Bronchoscopic-Guided Selective Endobronchial Injection of Methylene Blue Solution for Localising Peripheral Bronchopleural Fistulas During Pleural Debridement in a Post-Lobectomy Patient With Bronchopleural Fistula and Empyema
Funding: This work was supported by Zhejiang Provincial Natural Science Foundation of China (LGD21C040002).
ABSTRACT
The bronchopleural fistula (BPF) is a pathological passageway between the bronchus and the pleural cavity. Diagnosing and localising BPF can be challenging, and the traditional retrograde methylene blue (MB) perfusion method may fail to identify multifocal BPFs. This article reports a novel method for locating multifocal BPFs in patients undergoing concurrent empyema debridement. Initially, selective endobronchial injection of MB solution is performed under bronchoscopic guidance. Subsequently, the thoracic surgeon determines the location of BPFs as the MB solution flows into the pleural cavity. This process of injecting MB within the bronchus is repeated to locate any additional BPFs until no new ones are found. We report a case where this new method was used to identify multifocal BPFs, ultimately diagnosing three BPFs' presence. Following treatment with bronchopleural fistula occlusion, no recurrence of BPFs was observed during the follow-up period.
1 Introduction
The bronchopleural fistula (BPF) is a pathological passageway between the bronchus and the pleural cavity. Although BPF is a rare complication following lung resection, with a retrospective analysis indicating a postoperative incidence rate of 0.44% [1], it can pose a life-threatening risk when accompanied by severe complications including empyema, aspiration pneumonia, and respiratory failure. The mortality rate associated with BPF ranges from 12.5% to 71.2% [2].
Localising BPF in multiple sites or peripheral types can be challenging since the bronchoscope often fails to accurately visualise their locations. Accurate localization of BPF is crucial for effective bronchoscopic interventional treatment. In this report, we introduce a novel procedure for identifying multifocal BPFs.
2 Case Report
A 57-year-old male patient underwent a left upper lobectomy due to a nodule in the left upper lobe. Postoperative pathology indicated minimally invasive adenocarcinoma. The patient experienced a cough with purulent sputum and chest pain 1 month after surgery, and no BPF was detected by bronchoscopy. Chest CT showed hydropneumothorax on the left side. After ineffective thoracic drainage and anti-infection treatment, the patient received pleural debridement combined with bronchoscopy.
When the bronchoscope was close to the bronchial stump of the left upper lobe, no bronchial stump fistula (BSF) was found. A guidewire detection, as described in a previous paper [3], was performed. Briefly, the guidewire (Boston Scientific Corporation, Natick, MA, USA) was inserted into the airway and pushed towards a potential micro BSF. When the top of the guidewire entered the wall of the bronchial stump and penetrated through the bronchial wall into the pleural space, this location was identified as a micro BSF. Otherwise, it was not. The guidewire detection revealed a micro BSF in the lingular bronchial stump. After clearing the secretions in the left lower lobe bronchus, 20 mL of diluted MB solution (10 mg MB/250 mL saline) was injected into LB6. The thoracic surgeon observed extravasation of MB solution from the visceral pleura into the thoracic cavity (Figure 1), revealing BPF distal to LB6. After cleaning the MB solution in the bronchus and thoracic cavity, the same procedure was repeated in LB7+8, LB9, and LB10, but no BPFs were identified except for one in LB10. In total, three BPFs were identified: a micro BSF in the lingular bronchial stump, two BPFs in LB6 and LB10. Subsequently, an endobronchial valve (EBV, Zephyr endobronchial valve, Pulmonx Inc., USA) with version 5.5 was placed in LB6 while an EBV with version 4.0 was placed inLB10 (Figure 2). Closure of micro BSF was then performed as described in the previous paper [3].
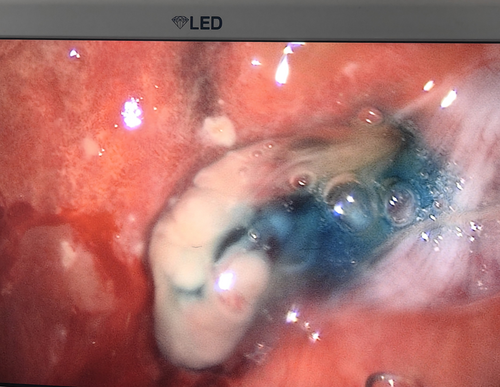
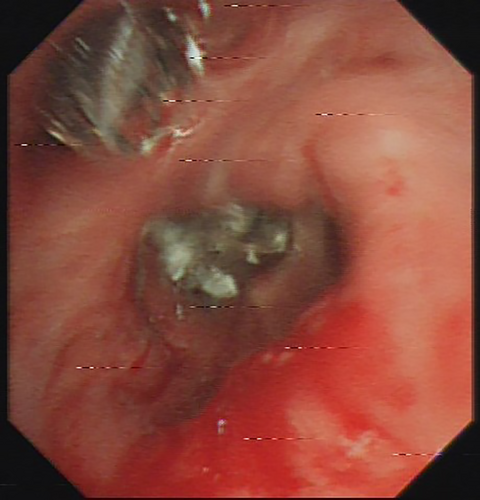
The closed thoracic drainage system showed no air leakage postoperatively. The patient recovered well without any postoperative complications, and the drainage was removed on the 6th day after treatment. There was no recurrence of BPF or empyema during the six-month follow-up period.
3 Discussion
BPF is a refractory disease characterised by the persistent non-healing of the fistula, which allows continuous entry of air from the lungs and mucus from the bronchi into the pleural cavity. This leads to intractable pneumothorax, lung infections, and empyema. Accurate localization of BPF is important before adopting precise treatment strategies.
Currently, there are various technical methods available for diagnosing BPFs. Among them, retrograde MB instillation procedure which introduce MB solution into thoracic cavity and observe whether MB solution flows into bronchus is now widely used because it is more effective, inexpensive, quick, and easy to perform [4]. However, this method may not locate multifocal BPFs [5]. Chronic empyema patients often have multiple BPFs due to lung tissue damage at several sites caused by pus immersion; therefore, retrograde MB instillation is not appropriate for these patients as it may result in missed diagnosis.
Not long ago, we reported a modified procedure for locating multifocal BPFs that combines reverse placement of EBVs and retrograde MB instillation [5]. This procedure is more effective but also more complex and time-consuming. It requires the operator to place an EBV in reverse to occlude the first BPF identified by retrograde MB instillation, then repeat retrograde MB instillation and reverse placement of EBVs to locate any additional BPFs until no new ones are found, and finally remove all the EBVs and place them in the correct orientations to occlude the BPFs. Furthermore, for BPF patients with empyema complications, using the retrograde instillation method is more likely to result in or worsen lung infection.
The present case was complicated by empyema with BPFs. During pleural debridement, the surgeon can inject MB via a bronchoscope and observe for any leakage from the thoracic cavity side, which is relatively quick and intuitive. After removing the MB in the bronchus and thoracic cavity, the pulmonologist can swiftly switch to a new bronchus for repeated injections and observations, achieving the identification of multiple fistulas. In the present case, this procedure revealed two peripheral BPFs.
When comparing the two mentioned retrograde MB infusion methods, selective endobronchial MB injection for locating multifocal BPFs is more convenient, precise, accurate, and easy to perform. The only drawback of this approach seems to be the requirement for simultaneous thoracotomy or thoracoscopy in the ipsilateral chest. However, it is this feature of simultaneous ipsilateral thoracic observation that has two unique advantages. Firstly, in patients with BPFs complicated by empyema, bronchoscopy can be performed during empyema debridement under a single session of general anaesthesia, reducing the use of total intravenous anaesthesia and benefiting the patient. Secondly, and more importantly, this procedure not only allows for locating the site of BPFs on the side of the bronchus but also enables identification of BPFs on the side of the thoracic cavity, providing accurate positioning for possible treatment from within.
As described in the previous paper [3], post-resectional BPFs are classified into three groups: bronchoscopically accessible and visible BPFs (visible BSF), accessible but invisible BPFs (micro BSF), and inaccessible and invisible BPFs (peripheral BPF). Among them, visible BSFs can be directly identified through bronchoscopic observation, micro BSFs are diagnosed using guidewire exploration, and peripheral BPFs can be located by performing bronchoscopic-guided selective endobronchial injection of MB solution. Therefore, all types of BPFs, including central and peripheral ones, can be accurately detected by combining these three procedures without any misdiagnosis.
In summary, for post-resection patients with the complication of BPFs and empyema, the application of bronchoscopic-guided selective endobronchial injection of MB solution during pleural debridement is effective in accurately locating multiple peripheral BPFs. This method is convenient, precise, and easy to perform. It also has a high probability of reducing pulmonary infection caused by retrograde MB infusion and provides the possibility of blocking BPFs from the thoracic side.
Author Contributions
Yi Wei: writing the manuscript, investigation, methodology. Ke Wang: collecting the relevant clinical data, revising the manuscript, investigation. Honglei Tao: ensuring the accuracy or integrity of the operation, methodology. Zhongliang He: designing the operation, methodology. Weihua Xu: designing the operation, final approval of the version to be published, methodology.
Acknowledgements
Many thanks should be expressed to Dr. Congbin Peng (Anesthesiology Department, Tongde Hospital of Zhejiang Province, Hangzhou, China) for providing helpful suggestions in clinical anaesthesia.
Ethics Statement
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and accompanying images.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




