Mediators and clinical treatment for cancer cachexia: a systematic review
Abstract
Background
Cachexia, a complex multi-organ syndrome, shortens survival time of patients, particularly those with cancer. Many studies and clinical trials have been carried out to identify cachexia-inducing factors and potential treatments for cancer cachexia over the last 20 years. Of these factors, some are promising targets for treatment in humans, owing to their expression profiles in patients. Several clinical interventions, which act on either cachexia-inducing factors or tissues affected by cachexia, have been developed. Some have had positive effects in the treatment of cancer cachexia; however, the question remains whether these interventions reverse cancer cachexia and could be used as standard interventions for disease treatment. The aim of this review is to understand the basic mechanisms and factors that induce cancer cachexia and their efficacies in clinical trials, providing a better outlook for future studies of cancer cachexia.
Methods
A systematic search was performed using PubMed and ClinicalTrials.gov databases for cachexia mediators and clinical trials.
Results
Of all databases and peer-reviewed facts considered, 256 papers and 35 clinical trials were included in this systematic review. Twenty-one mediators were identified, and 17 clinical interventions were reported in these studies. Outcomes of these clinical trials were assessed on changes in overall survival, body weight, lean body mass, appetite, muscle strength, muscle function, quality of life, and cytokine levels.
Conclusions
There is no current standard or successful intervention for treating cancer cachexia. Further research is needed to improve our understanding of initiators of cachexia to achieve successful outcomes in cachexia clinical trials.
Introduction
Cachexia, originally derived from the Greek words ‘kakos’ (bad) and ‘hexis’ (condition), was previously understood simply as wasting. In December of 2006, scientists and clinicians in Washington DC agreed on a definition of cachexia: ‘cachexia, is a complex metabolic syndrome associated with underlying illness and characterised by loss of muscle with or without loss of fat mass’.1 Cachexia occurs in various medical conditions, such as chronic obstructive pulmonary disease (COPD), cancer, heart failure, chronic renal failure, and rheumatoid arthritis.2 Whether the mechanism of this wasting condition is the same for all conditions in which it is found has not been determined, as the mechanisms are still poorly understood. It is therefore possible, even probable, that there are multiple causes of this condition and there exists a need for multiple potential treatments, specific to each sub-type of cachexia. Cancer-induced cachexia, as one of the main causes of death resulting from cancer, needs to be fully understood in order to develop effective treatments for this condition. Cancer cachexia is a complex syndrome compromising quality of life (QOL) and survival in patients. It is characterized mainly by weight loss, attributed to a catastrophic muscle loss, with or without adipose tissue loss.3 It occurs in up to 80% of cancer patients, depending on the type of cancer, and results in at least 20% of cancer-associated death.4, 5 Cachexia has the highest prevalence in patients with pancreatic and gastric cancer, while the rate is lower in breast cancer patients and is rarely observed in blood cancers.6, 7 In most cancer patients with cachexia, reduced physical function and tolerance to radiotherapies and chemotherapies have been observed, which potentially limits treatment. The most noticeable symptoms of cancer cachexia are muscle and adipose tissue wasting, which cannot be alleviated by an increase in appetite or caloric intake8, 9 as weight loss in cancer-cachexia and starvation occur by different mechanisms. Differences between cachexia and anorexia have been shown by several studies in animals and human patients. For example, multiple drugs aimed at increasing food intake in cancer cachexia patients could not completely reverse cancer cachexia in clinical trials, although, increasing appetite in some instances did increase lean body mass but had marginal impact on improving QOL.10 Furthermore, cancer cachexia is also distinct from sarcopenia, which is mostly associated with the ageing process or prolonged restriction of exercise and which results most prominently in muscle, but not fat loss. As such, cancer cachexia can be defined as a disease distinct from anorexia and sarcopenia, which—in addition to causing significant muscle and fat loss—causes other adverse events, such as organ failure, fatigue, and weakness.3
Symptoms of cancer cachexia
An invariable consequence of cancer cachexia is muscle wasting, usually a result of loss of skeletal muscle and is the first manifestation in patients with cancer cachexia.11 Many studies have been carried out to understand the mechanisms and pathways of muscle wasting in cancer cachexia. Protein synthesis and degradation are switches for regulating muscle mass, and muscle wasting is caused by increased protein degradation.12 In healthy conditions, proteolysis is essential for rapid removal of damaged and misfolded proteins, preventing the accumulation of non-functional and toxic proteins that may be produced naturally or under some anomalous circumstances, such as oxidative stress.12 Two chief intracellular protein degradation systems, the ubiquitin–proteasome system (UPS)13 and autophagy–lysosome pathway,14 are both implicated in cancer cachexia-induced muscle wasting. In muscle diseases, particularly muscle hypertrophy and atrophy, the imbalance of muscle protein synthesis and degradation is a primary culprit.15
In the muscle wasting mechanisms linked with the UPS, two E3 ubiquitin ligases16, 17—muscle RING-finger protein-1 (MuRF1) and muscle atrophy F-box (MAFbx)/Atrogin-1—have been shown to be up-regulated in muscle tissues in different muscle atrophy models. Deletions of these two proteins attenuate wasting symptoms in muscle atrophy induced by IL-1 and the glucocorticoid hormone, dexamethasone, highlighting their importance in muscle wasting. Up-regulation of these enzymes has been found in wasting muscles from patients with malignant disease.18 However, a recent study suggested that up-regulation of MuRF1 and Atrogin-1 in muscle was only in preclinical C-26 and Lewis lung carcinoma (LLC) xenograft models but not in cachectic pancreatic cancer patients.19 This raises doubt regarding the importance of MuRF1 and Atrogin-1 in clinical cancer cachexia. Transcription factors, such as Forkhead (FOXO1 and FOXO3a),20 NF-κB (p50, p52, and p65),21 and glucocorticoid receptor,22, 23 have been shown to interact with the promoter regions of the MuRF1 or Atrogin-1 genes, leading to their increased expression in muscle cells. Autophagy–lysosome pathway, as the second major system for muscle atrophy, has also been shown to be associated with muscle wasting in various preclinical cancer cachexia models14 and patients.24, 25 This is characterized by increased expression of autophagy-specific proteins, such as Beclin-1, LC3B, and p62/SQSTM1. Muscle protein synthesis is significantly suppressed in both cachectic mice and cancer cachexia patients, primarily through a decrease in the mammalian target of rapamycin (mTOR) pathway, one of the most important signalling pathways in protein synthesis.26-29
Wasting of adipose tissue is common but not universal in cancer cachexia and manifests itself through increased lipolysis, thermogenesis, and browning of white adipose tissue (WAT). Lipolysis is mainly regulated by two key lipolytic enzymes, hormone-sensitive lipase (HSL) and adipocyte triglyceride lipase (ATGL), which are generally highly expressed in cachectic WAT.30, 31 ATGL processes triglycerides into diglycerides, which can be further degraded into monoglycerides by HSL with the final products of lipolysis, glycerol, and fatty acids released into circulation, where it has been shown to disrupt liver function.32 Thermogenesis, the main function of brown adipose tissue (BAT), is regulated by uncoupling protein 1 (UCP1) causing proton leakage across the mitochondrial inner membrane. This results in generation of heat, rather than adenosine triphosphate (ATP).33 Increased UCP1 expression has been observed in various cancer cachexia models, while it has also been suggested that UCP1 is dispensable in cancer cachexia.34-36 Whether lipolysis of WAT or thermogenesis of BAT is responsible for fat loss may depend on the cachexia model used or experimental conditions employed. In addition to UCP1, there are other proteins involved in thermogenesis, such as Type II iodothyronine deiodinase (Dio2) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), which are commonly used as markers for BAT thermogenesis. Browning of WAT generally occurs during the initial stage of cancer cachexia, via up-regulation of UCP1. Under normal conditions, UCP1 is expressed in mitochondria in BAT but not in WAT.37 However, WAT from both cachectic mice and patients presented with positive staining for UCP1.38 The exact mechanism of how UCP1 expression is enhanced in WAT browning remains unclear, although, transcription factor Zfp516 was found to bind the UCP1 promoter and activate UCP1 expression in the browning process.39
In addition to muscle and adipose tissue wasting, many other organs are impacted by cancer cachexia. Cardiac atrophy is a common symptom of cancer cachexia. A decrease in heart weight has been observed in both preclinical cancer cachexia models40 and patients.41 Overall, like skeletal muscle wasting, decreased protein synthesis and increased protein degradation, especially sarcomere proteins, cause a decrease in cardiomyocyte size.42 Liver is the central organ of metabolism, and it is therefore unsurprising that it is also significantly affected by cancer cachexia. The liver is a major source of glucose—via gluconeogenesis, and of fatty acids, amino acids, and hormones as well as being responsible for the detoxification of ammonia.43 It is also a major sink for lipids in cachexia, as there is a shift in lipid storage from adipose tissue to the liver, causing the development of a fatty liver, with a major implication on the normal homeostatic functions of the organ. Instead of losing mass, it increases in size during cancer cachexia, accompanied by an increased energy expenditure.44 The increase of liver mass may in part be due to synthesis of acute-phase protein (APP) and metabolic recycling, which are activated by IL-6.45 Other studies have demonstrated the contribution of hepatic steatosis to cancer cachexia, caused by an increased uptake of fatty acids derived from adipose tissue.46-48
The brain also contributes to cancer cachexia, mainly by controlling food intake and the energy expenditure of the body. Loss of appetite, hyposmia, and hypogeusia are common features in cancer cachexia.49 Although cancer anorexia may not be a major element in cancer cachexia, decreased food intake worsens the condition of the body in cancer cachexia—especially in end-stage patients, and as such, increasing appetite could be helpful in treating cancer cachexia. Appetite is regulated by complex hormonal networks such as ghrelin,50 insulin and leptin,51 and the hypothalamus52 all play a pivotal role in controlling food intake in cancer cachexia. Resistance to hormones promoting food intake has been shown in preclinical models and patients.50, 53-55 However, decreased food intake is more likely the outcome rather than the cause of cancer cachexia, for as discussed earlier, increasing appetite appears not to ameliorate all the symptoms of cancer cachexia. Other tissues, such as the pancreas, gut, and kidney, also appear to be involved in cancer cachexia.56, 57 However, the contributions and changes of these tissues to cachexia are not as well defined as those described earlier. In summary, cancer cachexia is a multi-organ syndrome, with widespread effects on many organs.
Although the symptoms and effects of cancer cachexia have been well studied, efforts to identify the inducers and mediators of cancer cachexia continue apace, leading to the identification of some promising clinical targets. While there have been several proposed treatments targeting different factors or tissues, none of these have been successfully translated to the clinic and demonstrated as effective in humans. The following summarizes factors that have been identified and tested as potential clinical treatments for cachexia and others, which may prove to be potential targets for future treatment.
Mediators of cancer cachexia
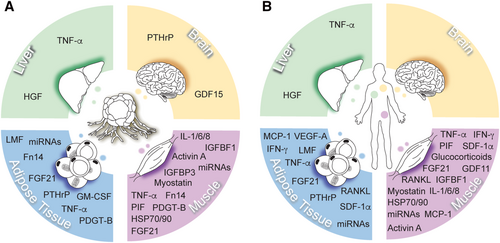
Cancer cachexia is driven by a variety of mediators that are derived from either tumours or host tissues (Figure 1). These mediators directly or indirectly induce various symptoms of cancer cachexia through different pathways (Table 1). Over the past few decades, many mediators have been identified in the hope of developing novel therapeutics to target tumour-induced muscle and adipose tissue loss. These mediators, which include many inflammatory cytokines, are elevated in cancer patients.58, 59 However, few of the identified mediators have shown promise for treating cachexia. More likely, most of these mediators are the result of, rather than the cause of, cachexia and therefore have little therapeutic relevance.
| Mediators | Mechanism | Potential drugs | Drug effects in preclinical models | References |
|---|---|---|---|---|
| TNF-α | Induction of MuRF1 and Atrogin-1 expression in muscle cells | Anti-TNF-α antibody | Inhibition of tumour growth and progression of cancer cachexia | Gelin et al.72 |
| IL-6 | Activation of JAK/STAT3 pathway | Anti-IL-6 antibody | Blocks cancer cachexia | Zaki et al.79 |
| Myostatin | Activation of Alk4/5/7/Smad pathways. Inhibition PI3K/AKT/mTOR pathways | Anti-myostatin antibody or anti-activin type II receptor antibody | Inhibition of muscle atrophy | Lee et al.102 |
| Activin A | Activation of Alk4/5/7/Smad pathways. Inhibition of PI3K/AKT/mTOR pathways | Anti-activin A antibody or anti-activin type II receptor antibody | Reversal of cancer cachexia; prolonging survival | Zhou et al. and Hatakeyama et al.109, 110 |
| GDF15 | Activation of GFRAL receptor and appetite; attenuation of lipid oxidation | Anti-GDF15 antibody | Reversal of weight loss and increase in food intake | Suriben et al.125 |
| PIF | Activation of NF-κB and STAT3; induction of proteolysis in muscle cells | Anti-PIF receptor antibody | Attenuation of protein degradation in muscle cells | Mirza and Tisdale131 |
| IGFBP-3 | Increase in muscle cells proteolysis | Anti-IGFBP3 antibody | Attenuation of muscle wasting | Ranke141 |
| PTHrP | Induction of UCP1 expression of lipolysis genes | Anti-PTHrP antibody | Reversal of muscle and adipose tissue loss | Hong et al.151 |
| Glucocorticoids | Induction of MuRF1 and Atrogin-1 expression in muscle cells | Glucocorticoid receptor antagonist | No effect | Lemmey et al.156 |
| Fn14 | Unknown | Anti-Fn14 antibody | Reversal of cancer cachexia; prolongs survival | Bilir et al.188 |
TNF-α
TNF-α, also called cachectin or cachexin in the past, is a pleiotropic cytokine responsible for systemic inflammation. TNF-α was shown to induce cachexia in rabbits and mice by daily injections of macrophage-conditioned media containing TNF-α, or using purified TNF-α.60, 61 When added to proliferating myoblasts, TNF-α inhibited expression of the muscle-specific gene α-cardiac actin and prevented differentiation by down-regulating the expression of genes, such as those encoding myosin heavy chain.62 Addition of TNF-α was shown to promote atrophy of cultured myotubes, through the induction of MuRF1 and Atrogin-1, which mediated the breakdown of myofibrillar proteins.63, 64 TNF-like weak inducer of apoptosis (TWEAK), a structural homologue of TNF-α, was also able to promote muscle atrophy by the induction of MuRF1 and the subsequent degradation of myosin heavy chain at the thick filament of the sarcomere.65, 66 The expression of the TNF-α receptor gene was also increased in skeletal muscle and adipose tissue in an animal model of cancer cachexia.67 TNF-α was also shown to be responsible for increased gluconeogenesis and non-phosphorylation energy wasting in the liver68, 69 and may be a key mediator of metabolic changes in the liver during cancer cachexia.
Nevertheless, therapies targeting TNF-α suggest that it may not be a central component in the development of cancer cachexia. As a multifunctional cytokine, TNF-α is chiefly synthesized and secreted by activated macrophages, which can infiltrate adipose and liver tissue.70, 71 Although anti-TNF-α treatment showed inhibition of tumour growth and the progression of cancer cachexia in a mouse model,72 clinical trials showed that blocking TNF-α with an anti-TNF-α antibody did not prevent weight loss in cancer cachexia patients.73 This suggests that an increase in TNF-α is insufficient to induce cachexia and that there are potential differences between mouse models and human patients. Side effects of anti-TNF-α treatment need to be considered as it is a pleiotropic cytokine making it unlikely that TNF-α would be an ideal target for cancer cachexia treatment.
IL-6 and other interleukins
IL-6 is another pleiotropic cytokine, which is secreted by various cells, including fibroblasts, monocytes, and B cells. It is involved not only in host defences and pathological processes but also in cancer cachexia. The relationship between IL-6 and weight loss has been well documented, both in preclinical models and human patients.58, 74-76 Resection of primary tumours from cachectic mice significantly decreased circulating IL-6 levels and returned body mass to baseline.77 Mouse C-26 tumour cells expressing IL-6 cause cachexia, while the same tumour cells that did not express IL-6 had no effect on wasting,78 suggesting that tumour, rather than host-derived IL-6, is implicated in the cause of cachexia. This is supported by another experiment where human tumours expressing IL-6 induced cachexia in mice, which was blocked by a human anti-IL-6 antibody but not a mouse anti-IL-6 antibody.79 Circulating IL-6 was shown to be elevated in cachectic patients, compared with non-cachectic cancer patients,80 and was also shown to correlate with patient survival.81 The JAK/STAT3 pathway is believed to be activated by IL-6 receptor activation. Inhibition of the pathway was able to block skeletal muscle wasting downstream of IL-6 in cancer.82 Notably, the anti-cachexia effect was not an anti-cancer effect.
As targeting IL-6 proved to be effective in preclinical models, clinical trials aimed at IL-6 were also carried out in human cancer cachexia patients. Treating cachectic cancer patients with non-small cell lung cancer with an anti-IL-6 antibody ameliorated anorexia, anaemia, and weight loss.83, 84 Nevertheless, many other trials have shown no efficacy of IL-6 inhibitors in various cancers, including multiple myeloma and prostate cancer.85, 86 Thus, anti-IL6 therapies may not be an effective treatment for cachexia in all cancer patients, and the induction of IL-6 is not universal in all cancer cachexia patients.
IL-1 and IL-8 also contribute to cancer cachexia. IL-1 is mainly secreted by macrophages and can also be produced by different tumour cell lines. Like TNF-α, IL-1 can also induce protein degradation in muscle cells in preclinical models, while daily injection of IL-1 resulted in peripheral protein wasting.87 IL-1 interacts with its cognate receptor on tumour cells, which was shown to result in the amplification of IL-6 secretion.88 Levels of circulating IL-8 also correlate with cancer cachexia,89, 90 but no anti-IL-8 trials have been carried out to assess its efficacy in cancer cachexia.
Myostatin
Myostatin, also known as growth differentiation factor 8 (GDF-8), is a member of the TGF-β family. It was identified as a negative regulator of myogenesis, which acts by inhibiting myoblast proliferation.91 Mutations of the myostatin gene in animals and humans induce muscle hypertrophy,92, 93 demonstrating its role in controlling muscle mass. Mutation of the myostatin gene is responsible for enhanced racing performance of the whippet dog breed in heterozygotes.93 Complete knockout of myostatin in animals increased myogenesis but decreased adipogenesis, resulting in increased muscle mass and decreased fat mass.94, 95 Myostatin knockout animals also have impaired force generation, despite an increase in muscle mass.96 The mechanisms by which deletion of the myostatin gene leads to impaired force generation are still unclear. As an autocrine protein, myostatin is produced and secreted mainly by muscle cells. Myostatin signals through the activin type II receptor on muscle cells, which then recruits Alk4/5 kinase, forming an activin receptor IIB/Alk4/5 heterodimer.97 This heterodimer activates the transcription factors Smad2 and Smad3, resulting in the formation of the Smad complex, which translocates to the nucleus and regulates expression of target genes.98, 99 The activin receptor IIB/Alk4/5 heterodimer also inhibits the PI3K/AKT/mTOR pathway,100 which is important for protein synthesis, thus reducing protein content and causing muscle atrophy. Overexpression or systemic administration of myostatin caused muscle atrophy, while myostatin deletion attenuated muscle wasting in cancer.101-103 However, in a clinical study for anti-myostatin antibody (LY2495655), pancreatic cancer patients with LY2495655 treatment showed increased fatigue and anorexia and earlier death.104 This clinical trial was terminated due adverse effects of LY2495655.
The role of myostatin in cancer cachexia is controversial. Indeed, myostatin levels showed a negative correlation with muscle mass in some of the animal studies described earlier. Also, the circulating myostatin level was reduced, rather than increased, in some cancer cachexia patients,105 suggesting that myostatin may not be a universal or vital trigger for inducing cancer cachexia nor a reliable circulating marker or ideal target for treating cancer cachexia.
Activin A
Activin A, a member of the TGF-β protein family, has also been found to correlate with cancer-induced weight loss. Circulating activin A is elevated in cancer cachexia patients,105 and overexpression of activin A promoted muscle wasting and cachexia in an animal model.106 As a dimeric protein, activin A is formed by two inhibin β A monomers linked by a single disulphide bond.107 Like myostatin, activin A also signals through the activin type II receptor on muscle cells, which recruits Alk4/7 kinase to form dimers. Activated Alk4/7 then phosphorylates Smad2/3, forming the Smad transcription complex with Smad4, where it regulates target gene expression, such as MuRF1 and Atrogin-1.108 Anti-activin A antibody treatment was shown to reverse cancer cachexia and prolong survival in animals.109 Blocking the activin type II receptor using an antibody also had a similar effect to anti-activin A antibody treatment and led to resistance against cancer cachexia, prolonging survival in a cachexia animal model.110
Although activin A and myostatin behave similarly in most cancer cachexia animal models, there is a significant difference in the circulating levels of these proteins in cancer cachexia patients. In a human study, circulating activin A levels were positively correlated with weight loss, while myostatin was not.105 Another study showed that activin A more conspicuously regulated muscle mass in primates compared with myostatin.111 However, another study revealed that activin A also plays a critical role in promoting proliferation and differentiation of human adipose progenitors,112 suggesting that suppression of activin A expression could potentially intensify fat loss in cancer cachexia patients.
GDF11
Recently, supraphysiologic GDF11 has been found to induce cachexia, anorexia, and muscle atrophy in vitro and in vivo.113-115 It is a TGF-β family member with similarities to myostatin, which also signals via the activin type II receptor/Alk4/5/7/Smad pathway. However, in addition to regulating Atrogin-1 and MuRF1 expression, GDF11 was also able to induce activin A and GDF15 expression.113 Activin A can induce muscle wasting symptoms, while GDF15 has the potential to cause anorexia via a GDNF family receptor α-like (GFRAL)/RET receptor.113, 116 So far, data confirming a role of GDF11 in cancer cachexia are limited.
As myostatin, activin A and GDF11 all have the potential to influence the development of cancer cachexia by a similar pathway, blocking each factor individually may not stop the progression of and reverse cancer cachexia. In view of this, the approach of targeting the shared activin type II receptor has been tested. Blocking the activin type II receptor did induce muscle hypertrophy in healthy mice and reversed muscle wasting in mouse cancer cachexia models.109, 117, 118 Nonetheless, a recent clinical trial revealed that blocking the activin type II receptor increased skeletal muscle mass but did not improve its functional capacity, determined by a 6 min walk test.119 As targeting the activin type II receptor appears to only improve muscle mass but not function, the effect of this treatment may be of limited use in treating cancer cachexia. In future, if therapies improving muscle function are found, they could potentially be combined with anti-activin type II receptor treatment for treating cancer cachexia.
GDF15
Circulating levels of GDF15 have been shown to positively correlate with weight loss in both cancer cachexia mouse models and patients with metastatic lung or exocrine pancreatic cancer.120 One study demonstrated that the MAP 3K11/GDF15 axis was a critical driver for weight loss in cancer cachexia, while inhibition of GDF15 with a specific antibody was shown to reverse weight loss induced by cancer in mice.121 However, these mice had reduced food intake, and anti-GDF15 treatment strongly increased their food intake. Further studies have suggested that GDF15 is a nutrition-related factor to suppress appetite, via signalling through GFRAL receptor in the hindbrain.122-124 Thus, the positive effect of inhibiting GDF15 is more suitable for treating anorexia, rather than cachexia. A recent study found that an antagonistic antibody targeting GFRAL could reverse excessive lipid oxidation and prevent cancer cachexia in mice, even under calorie-restricted conditions.125 This suggests that other mechanisms are likely involved in GDF15-induced weight loss.
Proteolysis-inducing factor
Proteolysis-inducing factor (PIF) has been identified as a tumour-derived protein that induces cachexia and stimulates protein degradation by induction of the UPS in muscle cells.126, 127 Activation of transcription factors NF-κB and STAT3 was shown to be related to PIF-induced muscle loss.128, 129 In addition to directly inducing protein degradation, PIF increased expression of pro-inflammatory cytokines such as IL-6 and IL-8—which can also induce muscle wasting.129 As a secreted protein, PIF functions by binding to the PIF receptor on skeletal muscle, which has been shown to play a role in muscle atrophy in cachexia.130 In vitro, PIF-induced activation of the ubiquitin-proteasome pathway was attenuated by anti-PIF receptor antibody treatment.131 However, the role of PIF in human cancer cachexia is unclear, as human PIF is apparently only detected in a few cancer types.126, 132
Lipid-mobilizing factor
Lipid-mobilizing factor (LMF), also called zinc-α2-glycoprotein (ZAG), is another tumour-derived protein, which has been shown to induce cachexia through adipose tissue degradation. It has been found to be expressed and secreted by both cachectic tumours and WAT of the host.133 Elevation of LMF levels was observed in different cancer cachexia mouse models.134, 135 In cancer patients, enhanced LMF expression in adipose tissue was positively correlated with weight loss, while the circulating LMF level was unaffected,136 while recombinant LMF has been found to stimulate lipolysis in both mouse and human adipocytes.135, 136 LMF acts via β-adrenergic receptors, including the β1−, β2− and β3− receptors, activating protein kinase A (PKA) to increase the phosphorylation of HSL and Perilipin-1, which are important in WAT lipolysis.137 More recently, LMF has been shown to promote WAT browning and thermogenesis by increasing UCP1 expression through stimulation of PPARγ and PPARγ-coactivator 1α, that can bind the UCP1 promoter.138 LMF may not be an ideal target for cancer cachexia therapies as it is only responsible for adipose tissue wasting and circulating levels were shown not to change in human patients with cachexia. These findings suggest that the effect of LMF in adipose tissue wasting may not come from tumours, but rather that it is an autocrine product from WAT that induces lipolysis, even when tumours express it highly.
Insulin-like growth factor binding protein-3
Insulin-like growth factor binding protein-3 (IGFBP-3) is an antagonistic protein for insulin-like growth factor 1 (IGF-1) in its normal role in the IGF-1/PI3K/Akt signalling pathway,139 which is essential for regulating muscle protein balance. The normal signalling pathway, when arrested through overexpression or exogenous treatment of IGFBP-3, increased proteolysis in muscle cells.140 This study also found that IGFBP-3 was up-regulated in tumour samples from pancreatic cancer patients, suggesting that it could be a potential mediator for muscle wasting in cancer cachexia. Both anti-IGFBP-3 antibody treatment and IGFBP-3 knock-down experiments showed positive effects in attenuating muscle wasting in vitro.140 However, in vivo validation of such effects has so far not been demonstrated. In tumour-free conditions, circulating IGFBP-3 is mainly produced from hepatocytes, while other tissues such as the kidney, placenta, and uterus are alternative sources for healthy individuals.141 Thus, other functions of IGFBP-3 would also need to be fully investigated before it can be confirmed as a target for cancer cachexia treatment in the clinic.
PDGF-related and VEGF-related factor 1 (PVF1, Drosophila melanogaster) and neural/ectodermal development factor IMP-L2 (IMPL2, Drosophila melanogaster)
As homologues for PDGF/VEGF and IGFBP in human, PDGF-related and VEGF-related factor 1 (PVF1)142 and IMPL2143 have recently been identified as two tumour-derived factors inducing host wasting in the fruit fly. Induction of an oncogene yorkie, the homologue of human Yap1, led to the development of a novel cancer cachexia model in Drosophila, which displays most wasting phenotypes such as muscle dysfunction, lipid loss, and hyperglycaemia.142 Using RNA-seq and RNAi screening, 17 potential ligands were identified as responsible for the wasting condition. PVF1 was shown as the only ligand for inducing MEK signalling in host tissue, and IMPL1 was found to inhibit systemic insulin/IGF signalling.142, 143 This new model shows promise as a means for identifying cachexia-inducing factors. The homologues of these factors need to be further tested in mammalian cancer cachexia models, as the tumour microenvironment and crosstalk among tissues may be different in fruit fly and mammals.
Fibroblast growth factor 21
Elevated fibroblast growth factor 21 (FGF21) was found to be associated with cachexia, independent of age and body mass index.144 Serum FGF21 levels were also shown to be related with BAT activity in humans,145 suggesting that it could be a factor inducing thermogenesis in cancer cachexia. Muscle FGF21 was shown to be essential for controlling mitophagy and muscle mass in mice.146 In addition, FGF21 was also shown to be associated with aggressiveness in cancer and could serve as a biomarker for predicting tumour progression, although the main source of FGF21 was shown to be host derived.147, 148
Parathyroid hormone-related protein
Parathyroid hormone-related protein (PTHrP) has been identified as a tumour-derived protein that triggers WAT browning and cancer cachexia.149 Tumour-derived PTHrP induced muscle and adipose tissue loss in a preclinical cachexia model, which could be reversed by an anti-PTHrP antibody.150 Circulating PTHrP levels were also shown to positively correlate with weight loss in patients.151 PTHrP signals through the PTH/PTHrP receptor, which is present on adipose cells and transduces the signal into the interior of WAT to promote the expression of the thermogenic gene UCP1 and lipolysis genes.149, 152 PTHrP was also found to induce leukaemia inhibitory factor (LIF) and IL-6 expression,153 both of which are cancer cachexia inducers. PTHrP is also present in some regions of the brain,154 suggesting that it may also act on the hypothalamus to induce symptoms of cancer cachexia.
A potential role for PTHrP in cancer cachexia was also demonstrated by PTH receptor depletion in mice, which resulted in resistance to browning and atrophy in adipose tissue and skeletal muscle.152 Therefore, PTHrP and PTH receptors are potential targets for cancer cachexia treatment. Because PTHrP and PTH operate through the same receptor, developing a drug specifically targeting PTHrP but not PTH will be challenging. The PTH receptor is also widely expressed in other tissues, which raises the possibility of undesirable side effects.
Glucocorticoids
Glucocorticoids are a class of steroid hormones, mainly synthesized and secreted by the adrenal cortex. Elevated circulating glucocorticoid hormone levels have been observed in both murine cancer cachexia models and patients.155 Administration of glucocorticoid hormones caused muscle loss in human patients.156 Glucocorticoids act by binding to intracellular glucocorticoid receptors, forming a complex that can translocate to the nucleus and interact with the glucocorticoid binding site in the promoter regions of target genes.157 In muscle atrophy, glucocorticoids can activate expression of E3 ubiquitin ligases, such as Atrogin-1 and MuRF1, thus inducing protein degradation and reducing muscle mass.158 Glucocorticoids can also induce LMF expression in adipose tissue, causing adipose tissue wasting in cancer cachexia.159 Muscle-specific deletion of the glucocorticoid receptor was shown to protect against muscle atrophy induced by LPS and tumours,160 supporting a role for an intact glucocorticoid signalling pathway in muscle atrophy. However, glucocorticoid blockade by a glucocorticoid receptor antagonist did not stop cancer cachexia in a murine model,155 raising doubt about the role of glucocorticoids in cancer cachexia. Additional studies are required to ascertain the role of glucocorticoids in human cancer cachexia. Exclusively targeting the intracellular pathway may also be a concern for developing therapies based on glucocorticoids.
MicroRNAs
MicroRNAs (miRNAs) have recently been found to be involved in cancer cachexia progression. A class of short noncoding RNAs (19–25 nucleotides), miRNAs are found in all eukaryotic cells and function as regulatory molecules for many different pathways and process, regulating proliferation, migration, and apoptosis.161 Recently, miRNAs have been identified to play a role in cancer cachexia. MiRNA-206 and miRNA-21 are the two most highly induced miRNAs in wasting muscle cells, which have been shown to act on the downstream targets, transcription factor YY1, and the translational initiator factor eIF4E3, promoting muscle wasting.162 Elevated expression of miRNA-21 was found in tumour-secreted micro-vesicles, which have the potential to be transported by the circulatory system and taken up by muscle cells.163 Two recent studies profiling expression of miRNAs in human skeletal muscle revealed more miRNAs that were differentially expressed in cachectic and non-cachectic muscles164, 165 and tumour-originating exosomal miRNA-155 has been shown to promote BAT differentiation by down-regulating PPARγ expression.166 Despite the recent progress in this area, what is currently known about miRNAs is only the tip of the iceberg and in the future, miRNAs could be promising targets for cancer cachexia treatment.
HSP70 and HSP90
HSP70 and HSP90 are intracellular molecular chaperones under normal physiological conditions, which can also be associated with the plasma membrane under stress conditions.167, 168 High expression of cell surface HSP70 and HSP90 was observed in several different cancer cell lines and tumours.169-171 Exosomal HSP70 and HSP90, derived from tumours, have been shown to prevent the anti-tumour effect of natural killer cells, causing immunosuppression in cancer.172-174 Tumour-released extracellular vesicles expressing HSP70 and HSP90 have been found to induce muscle wasting via TLR4 on muscle cells and systemic inflammation by increasing circulating cytokine levels.175 Changes in circulating levels of HSP70 and HSP90 in cancer cachexia patients still need to be confirmed. Additionally, as these molecular chaperones play a broad role in cellular function, any treatment based on targeting HSP70 and HSP90 could be detrimental to normal physiological function.
Monocyte chemotactic protein-1
Monocyte chemotactic protein-1 (MCP-1) is considered a chemotactic factor that recruits inflammatory cells to sites of inflammation during infection. Recent findings have shown that MCP-1 could play a role in cancer cachexia. Deletion of host MCP-1 showed attenuated bone loss in mice bearing LLC tumours.176 MCP-1 has also been shown to induce muscle inflammation, although it had no effect on host insulin sensitivity.177 Recent studies in cancer cachexia found that circulating levels of MCP-1 were elevated in a C26 cachexia model in addition to pancreatic cancer patients,178, 179 suggesting that MCP-1 could be a potential biomarker for cancer cachexia. Interestingly, circulating levels of other cytokines or growth factors, such as FGF-2, PDGF-B, and VEGF-A, did not correlate with cancer cachexia in pancreatic cancer patients.179
TNF-like weak inducer of apoptosis and Fn14
TNF-like weak inducer of apoptosis (TWEAK), also known as tumour necrosis factor ligand superfamily member 12a, is the ligand for the TWEAK receptor, which is also called Fn14. As a multifunctional cytokine, TWEAK is involved in many different processes, such as inflammation,180 wound repair,181 proliferation,182 and apoptosis.183 TWEAK is mainly synthesized and secreted by leukocytes, including monocytes, lymphocytes, and granulocytes.184 TWEAK was thought to induce skeletal muscle wasting by activating the UPS, autophagy, and caspases.65, 185 Overexpression of TWEAK in skeletal muscle cells caused muscle atrophy in transgenic mice, while TWEAK KO mice and mice treated with anti-TWEAK antibodies showed protection against muscle wasting.186 However, TWEAK-KO mice still succumb to cancer cachexia, and anti-TWEAK antibody treatment had no inhibitory effect on cancer cachexia.187 Indeed, levels of circulating TWEAK in cancer cachexia patients are not significantly different from control patients,188 suggesting that TWEAK may not be a major mediator of inducing cancer cachexia in humans.
Fn14, the receptor of TWEAK, has been shown to be involved in causing cancer cachexia. Based on the Human Protein Atlas, Fn14 is highly expressed in many solid tumours, including pancreatic and colorectal cancers. It was been shown that tumours expressing Fn14 cause cancer cachexia, while the same tumours without Fn14 did not cause cachexia.187 Significantly, host Fn14 had no effect on cancer cachexia development, as Fn14 KO mice developed cachexia normally when implanted with tumour cells expressing human Fn14,187 showing that the signal for cachexia originates in the tumour. The expression of Fn14 on tumours also had a significant effect on muscle function and adipose tissue loss and increased the levels of circulating pro-inflammatory cytokines, while treatment of cachectic mice with antagonistic anti-Fn14 antibodies blocked both muscle and adipose tissue wasting, improved muscle function, and reversed the release of inflammatory cytokines.187 Aside from its effect on blocking cachexia, anti-Fn14 antibodies have also been shown to inhibit tumour growth in some human cancers.189, 190
Considering that TWEAK KO tumours can induce cachexia in TWEAK KO mice and only tumour Fn14 expression is essential for developing cancer cachexia, a TWEAK-independent tumour Fn14 pathway must exist and be responsible for the observed cancer cachexia. There are either other unknown factors activating the Fn14 pathway, or the Fn14 pathway is auto activated in tumours. Understanding how the Fn14 pathway is activated in tumour cells is clearly a priority for understanding how cachexia is induced and initiated by cancer. The observation that all downstream effects of cachexia can be prevented and reversed in preclinical models of cancer cachexia by the administration of antagonistic antibodies against Fn14 suggests that targeting Fn14 on tumours could be a universal and promising therapy for cancer cachexia.
In summary, there are many identified mediators involved in cancer cachexia, which act by a range of distinct mechanisms (Table 1). To identify more such factors, reporter cell lines that can be activated by any factors inducing muscle wasting have been developed.191 The circulating levels of some of these mediators change significantly in cancer cachexia patients and could potentially serve as circulating biomarkers for evaluating cachexia in clinical applications and to assess the therapeutic effect of new clinical therapies. However, one of the criteria for these mediators serving as biomarkers is that they must be consistently elevated in cancer cachexia and not in other wasting conditions, while being detectable in all cancer cachexia models and patients. Identifying a biomarker with these properties would be invaluable in the study of cancer cachexia. It should be emphasized that expression of these mediators could be tumour specific. A recent study revealed that these mediators or cachexia-inducing factors are differentially expressed in different cancer types, in which the number of up-regulated mediators was correlated with the prevalence of cachexia in these cancer types.192 Therefore, the combination of these mediators or cachexia-inducing factors could serve as a potential diagnostic indicator for cancer cachexia. On the other hand, this study also suggests that developing a sole therapeutic treatment for cachexia of different cancer types will be challenging, as there is no universal mediator for all cancer patients with cachexia.
Clinical trials for anti-cachexia therapeutics
Many clinical trials have been performed to test treatments for cancer cachexia. In the last 10 years, more than 300 clinical studies have been specifically carried out for cachexia, of which 67 are still ongoing (statistics from NIH clinicalTrial.gov). Targets of these interventions vary from the symptoms of cancer cachexia, such as reduced appetite, muscle, and adipose tissue wasting, to factors inducing these symptoms (Table 2). Unfortunately, although most of these treatments were considered effective in animal models, few of them have succeeded in human studies. The following examines the reasons for the low frequency of successful translation into the clinic.
| Target | Interventions | Phase | OS | BW | LBM | Appetite | Strength | Function | QOL | Cytokines |
|---|---|---|---|---|---|---|---|---|---|---|
| Appetite | MA | II/III | × | √ | NA | √ | NA | NA | × | NA |
| Appetite | EPA | II/III | × | × | × | × | NA | NA | × | NA |
| Appetite | MA + EPA | III | × | √ | NA | √ | NA | NA | × | NA |
| Appetite | Nabilone | III | NA | NA | NA | √ | NA | NA | √ | NA |
| Appetite | Melatonin | II | × | × | √ | × | NA | NA | × | NA |
| Appetite | Anamorelin | II/III | × | √ | √ | √ | × | × | √ | × |
| Androgen receptor | Enobosarm | III | NA | NA | √ | NA | NA | NA | NA | NA |
| Adrenoceptor | Formoterol + MA | IIa | NA | NA | √ | √ | × | NA | NA | NA |
| Adrenoceptor | Espindolol | II | × | √ | √ | NA | √ | × | NA | NA |
| TNF-α | Etanercept | III | × | × | × | × | × | × | NA | NA |
| TNF-α | Infliximab | II | × | × | × | × | × | × | × | NA |
| TNF-α | Thalidomide | II | × | √ | √ | √ | NA | NA | × | IL-6, TNF-α |
| TNF-α | Pentoxifylline | I | × | × | × | × | × | × | × | NA |
| IL-6 | Clazakizumab | II | × | NA | √ | NA | NA | NA | NA | NA |
| IL-1α | MABp1 | I/III | √ | NA | √ | × | × | √ | NA | IL-1, IL-6 |
| Myostatin | LY2495655 | II | × | × | × | × | × | NA | NA | NA |
| ActRII | BYM337 | II | NA | × | √ | NA | √ | √ | NA | NA |
- BW, body weight; EPA, eicosapentaenoic acid; LBM, lean body mass; MA, megestrol acetate; OS, overall survival; QOL, quality of life. × represents no significant change; √ represents significant change; NA represents no records.
Appetite stimulators
Often, cancer cachexia patients also develop anorexia, which was deemed, in the last decade, as a promising target for cancer cachexia treatment. Thus, many clinical trials have been conceived, aimed at increasing food intake to reverse cachexia symptoms with the assumption that anorexia is at least part of the cause of cancer cachexia.
Megestrol acetate
As a synthetic derivative of progesterone, megestrol acetate (MA) has been shown to be well tolerated in patients with advanced malignant diseases, such as cancer193 and HIV,194 and has been shown to have a positive effect on improving appetite and gaining body weight.193, 194 It acts primarily to inhibit intracellular androgen action, although it also has a cytotoxic effect at a high concentration.195-197 In 2005, MA was approved by the Food and Drug Administration (FDA) for treating anorexia, cachexia, or an unexplained significant weight loss in patients with a diagnosis of acquired immunodeficiency syndrome. Thus, it was considered as a potential drug for treating cancer cachexia, and several clinical trials were performed to assess this. In most of these studies, patients showed improved food intake, although no significant differences were observed for other measurements, such as body weight.198-200 However, long-term (>3 months) treatment did show a moderate increase of body weight in a proportion of enrolled patients.198 Improvement of QOL, based on questionnaires, gave variable results in these trails. Changes in muscle function and lean body mass were not analysed in these studies, even though it is regarded as an essential indicator for cancer cachexia. Due to the obvious benefit on increasing appetite, combinations of MA, and other interventions have also been widely investigated in treating cancer cachexia.
Eicosapentaenoic acid
Eicosapentaenoic acid (EPA), an omega-3 fatty acid, was found to modulate changes in concentration of several orexigenic and anorexigenic neuropeptides in the brain.201 Thus, it has been used to treat anorexia nervosa, mainly to improve appetite. The effect of EPA on cancer cachexia has also been investigated, but no significant improvement in body mass, survival, QOL, and appetite was observed.202-204 Another clinical study also showed that EPA, either alone or in combination with MA, does not improve body weight or appetite better than MA alone,205 leading to the conclusion that EPA has little or no benefit on treating cancer cachexia.
Cannabinoid derivatives
The endogenous cannabinoid system was found to regulate appetite via its receptors CB1 and CB2 at four different levels: (i) limbic system, (ii) hypothalamus, (iii) intestinal, and (iv) adipose tissue.206, 207 Cannabis sativa extract and its derivatives, such as tetrahydrocannabinol (THC) and cannabinoids, have been found to stimulate appetite and increase body weight.208, 209 Given this, a randomized controlled trial testing the effects of cannabis extract and THC on treating cachexia was conducted in cancer patients with more than 5% weight loss in the preceding 6 months.210 The outcomes showed no difference in appetite, weight loss, and QOL, and there was no observed toxicity.210 Nabilone, a synthetic analogue of THC, has also been tested for treating anorexia-related cancer cachexia. After 8 weeks of treatment, patients receiving nabilone had increased food intake and showed an improvement in QOL,211 suggesting that it is an option for treating anorexia. Still, other important measurements for cancer cachexia, such as muscle function and overall survival, were not measured in this trial. These assessments should be carried out in order to evaluate its overall impact as a treatment for cancer cachexia.
Melatonin
Melatonin, produced mainly in the pineal gland, is a hormone regulating the sleep–wake cycle, which has been shown to stimulate appetite in animals by modulating some metabolic hormones such as insulin, ghrelin, and thyroid hormone.207, 212, 213 The effects of melatonin on appetite and other cancer cachexia symptoms were assessed in a double-blind placebo-controlled trial. The study, terminated after an interim analysis of enrolled patients, showed no significant differences in appetite, body weight, overall survival, and QOL between groups treated with melatonin or placebo.214 Thus, even though melatonin can stimulate appetite in animal models, it appears to have no effect on increasing food intake in cancer cachexia patients.
Anamorelin
Anamorelin is an orally active, selective agonist for the ghrelin receptor, which can enhance appetite and have anabolic effects. A preclinical study found that anamorelin showed significant binding activity with the ghrelin receptor, leading to increased secretion of growth hormone in vitro.215 An in vivo study suggested that anamorelin significantly increased food intake and body weight in rats. Increased levels of growth hormone and IGF-1 were also observed in pigs administrated with anamorelin in the same study.215 Several clinical trials for anamorelin have been carried out in patients with different cancers. Two phase II, placebo-controlled, double-blind clinical trials found that anamorelin treatment for 12 weeks had a favourable clinical response profile for cancer anorexia–cachexia syndrome, with increased lean body mass, QOL, muscle strength, appetite, and circulating IGF-1.216 Other biochemical markers including C-reactive proteins, IL-6, and TNF-α did not differ significantly between groups, while adverse events such as fatigue, asthenia, atrial fibrillation, and dyspnoea were observed in some patients. Overall survival was not investigated in these two trials. Likewise, another phase II randomized controlled trial showed improvement in appetite and QOL, while no differences were observed in levels of inflammatory markers (i.e. TNF-α and IL-6), handgrip strength or a 6 min walk test,217, 218 suggesting no improvement in muscle function for these patients. No differences in overall survival and tumour responses were observed between the anamorelin group and control groups, indicating this therapy had no effect on the progression of the disease. Additionally, there were more adverse drug reactions in the anamorelin group than the placebo group. The largest clinical trial for anamorelin was carried out at 93 sites in 19 countries, called ROMANA 1, 2, and 3.219, 220 These were phase III randomized controlled trials in patients with stage III/IV non-small-cell lung cancer. Like other clinical trials for anamorelin, patients significantly increased their body weight, while there were no differences in handgrip strength and overall survival between anamorelin and placebo groups.219, 220 In conclusion, anamorelin treatment did improve appetite, body weight, and QOL in cancer patients, yet did not improve overall survival and muscle function, as shown by the handgrip test and 6 min walk test. Subsequently, it was not approved as an anti-cachexia therapeutic treatment. To completely reverse cancer cachexia, muscle function needs to be restored. Thus, combining anamorelin with other therapies like physical exercise that can improve muscle function, could be an option for reversing cachexia in cancer patients.
Drugs targeting metabolism
Enobosarm
Enobosarm is a nonsteroidal selective modulator of the androgen receptor that has tissue-selective anabolic effects in muscle and bone. It was shown to improve lean body mass and physical function in healthy elderly men and postmenopausal women.221, 222 It was also tested for treating cancer cachexia. In a randomized controlled trial, patients receiving enobosarm showed significant increases in median lean body mass, while no improvement was observed in patients receiving placebo.223 Notably, there were no significant differences in handgrip strength, suggesting muscle function was not recovered by such treatment. Two larger clinical trials (ClinicalTrials.gov Identifier: NCT01355484 and NCT01355497) for treating cancer cachexia patients with enobosarm have also been completed but results have not yet been published.
Formoterol
Formoterol, a long-acting β2-selective adrenoceptor agonist with low cardiac toxicity, was shown to reverse muscle wasting in preclinical studies by activating protein synthesis and inhibiting proteolysis.224-226 Decreased muscle apoptosis and increased proliferation of muscle satellite cells were also observed with formoterol treatment.224, 227 The effect of the combination of formoterol and MA was investigated in a small phase IIa clinical study, with 13 cancer cachexia patients.228 Increased appetite was observed, as in other clinical trials for MA. Improved muscle size and function was also achieved with this combination of treatment. Yet, the result showed no significant improvement of muscle strength that was measured by quadriceps and handgrip strength, suggesting that muscle function was not fully recovered in this study. A larger clinical trial will be required to further investigate the effect of this treatment in patients with cancer cachexia.
Espindolol
Espindolol, a nonspecific β1/β2 adrenoceptor antagonist, exhibits pro-anabolic, anti-catabolic, and appetite-stimulating effects via β and central 5-HT1α receptors.229 In a phase II randomized placebo-controlled trial, patients receiving a high dose of espindolol (10 mg twice daily) showed significant increases in body weight, lean body mass, and handgrip strength, with no significant changes in fat mass, stair climbing power and a 6 min walk test.230 There were no significant differences in overall survival between the treatment groups and an excess of dyspnoea was observed with high dose espindolol compared with placebo. One animal study showed that espindolol increased muscle mass but decreased fat mass in old rats, suggesting that it could be a potential treatment for sarcopenia, because patients with this condition have decreased muscle mass and increased fat mass.231 However, espindolol may not be a suitable drug candidate for treating cancer cachexia, as loss of fat mass is also a common symptom of this condition.
Cytokine modulators
Etanercept
Etanercept, a dimeric fusion protein, blocks the interaction between TNF-α and its receptors by binding directly with TNF-α.232 Considering the role of TNF-α in development of cancer cachexia, etanercept was clinically tested for this condition. A placebo-controlled double-blind trial showed no significant differences in body weight change, appetite, median survival, and pathogen infection rates between etanercept and placebo treatment groups,233 suggesting it has no effect in palliation of cancer anorexia/weight loss syndrome in these patients. Another phase IIa clinical trial also found that etanercept has no significant benefit in treating cachexia-related symptoms in patients with advanced disease,234 further confirming the conclusions of the previous study. Another clinical study, in patients with cachexia induced by rheumatoid arthritis, showed that long-term etanercept therapy favours weight gain and ameliorates weight loss while no beneficial effect on appetite was observed.235
Infliximab
Infliximab, a TNF-specific monoclonal antibody, is approved by the FDA for treating a variety of disease, such as Crohn's disease236 and rheumatoid arthritis.237 The effect of infliximab on cancer cachexia has been investigated in two clinical trials. In a placebo-controlled, double-blind trial of infliximab for weight loss in elderly and cancer patients, infliximab did not show any benefits in improving body weight and overall survival compared with placebo.73 Instead, infliximab treated patients developed greater fatigue and poorer QOL scores. Another phase II study of infliximab and gemcitabine on cancer cachexia in patients with pancreatic cancer also showed that infliximab had no statistically significant improvements on cachectic symptoms.238
Thalidomide
Thalidomide, an immunomodulatory and anti-inflammatory drug, was also found to impede TNF-α production in vitro and inhibit the TNF response in vivo in animal models.239, 240 Several clinical trials have been completed to assess the efficacy of thalidomide in attenuating weight loss in cancer patients. An open study suggested that thalidomide was beneficial in improving the subjective symptoms of cancer cachexia and can increase appetite in these patients.241 A randomized placebo-controlled trial showed that thalidomide was effective at attenuating weight loss and increasing lean body mass in pancreatic cancer patients with cachexia but did not improve QOL or overall survival.242 In another study, thalidomide decreased levels of TNF-α and IL-8, but these decreases were not statistically significant compared with placebo treatment.10 Thalidomide was also shown to be a promising candidate drug for combination therapy alongside MA. In a study combining thalidomide and MA, patients showed significantly increased body weight, QOL, appetite, and handgrip strength, compared with MA alone.243 Additionally, decreases in circulating levels of cytokines, including IL-6 and TNF-α, were also observed. Therefore, thalidomide may prove to be beneficial as an add-on therapy rather than as a sole treatment.
Pentoxifylline
Pentoxifylline, a xanthine derivative used to treat muscle pain, was also found to inhibit the production and release of TNF-α.244 In a randomized controlled trial, cancer patients with cachexia receiving pentoxifylline showed no difference in weight gain, arm circumference, and QOL compared with placebo treatment.245 Thus, clinical trial data so far suggest that TNF-α and its receptor may not be a favourable target for treating cancer cachexia.
Clazakizumab (ALD-518)
Clazakizumab, a humanized IL-6-specific monoclonal antibody, was developed for treating cancer cachexia based on the observation that inhibiting IL-6 attenuated cachectic symptoms in animal models.246 Several anti-IL-6 drugs are under development and clazakizumab is the only anti-IL-6 agent tested in a clinical cancer cachexia trial so far. In a phase II randomized controlled trial, clazakizumab attenuated loss of lean body mass and reversed fatigue in cancer patients with cachexia.247 No significant differences in overall survival were observed in this trial. Increases in levels of haemoglobin, haematocrit, and albumin were shown in patients treated with clazakizumab from this same trial, suggesting that clazakizumab could be a novel non-erythropoietic stimulating agent for cancer-related anaemia. However, it should be noted that only a small number of patients completed the study, even though the number was enough to show statistical differences between groups. A larger scale of trials will be required to further confirm the role of clazakizumab in treating cancer cachexia.
MABp1
MABp1, a natural IgG1κ human monoclonal antibody targeting IL-1α, has recently been investigated as a new pharmacological treatment for cancer cachexia. This is based on the observation that IL-1α induces cancer cachexia in animal models and blocking IL-1α showed some attenuation of weight loss in these models.87, 248, 249 In an open-label, dose-escalation, phase I study for MABp1, no dose-limiting toxicities or immunogenicity were observed in patients that received MABp1, suggesting that it is well tolerated, despite some adverse events such as proteinuria, nausea, and fatigue.250 The same study also found decreased IL-6 levels and increased lean body mass in the MABp1 treated group. A larger, randomized, double-blind, placebo-controlled, phase III study evaluated the effect of MABp1 on treating advanced colorectal cancer. A higher proportion of patients in the MABp1 group achieved the primary endpoint, compared with the placebo group (33% vs. 19%).251 Significantly lower levels of circulating IL-6, thrombocytosis, and longer median survival were observed in the MABp1 group. The effect of MABp1 and MA on improving overall survival in advanced colorectal cancer patients was evaluated in another open-label, multicentre, phase III study.252 Longer median overall survival and stable physical functions were observed in the MABp1 group. Despite these promising results, additional research is required for the use of MABp1 as a drug for treating cancer cachexia, as the study lacked analysis of specific cancer cachexia parameters, such as muscle function tests and overall survival.
Landogrozumab (LY2495655)
LY2495655 is a humanized monoclonal antibody, which neutralizes myostatin and was designed for treating muscle wasting disorders.253 In a proof-of-concept, randomized, placebo-controlled, multicentre, phase II study, LY2495655 treatment was shown to increase lean body mass and slightly improve muscle function in elderly patients with muscle weakness,254 suggesting that it could be a treatment for sarcopenia. It was also evaluated for treating cachexia induced by pancreatic cancer. In a randomized, phase II trial, patients with stage 2–4 pancreatic cancer were treated with 300 mg LY2495655, 100 mg LY2495655, or placebo. The 300 mg LY2495655 treatment was terminated due to high death rates, and 100 mg LY2495655 treatment was also terminated as it showed no beneficial effects.104 Drug-related adverse events, including fatigue, diarrhoea, and anorexia were more common in patients administered LY2495655 than placebo. No benefits on overall survival and weight loss were observed in LY2495655-treated patients. Other outcome measurements for cancer cachexia, such as muscle function and QOL, were not evaluated in this study.
BYM338
In addition to directly inhibiting cytokines, their receptors have also been targeted for the clinical treatment of cancer cachexia. BYM338, a human monoclonal antibody blocking the activin type 2 receptors, was studied for treating muscle wasting induced by COPD and cancer cachexia.117 In a recently published clinical trial, BYM338 treatment significantly increased skeletal muscle mass in patients with COPD without improving muscle function, suggesting that it did not completely reverse muscle wasting in these patients.119 Another phase II clinical trial was also completed, investigating BYM338 as a treatment of cachexia induced by lung or pancreatic cancer (NCT01433263). Greater increases in thigh muscle volume, lean body mass, and physical activity were observed in patients treated with BYM338, compared with placebo treatment. Patients receiving BYM338 showed more weight loss than the placebo group, suggesting that it might have an adverse effect on other wasting symptoms in cancer patients (NCT01433263). This suggests that further study is required before confirming this as a potential treatment for cancer cachexia.
In addition to the earlier published clinical trials for cancer cachexia, there are also many completed but unpublished clinical studies. We are currently unable to review these trials, as no results have been publicly released. However, from reviewing and summarizing the published clinical studies, it is not possible to unequivocally conclude that an effective therapeutic intervention for cancer cachexia has been found to date, although a few drugs were effective for treating some cachexia-related symptoms. It is expected that more clinical trials for cancer cachexia will be carried out, as many novel targets have been confirmed in preclinical models. The trend of studies on cancer cachexia study has also shifted from improving appetite and wasting symptoms to targeting key factors that induce cancer cachexia, either directly or indirectly. Other interventions, combining drugs for treating different aspects for cancer cachexia, are also promising, and more of such treatments are under clinical assessment.
Conclusion and future perspectives
Cancer cachexia is a complex, multi-organ disease. Therefore, treatments should target this condition as one that affects the whole body. Drugs targeting wasting symptoms of one organ, like muscle wasting, may only benefit the specified tissue but fail to reverse the disease as a whole. It is important to recognize that the primary signal for establishing cancer cachexia comes from the tumour. This points to the activation of tumour genes encoding proteins or other factors, such as microRNAs, which are either directly responsible for the multiple symptoms of cachexia or activate genes in peripheral tissues that are responsible for the symptoms of cachexia. Irrespective of the pathway, the entire process originates in the tumour, so it follows that the best way to treat cancer cachexia is to target a causative factor at the base of the process, in the tumour, rather than downstream of the causative process, or at peripheral tissues.
The events that occur at the peripheral tissues are complicated by inter-organ communication, particularly crosstalk between muscle and non-muscle tissue. This crosstalk has been shown to influence metabolic homeostasis in cancer cachexia, via adipokines, myokines, cardiac factors, and gut peptides. These are involved in the inflammatory response observed in cancer cachexia and have been shown to be critically involved in activating muscle and adipose tissue wasting. Thus, targeting a single tumour-derived factor (e.g. PTHrP), or a single causative receptor (e.g. Fn14), may reverse multiple symptoms of cachexia in the hosts. Factors arising from peripheral tissues and involved in cancer cachexia have been identified, and solely inhibiting one factor has, in most cases, been effective only for alleviating parts of the overall process. Similarly, a number of these factors have been shown to be effective in attenuating cancer cachexia in animal models but were not effective in humans. It is likely that cachexia found in different clinical conditions may be driven by diverse mechanisms, and it is also possible that there are multiple causes even for the sub-class of cachexia. To ameliorate these potential problems, it will be important to discover specific biomarkers that can be used to develop diagnostic methods to test the applicability of a potential treatment for different types of cancers that are associated with cachexia. Such biomarkers would also be useful to stratify patients for clinical trials. Equally, it is important to ensure that these biomarkers are responsive to the treatment being tested, so the efficacy of the treatment may be monitored by changes in these specific biomarkers.
Many critical differences have been found between animal models and humans. The most common preclinical models for cancer cachexia are mainly syngeneic models, such as LLC, C26, MAC6, and AH-130 models. These models have distinct advantages for use in laboratory studies, such as short modelling time, high consistency, and reproducibility. Nevertheless, these models may also oversimplify the actual condition of cancer cachexia in humans. Most of these models are ectopic syngeneic models, leading to the uncertainty whether they can reflect the actual cancer cachexia process. Under such circumstances, using ectopic cancer cachexia models with human xenografts will help identify cachexia-inducing factors derived from human cancer cells; using an orthotopic cancer cachexia model is superior for imitating the effects of the tumour microenvironment. These spontaneous cancer cachexia models are an ideal choice for preclinical tests, but few such models have been established. Testing treatments in distinct types of cancer cachexia models may improve the success rate of human clinical trials for cancer cachexia.
Despite the many clinical trials for cancer cachexia that have been completed, improvements are still required for accurately evaluating different interventions—particularly for recruiting patients. Cancer cachexia has many similarities with anorexia and sarcopenia, making a definitive differential diagnosis difficult, as patients with advanced cancer may have all three conditions simultaneously. Thus, patients with advanced cancers are often bed ridden for extended periods of time, leading to significant muscle wasting, through sarcopenia. Similarly, patients often react poorly to chemotherapy, causing significant anorexia, perhaps accounting for the observations that improving appetite did show some benefit for attenuating weight loss in some clinical trials. However, other aspects of cancer cachexia, such as muscle function, were not restored by improving food intake. Preclinical studies also show that reduced food intake is not the cause of cancer cachexia in mice, as shown by paired feeding experiments.187, 255 Thus, improving food intake of patients with cancer cachexia may not be as effective as for patients with anorexia. Currently, the primary outcome measurements of most completed clinical trials of cancer cachexia are changes in body weight and lean body mass. Secondary outcome measurements generally vary between studies, including appetite, QOL, overall survival, muscle function, and performance. Changes in the levels of some cytokines have recently been employed as a secondary outcome measurement, as cytokines like IL-6 and GDF15 have shown potential as indicators for cancer cachexia. Recently, the FDA provided guidance on outcomes for cancer cachexia clinical trials, requiring traditional tangible measures, such as lean body mass, to be considered and measured alongside more robust quantitative measures like bioassays as a readout of patient improvement. Although some molecular markers have been evaluated in clinical trials, there are no reliable biomarkers identified for selection of patients for cachexia trials. Thus, developing reliable biomarkers as clinical indicators will not only improve the accuracy of these clinical trials but also accelerate the whole clinical trial process. Improvements in measuring muscle function should also be made, given the importance of restoring muscle function in reversing cancer cachexia. Currently, the most common test for measuring muscle function is the handgrip strength test, which may be affected by other conditions, such as arthritis and oedema. A reliable test for measuring leg power, such as stair climbing, should also be employed in future clinical trials. QOL measures could also be enhanced by using digital monitoring equipment to measure movement, such as devices now commonly used by athletes and the general public to measure steps and distance travelled.
In summary, additional studies are required to develop an ideal treatment for cancer cachexia. Future research for cancer cachexia must be focused on understanding cachectic mechanisms in tumours, establishing additional models to bridge the gap between animals and the clinic, developing biomarkers and suitable assessments, and differentiating cachexia from sarcopenia and anorexia. Cancer cachexia should be considered and treated as one of the major pathological conditions in the clinic, as preventing or inhibiting it could significantly improve the QOL for cancer patients and the cure rate of cancer.
Acknowledgements
Zhipeng Cao was supported by a La Trobe University Postgraduate Research Scholarship. The authors acknowledge funding from the Australian National Health and Medical Research Council, Victorian Cancer Agency, Victorian Department of Health and Human Services, and La Trobe University Research Focused Area (Understanding Disease). The authors certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle—Rapid Communications.256
Conflict of interest
The authors declare that they have no conflicts of interest.




