34 ± 0.07 | 1.06 | 54 ± 5 | 61 ± 8 | 298 500 ± 29 230 | 2.34 ± 0.03 | 100 | 0.97 ± 0.03 | 0.40 |
| F0 | ||||||||||||||
| F1 | 44 ± 4 | 49 ± 5 | 285 000 ± 10 840 | 1.3 ± 0.02 | 100 | 1.48 ± 0.09 | 1.14 | 54 ± 6 | 60 ± 5 | 305 000 ± 33 140 | 2.28 ± 0.02 | 100 | 0.80 ± 0.12 | 0.35 |
| F2 | 54 ± 5 | 59 ± 6 | 286 000 ± 11 090 | 2.32 ± 0.01 | 100 | 1.25 ± 0.08 | 0.54 | 54 ± 5 | 61 ± 6 | 295 400 ± 34 290 | 2.31 ± 0.02 | 100 | 0.98 ±0.02 | 0.42 |
| F3 | 46 ± 3 | 49 ± 6 | 258 200 ± 13 980 | 1.29 ± 0.01 | 100 | 1.57 ± 0.08 | 1.22 | 53 ± 5 | 63 ± 6 | 275 400 ±29 520 | 2.35 ± 0.03 | 100 | 0.99 ± 0.09 | 0.42 |
2.4 Morphological and Growth Effects
After 24 h exposure to pristine suspensions of Ag2S and Bulk Ag (in HH combo medium) reductions in the daphnid body length were observed with the F0 generation, being on average 8% (Ag2S) and 5% (Bulk Ag) smaller than the controls over the duration of the study (Figure 3). There were no significant body length differences for the F0 generation exposed to the pristine PVP Ag in HH combo medium, although negative effects were observed in the F1exp and F1rec generations, which had significantly larger body lengths than the controls, showing abnormal accelerated growth as a response to parental Ag NP exposure. Compared to all other Ag NP exposures, the pristine uncoated Ag NPs (in the HH combo medium) had the most negative effects on daphnid growth. The F0 populations were significantly smaller (p < 0.05 Appendix: Table AP.1, Supporting Information) from day 9, and on average were between 13–22% smaller than the control populations. The negative effect on growth was also observed in both the continuous exposure and recovery generations, with F1exp and F1rec both being up to 21% smaller than the controls, whereas the F2exp were up to 26% smaller (although the F2rec did show some recovery with no significant size difference after day 9) and the F3exp were 35% smaller than controls (Figure 3). Assessment of changes in the rate of growth of the daphnids were also determined using linear Log10 transformations to assess the total growth over time for all conditions as shown in[36] (Appendix Table AP.3, Supporting Information). A reduction/increase in growth rate coefficient relative to the control (which had values between 0.008–0.009) was considered as evidence of toxicity resulting from the NP exposure.
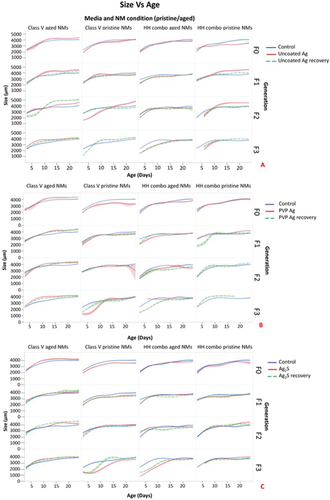
Exposure of the F0 generation to the pristine Ag NPs in class V water also resulted in the Daphnia being significantly smaller than the controls by up to 18% (Ag2S and Bulk Ag) and 21% (uncoated and PVP Ag) (Figure 3). On average, the F1-3exp generations and F1-2rec generations were always smaller than the controls. Whereas, the F3rec generations were significantly larger (Table SAP.1, Supporting Information) than the control sizes at the same measured time points (Figure 3). The results overall show that the reduced body sizes observed are a direct effect when exposed to Ag NPs, irrespective of their coating.
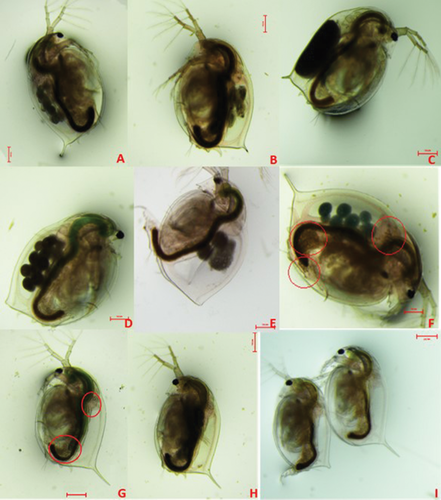
Morphological observations of the Daphnia (F1–F3) exposed to the pristine Ag NPs in HH combo exhibited malfunctions including loss of ocular developments (Figure 4B), altered tail-spine length (Figures 4B,C,I), and, in some cases, offspring were born with no tails (Figure 4D,I,E). Each of these phenotypical characteristics were epigenetic since they were observed in all F1-3 generations irrespective of whether they underwent continuous cross-generational exposure to pristine uncoated and PVP Ag NPs, or were part of the recovery generations removed following parental exposure. Heavy metal exposure has been well documented to have effects on carapace shedding which in turn affects growth.[37, 38] However, the recovery generations (F2rec and F3rec) were never directly exposed to the Ag NPs, thus, the tail abnormalities/losses can only be explained by inherited changes induced in their exposed parents. No tail development indicates that the Ag NPs may disrupt the embryonic development in exposed populations, since the tail spines are formed during mid-embryonic maturation.[39] The combination of altered tail spines and reduced sizes are usually a phenotypical trait of normal ageing. However, since these phenotypic effects were observed in juveniles, this suggests that Ag NP exposure accelerates phenotypical ageing effects, which correlates with the reproductive declines observed. Studies by Djekoun et al.[38] observed tail deformities to Daphnia when exposed to cadmium over time. In addition, they reported delayed brood releases, inhibition of various developmental stages, and abnormalities in the carapace, eyes and caudal spine, as also observed in the current study.
Moreover, trans-generational reductions in sizes and tail losses/tail length reductions were observed after 3 generations in both continuously exposed and recovery groups exposed to pristine Ag NPs in HH combo medium. Note, these morphological defects to the tails were also observed for all Ag NP exposures/removed for the F1-3 generations in the pristine Ag NP exposures in HH combo medium, but only in the F1exp generation exposed to pristine uncoated Ag NPs in class V water. There were no observable morphological defects (tail abnormalities), indicating that in this case the parental exposures did not result in epigenetic changes to daphnids exposed to the aged Ag NPs in the Class V water.
Exposure to environmental toxicants has been linked with the activity of lipid allocation resulting in transcriptional and metabolic changes and enhanced lipid deposition,[40] and accumulation of lipid deposits has been previously linked with the effects of senescent decline in multiple species.[41-44] Although we did not quantify lipid deposition, morphological analysis (Figure 4F,G and Figures S5–8, Supporting Information) identified fatty deposits located around the heart, brain and abdominal claw, similar to those identified previously.[45] These deposits were not present in the control populations at earlier developmental stages (up to day 18) but were present in later F1-3exp generations of D. magna. Lipid deposits were visible around the heart from Day 6 in F0 Daphnia exposed to aged Ag2S and aged uncoated Ag (Figure S6, Supporting Information) and from day 21 for those exposed to aged PVP Ag in HH combo medium. Lipid deposits were present from day 6 in the F1 generations, although there was no evidence of lipid deposits in any of the F2 and F3 generations for those exposed to aged Ag NPs in the HH combo medium or in the subsequent recovery generations. Lipid deposits were only visible in the F1exp bulk Ag, F1exp uncoated Ag and in both F1 exposed and recovery generations exposed to aged PVP Ag NPs in the Class V water (Figure S8, Supporting Information).
Based on the evidence of the life history parameters observed in the present study and the morphological indicators such as shortened tail lengths relative to the controls, ageing as a stress response was investigated. Previous work investigating Daphnia longevity and ageing found a correlation between tail length and Daphnia age[36] as one of the morphological indicators of the rate of aging. In this approach, the ages of healthy daphnids can be calculated using a linear calibration of tail length versus age compared to the untreated controls. On this basis, NP-induced changes in tail length should lead to predictions of daphnid ages higher than their actual ages, indicative of an accelerated ageing phenotype. Using the equation of the line of best fit from the plot of tail length versus age measurements of healthy daphnids (i.e., the control populations in this study, Figure S15 and Table S12, Supporting Information) the apparent ages of the NP-exposed daphnids were determined relative to their actual ages to produce the predicted age values (Appendix: Tables AP.3–7, Supporting Information). Using this assessment, the predicted ages of the exposed daphnids were considerably higher than their actual ages based on the measured tail lengths compared to the control populations.
2.5 Bioaccumulation of Ag NPs
After 7 days exposure the total Ag body burden in the Daphnia generations (consisting of both ions and Ag NPs), the average Ag NP size in the media, Ag NP concentration (particles per mL) and dissolved ion concentrations (µg L−1) left in solution were determined by spICP-MS and total Ag concentration by ICP-MS (µg per daphnid) (Table 2). The pristine Ag2S NPs exposed in the HH combo medium had the highest measured uptake of Ag (F0 = 0.72, F1 = 0.66, F2 = 0.67, and F3 = 0.83 µg per daphnid), yet little toxicity was observed in terms of the daphnid survival, growth and reproduction across the F0-F3exp generations. The pristine uncoated Ag NPs resulted in the lowest uptake concentrations of total Ag in the daphnids (F0 = 0.39, F1 = 0.46, F2 = 0.35 and F3 = 0.71 µg per daphnid) across the F0–F3exp generations, and had the lowest NP concentration (particles per mL) left in solution and the largest mean Ag NP size (Table 2). However, these exposures had the most negative effects on daphnid growth, longevity and reproduction throughout the exposed generations, suggesting strong effects from Ag+. Interestingly, the dissolved concentrations of Ag+ in the medium were highest in the pristine uncoated Ag NP exposure conditions, highlighting that the observed toxicity is linked to ionic exposure via the dissolution of the Ag NPs.[12] Differences in surface coating will determine the Ag NP stability and solubility in media. Uncoated NPs will dissolve more readily than those with strongly bound coatings like PVP[15] as evidenced in the present study (Table 2).
When the uncoated Ag NPs were aged in the HH combo medium, the NP and dissolved concentrations in solution at day 7 were reduced, whereas the internalized Ag concentrations increased (between 0.7–0.78 µg per daphnid across the F0–F3exp generations) as did the mean NP sizes. However, the internalized Ag concentrations for the aged PVP and Ag2S NPs under the same conditions were reduced when compared to the pristine Ag NPs exposures. In all cases, despite the differences in uptake concentrations, surface coating and media effects, ageing of the Ag NPs played a clear role in reducing their overall toxicity.
In the environmentally realistic water (Class V), the pristine Ag NPs underwent quite different transformations. The dissolved Ag+ concentrations in the tissue were lower for the uncoated (0.34–0.55 µg per daphnid) and PVP Ag (0.55–0.97 µg per daphnid) NPs than those in the HH combo medium, whereas the Ag2S NPs had the highest uptake of both NPs and dissolved ions (Table 2) yet the pristine uncoated Ag NPs were still the most toxic to the Daphnia. Ageing the Ag NPs in the Class V water reduced the NP concentrations (particles per mL) and the dissolved Ag in solution for the uncoated and PVP Ag NPs. Furthermore, the uptake of the total Ag by the daphnids exposed to the uncoated and PVP Ag NPs was also reduced when compared to all the previous conditions. In all cases, ageing the Ag NPs in the realistic water dramatically reduced their toxicity, when compared to all other exposures.
The TEM cross sections of exposed daphnids (F0) are presented in Figure 5A-I and Figure S9 (Supporting Information) to illustrate both bioaccumulation of the Ag NPs and its impacts on the gut and surrounding histopathology. Sections of the control populations present typical gut structure, including mitochondria (M), cell junctions (CJ), microvilli (MV) and the peritrophic membrane (PTM) for daphnids cultured in each media (Figure 5A,B for HH combo and Class V water, respectively). Figure 5D presents a cross section of the daphnid gut (F0) exposed to pristine uncoated Ag NPs in HH combo media showing the interaction of the NPs with the cell, and the formation of lipid like cytoplasmic inclusions and large vacuole structures. The PTM is damaged and Ag NPs are present within the cellular matrix. The purpose of the PTM is to provide a cellular layer that protects the epithelial cells of the gut and regulates the exchange of endogenous substances and nutrients.[46] Internalization of the pristine uncoated Ag NPs is strongly correlated with the most toxic effects observed to growth, morphology and survival as previously discussed, potentially via the Trojan horse effect whereby dissolution following uptake releases high local concentrations of Ag+. The irregular organization of the organelle shapes, with the presence of large vacuole structures (Figure 5G), may be due to oxidative damage, mediated by the generation of reactive oxygen species (ROS). ROS has a direct and localized effect arising from the internalization of the Ag NPs and associated dissolved Ag, resulting in dysfunctional order and development of the cells/tissues leading to morphological changes[47] which may also have disturbed metabolism and chemical transformations.[48]
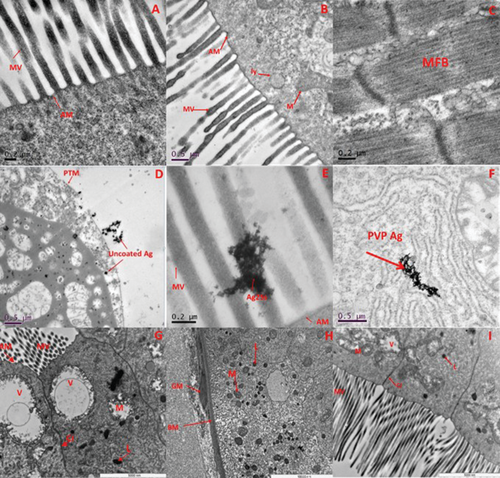
Pristine PVP Ag and Ag2S NPs (exposed in HH combo medium) can be seen clearly as agglomerates in the gut located in the lumen and MV areas (Figure 5E,F). However, there was no evidence (from the sections viewed) to suggest that either particle type was translocated into the cellular matrix. Similar distributions of NPs in Daphnia are reported in the literature for exposure to Fe3O4, α-Fe2O3[48] and CuO[49] where they are not internalized.
The cross sections from the Daphnia exposed to aged uncoated Ag in the HH combo medium (Figure S9E, Supporting Information) and Class V water (Figure 5G), are representative images showing disorganization of the mitochondria organelles. These appear lysed with empty internal spaces and disorganization of their cristae and the presence of large vacuoles. Despite being chemically aged, there are localized effects which may explain why the Daphnia were most sensitive to the uncoated Ag NPs due to their potential dissolution in vivo, although toxic effects were dramatically reduced in the class V water. In addition, the Bioconcentration Factor (BCF) was calculated (Table 2) to identify the bioaccumulation of total (dissolved vs particulate) Ag in the Daphnia relative to the dissolved Ag in each of the media, with the assumption that concentrations in excess of the background dissolved ions are indicative of uptake of Ag particles. In general, the BCF is lower (between 0.63–1.04 across the generations) in the pristine uncoated Ag samples (HH combo media) compared to the aged uncoated Ag NPs (HH combo) with BCF values between 0.96–2.92 despite the higher dissolved Ag+ content detected in the pristine media (especially HH Combo) (see Table 2). Therefore, although the bioaccumulation was higher in the daphnids exposed to the aged NPs, we can assume that the daphnids ingested the (transformed) aggregated aged Ag NPs which were less toxic compared to the mainly dissolved Ag+ ingested from the pristine exposures.
In both HH combo and Class V water, the identification of Ag NPs in vivo from the TEM images and associated damage is consistent with the increased dissolution of the pristine Ag NPs, compared to the lower dissolution in their aged counterparts (Table 2), confirming also that ingestion of the aged Ag NPs occurred as Ag NPs.
2.6 Gene Expression
Prior to the exposures a set of 8 genes were selected based on literature analysis. The genes selected represent pathways encoding for cellular functions known to be induced by other pollutants, including xenobiotic and metal detoxification, oxidative stress, transport and energy production, DNA repair (Poynton et al., 2007) and general maintenance.[50, 51] The specific genes were chosen to provide mechanistic insights into NP-organism interactions, and provide a way to group NPs by their molecular level effects. Furthermore, the genes expressed may indicate both nonspecific and specific modes of action as well as underlying mechanisms for toxicity of Ag NPs with particular physiochemical properties.[51] For all Ag NP exposures irrespective of media, aging or generation (F0–F3) there were significant differences between the exposed daphnids populations compared to the controls. The gene expression patterns are shown in Figure 6 for the uncoated Ag NP exposure and Figures S10–S12 (Supporting Information) for each of the differently surface coated Ag NPs and bulk Ag in each water condition (pristine/aged in HH combo/Class V). Differential gene expression patterns were observed for the different surface-coated Ag NPs and the most important factor in determining Daphnia sensitivity was whether the NPs were exposed as pristine or aged forms. Significant gene expression changes were induced by all pristine Ag NPs, and those effects were broadly remediated by ageing the NPs in the Class V water. As expected, MET, GST, CAT, NADH and β-Actin were strongly induced in all the pristine NP exposures, though mainly only after the initial F0 generation. MET, GST, CAT all code for proteins involved with xenobiotic detoxification, oxidative stress and ROS.[24, 51] β-Actin encodes for proteins important to cytoskeleton and muscle fibril production. Studies have described an increase in actin concentration as a compensatory mechanism to maintain muscular and cellular performance in times of environmental stress,[51, 52] as observed in the present study.
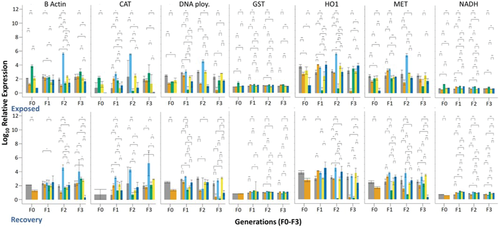
Daphnia exposed to the uncoated Ag NPs (Figure 6) had the most responses (upregulation compared to the controls) in both the continuously exposed and recovery populations for all conditions and all genes observed. The toxic responses from the uncoated Ag NPs may be due to particle stability, particularly in their pristine forms since the Ag NPs will be more susceptible to dissolution as shown by the high concentrations of internalized dissolved Ag from the pristine uncoated Ag exposures (Table 2). The dissolved species and high reactivity of the uncoated Ag are likely to contribute to the increase in mortality and decrease in overall health condition of daphnids, which may have led to a decrease in feeding and changes in physiology of the gut (Figure 5D). Irrespective of media composition the continuously exposed populations had significantly induced levels of CAT for the pristine uncoated Ag NPs in F1–F3 generations. Aging the NPs significantly reduced CAT expression. Recovery generations also had reduced gene expression for the aged and pristine uncoated Ag NPs, particularly for HH combo exposures.
For all Ag NP exposures, gene expression was dominated by NP ageing, NP surface coating and environmentally representative media and was reflective of the phenotype and life history data. Overall, the pristine NP exposures, irrespective of surface coating, were more stressful than NPs aged in Class V water and the continuously exposed daphnids were more stressed than the recovery generations. Recovery generations were less stressed overall, as expected, and the gene expression levels were mostly maintained throughout the generations.
3 Summary
To date, no paired studies of continuous exposure for 4 generations versus parental only exposure followed by recovery over an additional 3 generations have been published. Additionally, all other reproductive and multi-generation studies published to date were performed in salt-only medium using freshly dispersed (reactive) NPs. That said, our findings for the pristine NPs in HH combo medium are consistent with the emerging literature; for example,[53] did a comparative assessment of Ag NPs on three species of Daphnia, including D. magna and found that the toxicity of Ag NPs in chronic studies may be due to the presence of food and that feeding increases the accumulation of NPs in the gut of the daphnids. Studies on carbon nanotubes and multi-generational (three generations) exposures[54] have shown that parental exposure has considerable impacts on the survival and reproduction of subsequent generations. Similarly, multigenerational exposure (three generations) of daphnids to quantum dots[55] identified a decline in the reproductive output, and changes to gene expression of stress response genes in exposed daphnids. Furthermore, the effect of Ag NP transformations in a sewage sludge[56] identified that pristine Ag NPs were most toxic, reducing the number of offspring produced. When the Ag NPs were transformed by reacting in the sludge and collecting the effluent over 6–10 days, toxicity was reduced due to the formation of Ag2S with wastewater-borne Ag NPs having no effects on reproduction in any generation. Collectively these papers, and the data presented here, provide a growing body of evidence that complete risk assessment of NMs needs to consider both the transformed forms of NMs and multigenerational effects, ideally pairing continuous exposure and recovery generations to tease out epigenetic and adaptive effects.
4 Conclusions
Multi-generational exposure of Daphnia to acute EC30 concentrations of Ag NPs with various capping agents/surface chemistries (uncoated, PVP-coated, sulfidised Ag2S) induced impacts on all life history traits (growth, reproduction (brood delays, number of offspring, incidence of males and resting eggs)) in the directly exposed generations, inducing epigenetic changes that persisted in the subsequent three recovery generations when exposed in a standard high-hardness medium (HH combo) to both the pristine NPs and those aged for 6 months in the medium. While particle ageing in salt-only medium reduced the toxicity of the NPs compared to the pristine ones, it did not fully ameliorate NP toxicity. By contrast, ageing in the NOM-containing Class V water almost completed removed the NP toxicity.
The observed histology changes following Ag NP exposure in HH Combo medium correlated with the increase in expression of GST, which is indicative of an oxidative stress pathway, and with the up-regulation of CAT, GST and MET which are associated with metal detoxification. These genes remained elevated in the F2 and F3 recovery generations, whose F0 parent had been directly exposed, and where F1 were born into exposure but removed to fresh medium <24 h post birth. In the continuously exposed generations, by F3 an adaption to the Ag NPs was observed, particularly for the aged Ag NPs in class V water. The slow dissolution of the Ag NPs following uptake and internalization correlated with the histological changes and gene expression, and, as expected, toxicity was in the order Ag uncapped > Ag PVP > Ag2S and, for all NPs the toxicity induced by the pristine form exceeded that of the aged form.
Identical exposures of the Ag NPs in a representative freshwater (Class V water based on European water classifications) resulted in dramatically decreased effects on the Daphnia in both exposed and recovery generations, and with both the pristine and the 6-month aged variants. NOM is known to form a corona around NPs, and reduces the amount and rate of dissolution, thus driving the reduced toxicity observed. Life history traits were indistinguishable from the unexposed controls in all generations of the recovery generations, however at the genetic level, elevations and decreases in gene expressions were observed.
In all cases gene expression was dominated by NP ageing, NP surface coating and environmentally representative media and was reflective of the phenotype and life history data. The study confirms that exposure to pristine Ag NPs in simple high hardness medium results in very dramatic life history changes, with indications of accelerated ageing, and epigenetic effects that propagate though at least the subsequent three generations. The Ag NP effects were consistent with an accelerated ageing phenotype, evidenced by shortened tail lengths and lipid deposits, which will be investigated further in our ongoing research analyzing the complete genome. These effects are substantially ameliorated through utilization of a more environmentally realistic freshwater medium with natural organic matter and utilization of NP forms typical of the freshwater environment which will have acquired a biomolecule corona and undergone other physical and/or chemical transformations that generally reduce their toxicity to daphnia.
5 Experimental Section
The Supporting Information describes the methodology in more detail.
Materials
Commercially available chemicals, solvents and humic acid (HA) were purchased from Sigma-Aldrich (Dorset, UK) and were of analytical reagent grade. Ultrapure water (UPW) with a maximum resistivity of 18.2 MΩ cm−1 was used throughout. The NPs used in this study include uncoated bare Ag (61 ± 36 nm), a polyvinylpyrrolidone (PVP) coated Ag NP (18.2 ± 11 nm), and Ag2S PVP coated (43.6 ± 14 nm) to represent the chemically aged form. A micron sized Ag control was also included. All NPs were obtained from the EU H2020 NanoFASE project partners (Applied Nanoparticles (Ag2S PVP), Amepox (PVP Ag NPs) and Promethean (uncoated Ag NPs). Bulk Ag was purchased from Sigma (Sigma Aldrich UK).
Media and Representative Waters
Experiments were conducted in a high hardness culture medium (HH combo)[57] and in a synthetic Class V artificial river water standard.[28] The HH combo medium is designed to match the total hardness of water found in the environment without any natural organic matter. The Class V river water has high alkalinity and high ionic strength and is more environmentally relevant as it contains natural organic matter. The Class V water typically describes waters found in the southern UK, Poland, Greece, France, Balearic countries and the Iberian Peninsula.[28] A description of the water combinations are given in Tables S5, 5A, 5B (Supporting Information).
Nanoparticle Characterization and Chemical Ageing
Dynamic light scattering was used to measure the hydrodynamic diameters of “pristine” and “aged” 6 months in each of the test media) Ag NPs using a Malvern Nanosizer 5000 (Table 1). Transmission electron microscopy analysis of the NPs (Table 1 and Figure S1, Supporting Information) was performed using JEOL 1200EX 80kV and JEOL 1400EX 80kV microscopes. Ag NPs were prepared by the drop casting method, depositing a 20 µL drop of the NP suspension onto a 300 mesh carbon-coated copper TEM grid (Agar Scientific, UK). Ageing of the NPs was achieved by preparing stock solutions (1000 mg L−1) in the HH combo and class V river water (Table S5, Supporting Information) and storing them for at least 6 months prior to the Daphnia exposures. Stock solutions were kept continuously refrigerated at 4 °C throughout the ageing process.
Maintenance and Culturing of the Daphnia
Initial stocks of D. magna were maintained using pools of 3rd broods of Bham2 strain (genetically identical) and were kept in a 20 °C temperature controlled environment with 12 h light and dark cycles. Daphnia were cultured for at least 3 generations in HH Combo media[57] and Class V river artificial river water[28] for acclimatization to the medium(s) which were refreshed weekly to ensure healthy culture maintenance. Cultures were fed Chollera vulgaris algae daily, to a total 0.5 mg carbon between days 0–7 (750 µL) and 0.75 mg (1.5 mL) carbon per day from day 7.
Range-Finding Study (Daphnia Acute Immobilization Test, OECD 202)
The acute immobilization tests were conducted on Daphnia exposed to the pristine NMs in the HH combo medium over 24–48 h.[58] An EC30 value (Figure S13, Supporting Information) was established as follows: 20 (±1) µg L−1 for uncoated Ag NPs, 20 (±2) µg L−1 for PVP Ag NPs, and 100 (±4) µg L−1 for Ag2S NPs, respectively. There is a difference between using environmental concentrations and effect concentrations (EC); the justification for using effect concentrations in this study is because regulation and environmental risks are assessed by characterizing the effects in biological receptors. The EC30 values were selected for the chronic studies in order to have some toxicity in the pristine NMs and thus allow for assessment of the reduction in toxicity arising from dispersing the NMs in the NOM-containing medium and from ageing the NMs, both of which potentially reduce the NMs' surface reactivity.
Survival, Growth and Reproduction
The experimental and control daphnids were checked daily for survival, egg production, and neonate release. Measurements of body size were taken every 3 days in accordance with moulting, and neonates were counted over the first 5 consecutive broods. Neonates of 3rd broods were used to set up the following generation and/or harvested for gene expression analysis. Body lengths were measured (days 3–24) from the apex of the helmet to the base of the apical spine using a Nikon (Japan) stereomicroscope, model SMZ800 Digital Sight fitted with a D5-Fi2 camera, using NIS Elements software.
Total Body Burden/Bioaccumulation/Metal Concentrations after 7 Days Exposure
TEM cross sections of F0 generations after 7 days of exposure were further prepared by the Centre for Electron Microscopy at the University of Birmingham (UK). Briefly whole Daphnia were euthanized and fixed immediately in a 2.5% glutaraldehyde in a 0.1 m phosphate buffer suspension. Daphnids were dehydrated in ethanol and embedded in epoxy resin before sectioning using a ultramicrotome to cut 0.1 µm sections with a diamond knife. Images were visualized using JEOL 1200EX 80kV and JEOL 1400EX 80kV microscopes. Quantification of the Ag+ and Ag NP concentrations in solutions was carried out after the first 7 days of exposure only, when the media was refreshed. Samples of the old media containing NPs were analyzed by single particle-ICP-MS (NexION 300D, Perkin Elmer). In all cases, characterization measurements were performed as 3 replicates.
Multigenerational Study Design
The multigenerational design is shown in Figure S14 (Supporting Information). Briefly, Ag NPs were exposed to an F0 parent generation and the 3rd broods (F1) from the F0 generation were split to produce a paired set of successive generations, half of which were continuously exposed (Fexp) over three further successive generations (F1exp, F2exp, and F3exp) and the other half of which were allowed to recover (Frec) for 3 generations (F1rec, F2rec, and F3rec) in medium only. The medium and NPs (if exposed) were refreshed weekly. Each generation (exposed/recovery) was monitored until the appearance of their fifth broods (28–32 days). Information on the timing of broods for each treatment group is presented in Tables S1–4 (Supporting Information).
Gene Expression
An Agencourt RNAdvance Tissue Kit (Beckman Coulter A47943) using paramagnetic bead-based technology was used for total RNA isolation and purification and was performed on a Beckman Coulter Biomek FxP. A total of 8 genes were selected for target-specific amplification using a mix of previously published primer sequences (Table S6, Supporting Information). Primer sequences were also checked using NCBI primer blast software (https://www.ncbi.nlm.nih.gov/gene) for the probability of amplifying nonspecific products. A Onestep qPCR kit (Qiagen) was used in accordance with the manufacturer's guidance for reverse transcription (Tables S7–S9, Supporting Information). Gene expression was conducted using Flex Six Integrated Fluidic Circuit (IFC) Delta Gene Assay (72 × 72) in combination with a HX Prime (153x) system and a Fluidigm BioMark (Standard) Real time PCR instrument, as per the manufacture's recommended protocol (Tables S10 and S11, Supporting Information). A detailed description of the method can be found in Section S2.4 in the Supporting Information.
Statistical Analysis
All experiments were repeated in triplicate, and the data was recorded as the mean with standard deviation. For the growth studies, a student's t-test was used to detect any significant difference between the control, treated and recovery groups. In all analyses, a p-value <0.005/0.05 was considered statistically significant (Appendix: Table AP.1, Supporting Information).
Growth data was analyzed using a linear regression model testing Log10 transformed age in days against time in order to determine the growth rate in the NP-exposed conditions relative to the controls, as per.[36] The linear model rate (slope) of daphnid growth between each population for their age versus time was analyzed in RStudio. A positive number shows an increase of the growth rate line and the further from 0 the slope is, the faster the rate of growth is (Appendix: Table AP.2, Supporting Information). Therefore rate of change coefficients with values closer to 1 grew at a slower rate compared to those with values closer to 0. Toxicity of the NPs was considered to be present if there was a change in the rate of change coefficient relative to the control groups (which had values between 0.008–0.009).
To assess whether the NPs were inducing accelerated aging, the apparent age of the continuously exposed and recovery daphnids were determined based on their measured tail lengths.[36] Average tail length (mm) was fit using the equation from the line of age versus tail length for the unexposed controls to produce the predicted age of the exposed daphnids, as shown in the example in the Supporting Information. (Table S12 and Figure S15) with the calculated predicted ages shown in the Appendix Tables AP.3–7, Supporting Information).
Gene expression levels were normalized to 18S expression levels as previously described.[60] Statistical significance of changes in gene expression were computed in RStudio, as follows: models were fit using lmfit, eBayes was used to compute the significance of parameters, resulting p-values were corrected for multiple testing (False Discovery Rate) using the Benjamini–Hochberg (BH) method. The main effects were also evaluated using ANOVA in R (and corrected for multiple testing as above). Significance thresholds were applied to BH-adjusted p-values (Appendix Table AP.7, Supporting Information–Excel file).
Acknowledgements
This work was funded via a Natural Environment Research Council grant (NE/N006569/1). The authors acknowledge use of the UoB Daphnia Research and the Environmental Genomics facilities, and the use of the School of Materials and Metallurgy TEM centre. The authors thank Paul Stanley and Theresa Morris (School of Materials and Metallurgy) for assisting with the TEM Daphnia specimen preparations.
Conflict of Interest
The authors declare no conflict of interest.




