Forest restoration decouple soil C:N:P stoichiometry but has little effects on microbial biodiversity globally
Abstract
Introduction
Forest restoration is an effective way to promote ecosystem functions and mitigate climate change. However, how forest restoration affect soil C:N:P stoichiometry and microbial biodiversity, as well as their linkage across contrasting forest types globally remains largely illusive.
Materials and Methods
Here we conducted a global meta-analysis by synthesizing 121 published papers with 1649 observations to explore how forest restoration affect soil C:N:P stoichiometry and microbial biodiversity globally.
Results
Forest restoration significantly increased soil total carbon (C), nitrogen (N) and phosphorus (P) content, whereas having no significant impact on most microbial diversity indicator, except for an enhancement in bacterial operational taxonomic unit and fungal Simpson. Meanwhile, forest restoration effects on soil C:N:P stoichiometry varied with different forest types, with promoting more soil C and P in ectomycorrhizal than those in arbuscular mycorrhizal forests. Meanwhile, forest restoration induced changes in soil N and P were positively correlated with microbial Shannon index. More importantly, forest restoration effects on soil C:N:P stoichiometry and microbial biodiversity were regulated by climate factors such as mean annual temperature and mean annual precipitation.
Conclusion
Our results highlight the crucial role of forest restoration in decoupling the biogeochemical cycles of C, N and P through changes in microbial biodiversity. Therefore, incorporating the decouple effects of forest restoration on soil C:N:P stoichiometry into Earth system models may improve predictions of climate–forest feedbacks in the Anthropocene.
1 INTRODUCTION
Over the past decades, forests have been exposed to anthropogenic pressure and rapid climate change, causing severe loss of ecosystems functions and biodiversity, and further threatening the sustainable development of forest ecosystems (Hanewinkel et al., 2013; Zhou et al., 2022). To stop this trend, numerous international initiatives such as the New York Declaration on Forests and the Bonn Challenge (UNEP Year Book, 2011) have established ambitious targets to promote the restoration of degraded ecosystems aiming at conserving biodiversity and ecosystem function (Bastin et al., 2019). Indeed, numerous individual studies and a few meta-analyses have confirmed that forest restoration could contribute to increase multiple ecosystem services such as plant biodiversity and soil carbon (C) sequestration (Gong et al., 2017; Yang et al., 2011; Zhou et al., 2022). Yet, despite the enthusiasm surrounding forest restoration, the extent to which it will lead to long-term global improvements in soil C:N:P stoichiometry and microbial biodiversity in degraded forest systems is still largely unknown.
Soil C:N:P stoichiometry changes dramatically during forest restoration, which influenced mainly by plant type, mycorrhizal type and climatic zone (Jia et al., 2005; Ouyang et al., 2017; Pan et al., 2021; Song et al., 2019; Zhang et al., 2018). It has been shown that soil C, N and P increased as the succession proceeds (Gao and Huang, 2020; Zhao et al., 2023; Zhou et al., 2017), whereas others showed that total phosphorus (TP) decreased as forest age increased in plantations (Yu et al., 2022; Zhang et al., 2021). Furthermore, forest restoration induced increase in soil C:P and N:P far more than that of C:N, suggesting that forests are primarily limited by P over time (Ouyang et al., 2017; Pan et al., 2021; Zhang et al., 2018; Zhou & Wang, 2016). More importantly, arbuscular mycorrhizal (AM) forests tend to capture more soil C than ectomycorrhizal (ECM) forests, whereas conifer forest has lower soil nutrients such as N and P than those in angiosperm forest (Zhang et al., 2023; Zhou et al., 2022). Whether and how these changes affect soil C:N:P stoichiometry during long-term forest restoration remain largely unknown.
Soil microbial communities are spatially dependent and highly responsive to changes in the environment (Ettema & Wardle, 2002; Ren et al., 2019; Zhou et al., 2023). Several studies have suggested that forest restoration may have different effects on microbial diversity (Castaño et al., 2019; Ren et al., 2019; Zhu et al., 2022), with increase in bacterial diversity (Zhang et al., 2016; Zhao et al., 2019), and decrease effects on fungal diversity (Liu et al., 2020) and no change on soil bacterial diversity (Liu et al., 2020; Zhou et al., 2017; Zhu et al., 2022). Contradictory responses of microbial biodiversity to forest restoration may be associated with the differences in the soil properties and forest types (Delgado-Baquerizo et al., 2017; Zhou et al., 2022), which are susceptible to the changes during forest restoration (Wang et al., 2022). Therefore, understanding the effects of forest restoration on soil C:N:P stoichiometry and microbial biodiversity has implications for us to better predict the future of forest restoration under global climate change.
Microbial biodiversity is closely associated with soil C:N:P stoichiometry (Chapin et al., 2011). Based on ecological stoichiometry theory (Mooshammer et al., 2014; Sterner & Elser, 2002), the elemental balance of an ecosystem can profoundly influence community structure and ecosystem function. The resource-ratio theory also suggested that the ratio of available resources can determine the relative abundance of various functional groups within a community (Tilman, 1982). Therefore, forest restoration induced balance of C, N and P is expected to shape the composition and functioning of decomposers communities (Delgado-Baquerizo et al., 2017; Liu et al., 2020; Ren et al., 2016). Microbial decomposers with varying resource demands can coexist when the resource availability matches their niche preference (Waldrop et al., 2006). For example, bacteria are typically more efficient at utilizing labile carbon sources, whereas fungi are more effective at breaking down complex carbon compounds like lignin (Boer et al., 2005). However, prior research have reported inconsistent results, with some showing positive correlations between C:N:P stoichiometry and microbial diversity (Ren et al., 2016; Yang et al., 2022; Zhang et al., 2020), whereas others report negative associations (Delgado-Baquerizo et al., 2017). These discrepancies highlight the need for further exploration on the underlying mechanisms of these relationships during forest restoration at a global scale.
To address these knowledge gaps, we conducted a global meta-analysis of 1649 observations from 121 studies to demonstrate the changes in soil C, N and P and their stoichiometry, as well as microbial diversity, respond to the increasing stand age during forest restoration. The main objectives of this study were to (1) quantify the global effect of forest restoration on soil C:N:P stoichiometry and microbial diversity and determine whether these changes across contrasting forest types; (2) investigate how forest restoration affects the relationship between soil C:N:P stoichiometry and microbial diversity; and (3) explore the potential factors regulate the effects of forest restoration on soil C:N:P stoichiometry and microbial biodiversity. More specifically, we tested two hypotheses: (1) forest restoration influences soil C:N:P stoichiometry and microbial diversity, which may be moderated by forest types and climate conditions; and (2) effects of forest restoration on microbial biodiversity would be regulated by soil C:N:P stoichiometry.
2 MATERIALS AND METHODS
2.1 Data source
We used Web of Science and China National Knowledge Infrastructure to search for peer-reviewed journal articles between January 1950 and August 2022 on the impact of forest restoration on soil carbon, nitrogen, phosphorus and microbial diversity. We used the search term (“forest restoration” OR “forest succession” OR “secondary succession” OR “natural regeneration” OR “tree plantations”) AND (“soil carbon” OR “soil nitrogen” OR “soil phosphorus” OR “C:N:P stoichiometry” OR “bacterial diversity” OR “fungal diversity” OR “microbial diversity” OR “richness” OR “Shannon” OR “Simpson” OR “Chao1” OR “OTU”). Studies were selected based on the following five criteria: (i) all forests were initially disturbed and are now on continuous secondary succession. (ii) At least one independent variable in the experiment was used to examine the effects of forest restoration on soil carbon, nitrogen, phosphorus, C:N:P stoichiometry and microbial diversity index. (iii) The variables in different ages were at same spatial scales. (iv) Forest restoration should be less than 150 years. (v) The mean value of the selected variables in different age groups were extracted directly from the tables, graphs, or text. Values from graphs were extracted using the software Getdata. In total, 121 published papers with 1649 observations distributed were selected (Figure 1, PRISMA in Supporting Information S1: Figure 1 and Text 1).
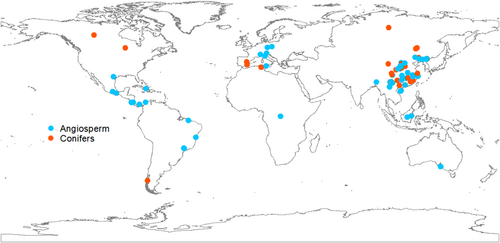
The selected studies had at least one of 16 variables related to forest restoration to be included in the database. The database included soil total carbon, nitrogen, phosphorus, C:N:P stoichiometry (i.e., C:N, N:P, C:P) and microbial diversity (i.e., bacterial Simpson, bacterial Shannon, bacterial Chao1, bacterial operational taxonomic unit (OTU), fungal Simpson, fungal Shannon, fungal Chao1, fungal OTU, microbial Simpson and microbial Shannon). Here, Shannon Index = , Simpson Index = , where S represents the total number of species and is the proportion of each species in the community (Whittaker, 1972). Meanwhile, we extracted the leaf life span (i.e., evergreen, deciduous and mixed) and plant functional type (i.e., angiosperm and conifers) of the dominant species, as well as stand age of forests. We also assembled mycorrhizal association information of dominant species, classifying them as AM fungi and ECM fungi at each study site, using the database of Wang and Qiu (2006), and Soudzilovskaia et al. (2020). In addition, we also recorded geographic and environmental variables from research articles or referenced sources, including longitude, latitude, mean annual temperature (MAT) and mean annual precipitation (MAP). If MAT and MAP were not available, we extracted the geographic coordinates of the experimental sites from the Global Climate Database (http://www.worldclim.org/).
2.2 Data analysis
We followed the method from Mooney et al. (2010) and Zhou et al. (2022) to quantify the effects of forest restoration on soil C:N:P stoichiometry and microbial biodiversity using the log response ratio (LnRR). Response ratio was calculated as LnRR = Ln (tx/t0), where tx and t0 were the mean values of a quantified variable in the older forest and youngest forest, respectively (Crouzeilles et al., 2016). If a study involved multiple forest ages, for example, young, medium and old forests, two combinations can be obtained when calculating the log response ratio of soil carbon, that is, young versus moderate and young versus old. A negative effect size means that the measured value in the older forest stand was lower than in the youngest forest.
A linear mixed model in the R package ‘lme 4’ was used for meta-analysis with LnRR as the dependent variable to obtain the average impact of forest restoration on each variable (Zhou et al., 2022). The importance of these models was tested by a likelihood ratio test. Using the restrictive maximum likelihood method, LnRR was extracted from 95% confidence intervals of the estimates obtained by the likelihood curve. To further explore the influence of mycorrhizal type and forest type, the following 12 parameters were classified by mycorrhizal type (i.e., AM and ECM), leaf seasonality (i.e., evergreen, deciduous and mixed) and plant type (i.e., angiosperm and conifers). Estimates (±95% confidence interval [CI]) of the log response ratio were calculated for soil total carbon, nitrogen, phosphorus, C:N, N:P, C:P, bacterial Simpson, bacterial Shannon, fungal Simpson, fungal Shannon, microbial Simpson and microbial Shannon, respectively. If the 95% CI did not overlap with zero, the effects of forest restoration were considered significant. The correlation between stand age and individual variables or within variables was evaluated through Spearman correlation analysis. In addition, partial correlation analyses were performed in SPSS to cross-verify the effects of climate (MAT and MAP) on the LnRR of variables, taking into account the control of LnRR of stand age. The statistical analyses were performed by R statistical software and SPSS 21.0 (IBM). The difference in p < 0.05 was considered statistically significant.
3 RESULTS
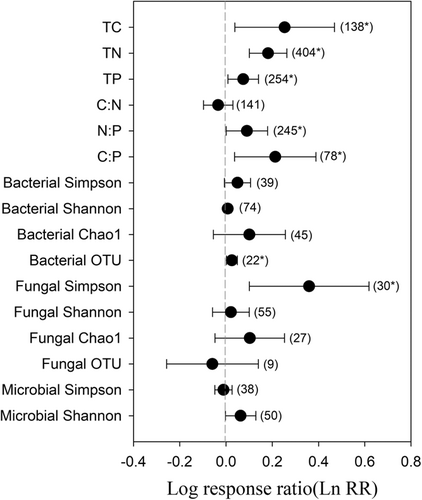
Forest restoration significantly affected soil C:N:P stoichiometry, bacterial OTU and fungal Simpson index, but did not alter fungal richness and most other indices of microbial diversity. Specifically, forest restoration significantly increased total carbon (TC), total nitrogen (TN), TP, N:P and C:P increased by 29%, 20%, 8%, 9% and 24%, respectively (Figure 2 and Supporting Information S1: Table 1). Among the variables, TC showed the largest increase in response to forest restoration compared to other variables. In contrast, forest restoration did not have significant effects on most of the diversity indices of soil bacteria and fungi, while significantly increased bacterial OTU and fungal Simpson by 3% and 43%, respectively (Figure 2).
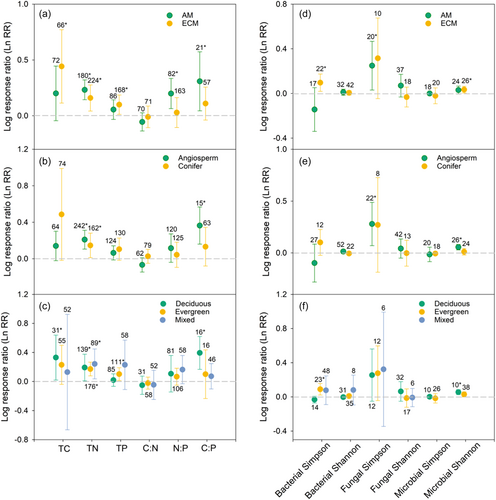
Plant functional group, leaf life span and mycorrhizal type significantly affected the responses of soil C:N:P stoichiometry and microbial biodiversity to forest restoration (Figure 3). For example, forest restoration significantly increased TC, TP, microbial Shannon in ECM forests but has no effect on that in AM forests (Figure 3a,d). The positive effects of forest restoration on soil C:P, fungal Simpson and microbial Shannon in angiosperm forests were greater than those in coniferous forests (Figure 3b,e). In addition, we also found that forest restoration significantly increased TP and bacterial Simpson were only significantly increased in evergreen forests, but not effects were found in both deciduous and mixed forests (Figure 3c,f).
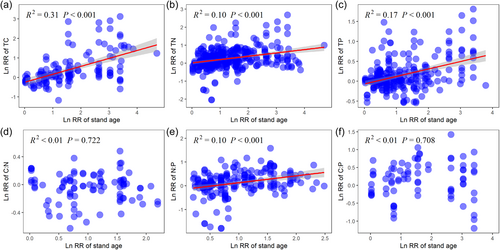
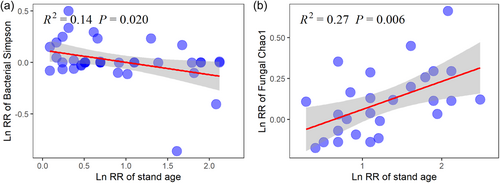
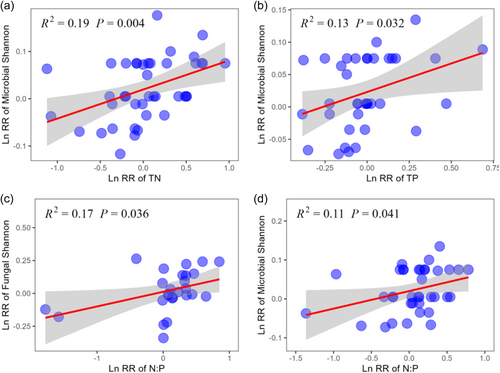
Forest restoration induced changes in TC, TN, TP and N:P were positively correlated with stand age, whereas no significant correlation between soil C:N, C:P and stand age were observed (Figure 4). Meanwhile, stand age was negatively correlated with the LnRR of bacterial Simpson, but positively correlated with the LnRR of fungal Chao1 (Figure 5). More importantly, we found that the effects of forest restoration on microbial biodiversity was regulated by soil C:N:P stoichiometry (Figure 6). Specifically, forest restoration induced change in microbial Shannon was positively correlated with TN, TP and N:P. In addition, fungal Shannon was positively correlated with N:P ratio (Figure 6).
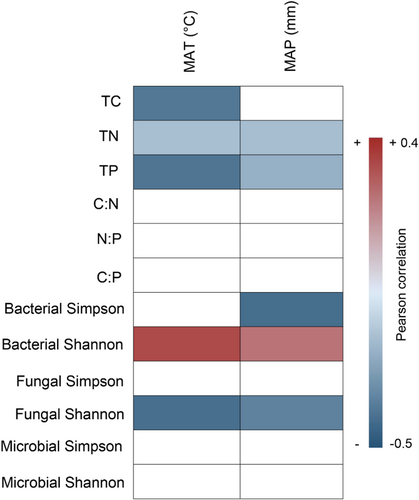
Climate was also an important factor in driving the response of soil C:N:P stoichiometry and microbial biodiversity to forest restoration (Figure 7). Specifically, we found that MAT was negatively correlated with the LnRR of TC, TN, TP and fungal Shannon, but positively correlated with bacterial Shannon. The LnRR of TN, TP, bacterial Simpson and fungal Shannon were negatively correlated with MAP, whereas positive correlation was found for bacterial Shannon (Figure 7).
4 DISCUSSION
In general, our study found that forest restoration significantly increased soil total carbon, nitrogen and phosphorus (Figure 2), which was consistent with several previous studies (Chen, Fang, et al., 2020; Zhang & Shangguan, 2018; Zhou et al., 2017; Zhu et al., 2021). The amount and quality of litterfall (leaves and twigs) and root biomass increase as forests mature, and their decomposition can promote the accumulation of organic matter and nutrients (Deng et al., 2016; Post & Kwon, 2000; Prescott, 2002). Otherwise, the closer symbiotic relationship between nitrogen-fixing plants and nitrogen-fixing bacteria with forest restoration, could further converse atmospheric nitrogen into plants-available forms, thereby increasing soil nitrogen content (Batterman et al., 2013; Li et al., 2022). Meanwhile, we also found that forest restoration significantly increased soils N:P and C:P globally but has no effect on soil C:N (Figure 2). As the accumulation rates of carbon and nitrogen is usually faster than that of phosphorus, which further leading to the soil C:N:P stoichiometry imbalances (Xu et al., 2018; Zhang et al., 2018). More importantly, our results showed that the effects of forest restoration on soil C:N:P stoichiometry were regulated by mycorrhizal type (Figure 3a). For example, forest restoration significantly increased soil N:P and C:P in AM forests, whereas it has no effect in ECM forest. It has been shown that AM fungi primarily enhance the uptake of phosphorus for host plants, leading to a higher phosphorus demand and ultimately result in increased soil N:P and C:P (Smith & Smith, 2011; Smith et al., 2011), whereas ECM fungi are more efficient at acquiring nitrogen, particularly organic nitrogen (Näsholm et al., 2009). These distinct nutrient acquisition strategies may explain why forest restoration had contrasted effect on soil N:P and C:P between AM and ECM forests.
However, our results found that forest restoration did not affect most indices of microbial diversity, which was similar with previous global work (Zhou et al., 2022). These results suggested that soil microbes had greater resistance to forest restoration than soil C:N:P stoichiometry. The higher context-dependent nature of ecological drivers may cause the stand age-microbial diversity relationship vary largely across ecosystems, leading to an overall nonsignificant effect at the global level (Delgado-Baquerizo et al., 2018; Fierer & Jackson, 2006). Meanwhile, we further found that forest stand age was negative correlated with bacterial diversity while positively correlated with fungal diversity (Figure 5). Decreasing bacterial diversity may result from less favourable environmental conditions, for example, increased soil acidity in older forests can reduce bacterial diversity (Lauber et al., 2009; Rousk et al., 2010; Xing et al., 2023). However, the accumulation of recalcitrant organic matter in older forests may favour fungi, which are more efficient at breaking down complex organic compounds than bacteria (Baldrian & Valášková, 2008; Boer et al., 2005). Fungi that are free-living in soil (e.g., soil saprobes) specialize in the decomposition of dead plant material (Crowther et al., 2012; Liu et al., 2023), leading to an increase in fungal diversity.
Soil nitrogen availability is an important factor in driving microbial diversity in forest ecosystems (He et al., 2016; Ren et al., 2019; Zhao et al., 2019). Our results showed that the change in microbial Shannon was positively correlated with TN, TP, as well as soil N:P (Figure 6), suggesting that microbial diversity was greatly depend on soil N availability during forest restoration. The enhanced accumulation and decomposition of organic matter may stimulate both soil nitrogen and phosphorus availability (Celi et al., 2013; Feng et al., 2019), which could greatly support the growth of microbial community via enhancing microbial interactions and niche differentiation. Meanwhile, the increased microbial diversity could also facilitate the release of nutrients from soil organic matter and mineral surfaces by producing a wider range of extracellular enzymes and organic acids (Allison & Vitousek, 2005; Kaiser et al., 2010), thereby affecting soil N:P ratio. Together, our study provides a new insight that enhancing the nutrients availability was an important step to enhance microbial diversity during long-term forest restoration, which could thereby affect multiple ecosystem functions such as climate regulation.
Despite forest restoration could significantly affect soil C:N:P stoichiometry, we also found that the magnitude of this influence is driven by climatic types (Figure 7). Specifically, we found that forest restoration induced change in soil C, N and P were negatively affected by MAT and MAP. It has been shown that the warmer ecosystems tend to stimulate the decomposition rates and enzyme activity, which could further decrease soil C and N content. Meanwhile, the higher MAP may greatly enhance soil saturation and water logging, which may further cause nutrient losses through leaching (Chapin et al., 2011; Woo & Seo, 2022). In addition, our study found that MAT and MAP were positively correlated with bacterial Shannon, but negatively correlated with fungal Shannon during forest restoration (Figure 7). The distinct responses of bacteria and fungi to climate factors may be attributed to the different utilization strategies, ecological strategies and environmental sensitivities (Wang et al., 2023). Specifically, fungi tend to have stronger dispersal limitations and exhibit lower network connectivity compared with bacteria (Chen, Wang, et al., 2020). Consequently, they face greater challenges in adapting to new environments, which further contributes to the decline in fungal Shannon diversity.
In summary, understanding how forest restoration affects soil C:N:P stoichiometry and microbial biodiversity is essential for predicting forest-climate change feedback (Zhou et al., 2022). Our comprehensive meta-analysis, synthesizing data from 121 published papers with 1649 observations, reveals that forest restoration has uneven impacts on soil C, N and P content, leading to a pronounced decoupling of soil C:N:P stoichiometry. In contrast, microbial biodiversity appears to exhibit limited response to these restoration efforts. Our findings further emphasize the role of plant functional groups, leaf life span and mycorrhizal types, in modulating the effects of forest restoration on soil characteristics and microbial communities. Meanwhile, forest restoration induced change in microbial diversity was closely associated with nutrients availability, especially soil nitrogen content. More importantly, our results also highlighted that the forest restoration effects on soil C:N:P stoichiometry and microbial biodiversity greatly depend on the climate condition such as temperature and precipitation. These insights will be invaluable in informing forest management practices in the context of future restoration initiatives, especially those aimed at mitigating climate change and supporting thriving human societies.
Furthermore, it is essential to recognize the inherent limitations in our study, which may impact the generalizability of our findings. Notably, the majority of our data was derived from the topsoil (0–20 cm), leaving unexplored the potential variations in response to forest restoration across different soil depths. Additionally, the complex interplay of factors associated with global change, including elevated CO2, warming, N addition and fire (Zhou et al., 2019), underscores the need for more comprehensive investigations to reveal the impacts of forest restoration on C:N:P stoichiometry and microbial biodiversity, as well as their complex interactions. Addressing these limitations and delving deeper into these factS1ors in future research is vital for a more comprehensive understanding of the ecological implications of forest restoration.
AUTHOR CONTRIBUTIONS
Guiyao Zhou designed the research. Ximei Han collected and analysed the data. Ximei Han and Guiyao Zhou wrote the first draft. All authors reviewed and edited manuscript together. All authors have read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
Guiyao Zhou was supported by Humboldt Research Foundation. This research was financially supported by the National Natural Science Foundation of China (grant numbers 32071593, 31930072, 31770559, 31600387, 31370489 and 42203076).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors confirm that they have adhered to the ethical policies of the journal.
Open Research
DATA AVAILABILITY STATEMENT
All the data used in this study are available from the corresponding author upon rational request.




