Development of a Novel, Pulmonary Endovascular Device to Treat Patients With Pulmonary Hypertension
ABSTRACT
Pulmonary hypertension (PH) carries a poor prognosis and a high mortality. Loss of pulmonary arterial compliance (PAC) plays a significant role in the development of PH and is an early predictor of mortality. Currently, there are no therapeutic options to overcome the loss of PAC. Aria CV (Saint Paul, MN) has developed a device to augment PAC. The device consists of a 20-cc balloon and anchor that can be implanted in the pulmonary artery using a minimally invasive procedure, a catheter, and a gas reservoir. Computed tomography imaging of 46 patients from the ASPIRE database and cadaver studies (n = 7) were used to ascertain device fit and optimize surgical procedure. Aria CV devices (n = 6) were tested for simulated use, durability, and PAC augmentation. Animal studies were conducted to demonstrate device safety in the deflated state (n = 9), and gas embolism due to simulated balloon rupture (n = 5). A chronic bovine model of PH was used to demonstrate PAC augmentation (n = 3). Chronic animal studies (n = 8, 30-days) were conducted to demonstrate long-term device safety and biocompatibility per ISO 10993 standards. In-silico fit and cadaver studies demonstrated that the device could be successfully implanted in the PA for a wide range of patients. In vitro and bovine models of PH demonstrated that the chronic Aria CV device enhanced PAC by > 0.4 ml/mmHg, which matched the PAC enhancement observed in 28 human patients with a short-term Aria CV device. The device passed all required durability, safety, and biocompatibility testing and is enrolling patients in a Food and Drug Administration (FDA) approved clinical trial (ASPIRE PH, NCT04555161).
1 Introduction/Background
Pulmonary hypertension (PH) is a progressive disease with a poor long-term prognosis that affects as many as 80 million people worldwide [1]. The median survival for PH is 3–7 years, depending on etiology, severity of disease, and response to therapy [2-4]. PH is classified into 5 groups based on etiology [5]. Although defined by a high pulmonary artery pressure (PAP), a common feature in all PH Groups is increased vascular stiffness (lower pulmonary arterial compliance [PAC]) in the pulmonary arteries with increased pulmonary vascular resistance (PVR) in most cases [6]. These changes result in abnormally high right ventricular (RV) afterload, initiating a series of progressive structural and functional changes culminating in right heart failure (RHF) and death [7].
PH is characterized by an early significant loss of vascular compliance, before a clinically significant increase in PVR [8-11]. The loss of vascular compliance leads to elevated pulmonary artery (PA) pulse pressure, peak systolic pressures, higher pulmonary arterial turbulence, restricted diastolic pulmonary arterial flow, pathologically altered shear stress, loss of normal PA reservoir function, augmented reflectance waves, and stimulation of mechanosensitive receptors, that result in progressive, pathologic vascular remodeling and elevated PVR [12-16]. While the pulsatile component of RV afterload is thought to constitute approximately 25% of afterload, it has been shown to have a consistent and outsized impact on survival [17, 18].
The increased arterial stiffness associated with loss of PA compliance results in (1) elevated RV afterload early in systole when the RV volume and wall stresses are high, (2) increased RV external work, and (3) alterations in RV pressure volume relationships [19-22]. Reduction in compliance results in the loss of Windkessel reservoir function and acceleration and magnification of PA reflectance waves, which increases RV afterload [23]. Wave reflections reach the native right ventricle when the pulmonic valve is open, resulting in further elevation of the ventricular ejection pressures and RV afterload [24]. Concurrently, elevated RV pressures compromise normal RV systolic coronary perfusion [25]. Unlike left ventricular coronary perfusion, healthy RV coronary perfusion is characterized by perfusion distributed throughout the entire cardiac cycle (systole and diastole) [26]. In PH, coronary flow is impaired, resulting in reduced myocardial oxygen uptake, lower coronary blood flow, reduced oxygen delivery, and functional ischemia. Thus, a reduction in RV systolic coronary perfusion contributes to a significant compromise of RV coronary reserve and the potential for myocardial hypoxia/ischemia in the presence of increased RV afterload [27-29].
In PH, the abnormally elevated RV afterload is comprised of two components: steady state and pulsatile load [8, 30]. Steady state load elevation is due to PVR increase and manifests as an elevated mean PAP Pulsatile load elevation is related to low PAC and results in elevated pulmonary pulse pressure. When the RV ejects into a normally compliant PA, one portion of the ejected blood flows through the pulmonary vasculature during systole and one fraction is temporarily stored due to the elastic deformation of the pulmonary arterial vasculature and is released during diastole. In patients with low pulmonary compliance, a significant amount of flow needs to pass through the pulmonary vasculature during the short systolic ejection phase with minimal diastolic flow due to a reduced storage component [8, 16]. Thus, low pulmonary vascular compliance results in high RV load that must be overcome during ejection.
Higher RV pressures and wall stresses cause ventricular pathologic remodeling ultimately resulting in RV dysfunction [31]. RV function and structure are highly dependent upon normal RV-PA coupling; which is the matching of contractility to afterload [18, 32, 33]. In the setting of increased RV afterload, RV-PA coupling is initially preserved, due to increased RV contractility, hypertrophy and dilatation, resulting in the maintenance of cardiac index, tricuspid annular plane systolic excursion (TAPSE), and right ventricular ejection fraction (RVEF) within a normal range [34].
RV-PA decoupling occurs when RV adaption cannot match the progressively increasing adverse remodeling associated with elevated RV afterload, resulting in RV dysfunction and RHF [35]. RV function is highly correlated with outcomes and RV function is the primary risk factor for mortality in patients with PH [36-39]. Nearly two-thirds of mortality associated with chronic PH has been attributed to RV failure [40]. Elevated systolic ventricular afterload alters RV contraction, resulting in dyssynchrony [41, 42]. RV isovolumetric contraction after aortic valve closure, due to a late leftward shift of the septum, augments dyssynchrony, impairs biventricular filling, and reduces stroke volume and cardiac output [43].
Currently employed pharmacologic options are mostly targeted to Group 1 physiology and predominantly lower PVR. However, research has shown that PVR is only weakly correlated with PH clinical outcomes [44]. Therapeutic options that dominantly target PVR do not address the other fundamental mechanical, functional, and structural aspects associated with PH, including the abnormally elevated pulmonary vascular stiffness (low compliance), adverse RV remodeling and dysfunction. While pharmacologic treatment results in PVR improvements, it does not abrogate progressive clinical deterioration leading to poor prognosis [45-48]. While the loss of compliance has been demonstrated to be an early and progressive predictor of mortality in humans [49, 50], a therapeutic option to specifically target the loss of PA compliance does not exist. To overcome this limitation, Aria CV Inc (St. Paul, MN) developed a chronic device that augments PA compliance and shields the RV from the increased pulsatile RV afterload. Previously, Aria CV had developed and implanted a short-term device in a human clinical trial. This manuscript presents the development and testing of the second-generation, chronic Aria CV device resulting in an FDA approved clinical study.
2 Methods
2.1 Device, Implantation, and Method of Action
The Aria CV device is a totally implanted, long-term, PH system that requires no external connection or power source. The device consists of a 20cc gas-filled balloon positioned in the main PA, a nitinol anchor located in the left PA, an implantable, subcutaneous gas-filled reservoir, and a 12 F catheter that connects the balloon to the gas reservoir and enables rapid flow of gas between them in response to changes in intravascular pressure during systole and diastole. (Figure 1). The balloon is designed to be non-obstructive as the diameter of the balloon is significantly smaller than the diameter of the main PA in patients with PH [51].
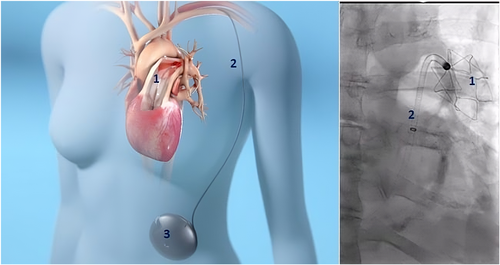
The device is implanted using a minimally invasive surgical technique. A small cut-down incision is made into the subclavian vein and a 22 F introducer is inserted. A standard guidewire is inserted through the subclavian vein and advanced into the left PA. A 20 Fr delivery sheath with a hemostatic valve is advanced to the main PA. The 12 Fr catheter with the attached balloon and nitinol anchor is advanced through the sheath. The sheath and introducer are removed, and hemostasis is achieved with purse-string sutures. The extravascular portion of the catheter is then tunneled subcutaneously to the abdomen. The gas reservoir is implanted subcutaneously on the anterior abdominal wall, and the tunneled catheter is connected. The device is then filled via needle puncture into the reservoir fill port with a proprietary mix of gases (primarily N2, O2, CO2) that has low permeability through the balloon. Any gas that diffuses through the polymer membranes is absorbed into the blood and exits through the lungs. The balloon catheter is designed to be detachable from the nitinol anchor if the device needs to be explanted after anchor ingrowth.
During systole, as the pressure rises in the PA, gas in the balloon is compressed and gas moves from the balloon through the conduit and into the reservoir. The progressive decrease in the volume of the balloon ensures the balloon is non-obstructive and makes space in the proximal PA for the incoming stroke volume of blood, like a healthy compliant PA that would stretch to make room for the RV stroke volume. The hemodynamic effect is to decrease systolic pressure and workload on the RV which can now eject more blood at lower pressure. During diastole, the pressure gradient between the reservoir and the pulmonary vasculature is reversed and the gas returns from the reservoir to fill the balloon. The diastolic increase in the volume of the balloon pushes blood into the distal pulmonary capillary bed augmenting diastolic PA flow. Thus, the Aria CV device compensates for the lack of compliance in the pulmonary arterial vasculature. The PA systolic pressure is reduced, and the diastolic pressure increases, resulting in a drop in PA pulse pressure.
2.2 Testing
The Aria CV device underwent extensive in silico, cadaver, in vitro, and acute and chronic animal studies to demonstrate anatomic fit, device durability, procedural and device safety, device efficacy, and to optimize the implantation procedure. These studies are presented next.
2.2.1 In Silico Fit Study
Anatomic data were extracted from clinically obtained computed tomography (CT) scans in PH patients of different size and gender (n = 46, Male = 26, 64.3 ± 13.4 years) from the ASPIRE registry [52]. The ASPIRE Registry includes deidentified data on consecutive patients undergoing investigation for suspected PH at the Sheffield Pulmonary Vascular Disease Unit. Ethical approval was granted by the Institutional Review Board and approved by the National Research Ethics Service (16/YH/0352 subsequently 22/EE/0011). The patient cohort included WHO Group 1 (n = 26), Group 2 (n = 4), and Group 3 (n = 5) patients. Mean PAP in the patient cohort was 46.9 ± 12.8 mmHg. PA left branch diameter and length from the pulmonary valve to the anchor location measurements were recorded because these measurements are critical to Aria CV device implant.
2.2.2 Cadaver Study
A cadaver study (n = 7) was conducted to evaluate and refine the surgical technique, evaluate potential forces generated during torso and arm movements that might affect the device catheter, and to optimize the tunneling pathway to minimize external forces or catheter deformation. The arm and torso movements included simulated shoulder flexion/extension (~ −30 to −180°), shoulder adduction/abduction (–180°), and torso rotation (~ 0–60°). Once the implant procedure was optimized, studies were conducted to evaluate ease of implant and quantify the forces due to torso and arm movements on the device.
2.2.3 In Vitro Studies
The Aria CV balloon, anchor, and device catheter (n = 6) were tested for simulated use, compliance enhancement, anchoring forces, durability, and compliance augmentation after gamma sterilization.
2.2.3.1 Simulated Use
In the simulated use testing, the sterilized Aria CV device (n = 6) was delivered in a 3D anatomical model of the vasculature (derived from previously obtained CT scans) from the subclavian vein to the PA in a body temperature water bath. The Aria CV delivery catheter was advanced over the guidewire to the PA and the balloon catheter and anchor deployed. The system flexibility, kink resistance, pushability, and trackability were examined. The implant positioning, deployment, recapture and redeployment and delivery system withdrawal were also assessed.
2.2.3.2 Pulse Duplicator for Compliance Testing
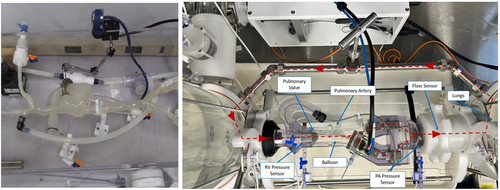
The pulse duplicator is a modified valve tester and consists of a mock right atrium, right ventricle, pulmonary valve, PA, and adjustable PA and lung compliances and resistances (Figure 2). The pulse duplicator was instrumented with pressure and flow sensors integrated with a custom data acquisition system. The Aria CV device was implanted in the mock PA. The pulse duplicator was filled with water and was tuned to mimic the PA hemodynamics and compliance of patients with PH. PA hemodynamic measurements were obtained during baseline with the device deactivated (gas was evacuated from the device, reservoir pressure at ~ mean pulmonary arterial pressure of the system) and with the device activated (gas cycling back and forth). PA compliance was calculated based on hemodynamic measurements before and after device activation.
2.2.3.3 Anchoring Forces
The nitinol anchor was crimped down in the loader and delivered through the 0.250” guiding catheter. A radial expansion force gauge (RX650, Machine Solutions Inc.) with an encoder for diameter accuracy was used for radial force measurement. The radial forces were measured at 16 mm and 22 mm (range of pulmonary vessel diameters where the device was anchored) in 37°C saline. The passing criterion was an anchoring force between 5 N to 9 N, a range shown not to cause damage in normotensive sheep pulmonary arteries.
2.2.3.4 Durability Tests
Durability tests were conducted following ISO 5840 standards and in consultation with the Food and Drug Administration for the early feasibility study.
2.2.3.4.1 Balloon
The balloon durability was tested in modified valve durability test units. The balloon displacement volume was representative of physiologic displacement. The balloon was frequently subjected to worst-case fatigue conditions and allowed to reach a fully depleted state during simulated end-systole. An additional 10 million cycles were conducted to simulate an overfilled balloon. The balloon was evaluated for the ability to disconnect from the anchor, balloon structural integrity, and helium permeance rate at the end of the test. Additionally, the ability of the balloon to add compliance ( ≥ 0.4 ml/mmHg) was evaluated in a hydrodynamic pulse duplicator before and after durability testing.
2.2.3.4.2 Anchor Deflection Testing
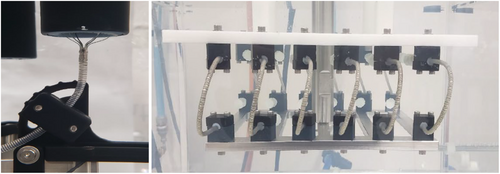
The loading profile and x-y displacement of the anchor was 0.25 ± 0.1 mm and was tested at 25 Hz per American Society for Testing and Materials (ASTM) standard (Figure 3). Following the completion, the x–y displacement was increased to a worst-case 0.68 ± 0.1 mm displacement and tested for 10 million additional cycles at 72 beats per minute. The anchor motion was recorded via high-speed camera and the deflection was measured in x-direction (lateral movement), y-direction (axial movement), and theta (rotational movement). The anchor was inspected visually for structural integrity, and anchor disconnection torque was measured at the end of the test.
2.2.3.4.3 Catheter Bend Testing
The 12 Fr balloon catheter was subjected to ±0.75 mm sinusoidal alternating displacement in 37oC saline at 30 Hz. The catheter was held at 57 mm vertical offset and 26 angular offset to mimic the in vivo positioning in a patient (Figure 3). The ± 0.75 mm cyclic displacement is representative of the intravascular motion of the catheter induced by the cardiac cycle to demonstrate long term durability of the implant. The catheter was evaluated for structural integrity at the end of the test.
2.3 Animal Studies
Bovine and ovine animal models were utilized to demonstrate device efficacy and safety. All animal studies were approved by the appropriate Institutional Animal Care and Use Committees (IACUC).
2.3.1 Efficacy Testing Using Hypoxic Bovine Model
Holstein (n = 6) and Angus (n = 3) calves, approximately 3 weeks of age were acclimated at ambient conditions for at least a week. Baseline measurements including right heart catheterization, transthoracic cardiac echo, and blood draws were collected. To replicate the naturally occurring high altitude “brisket” disease, the animals were housed in normobaric hypoxic stalls that simulated oxygen partial pressures equivalent to altitudes of up to 18,000 feet (Figure 4). The animals were exposed to gradually increasing altitudes (levels of hypoxia), in the chambers to simulate 12,000 feet of altitude at 90 days. Right heart catheterization, blood draws, and transthoracic cardiac echoes were performed monthly unless the observational status of the calves changed and assessment was deemed necessary. After reaching a state of dilated RV failure, the animals were anesthetized, and the Aria CV device implanted in the main PA. At the start of the procedure, a standard right heart catheterization was performed at baseline using a 7Fr. Swan Ganz catheter and thermodilution using jugular venous access. During baseline and device activation conditions, cardiac output (CO) was measured in quintuplicate by thermodilution and the results averaged. The clinically simplified stroke volume (SV)/pulse pressure (PP) formula was used to calculate PAC where SV was determined from CO/heart rate (HR). PVR was calculated using the formula: PVR = (mPAP − Pulmonary Capillary Wedge Pressure [PCWP])/CO. The PAP waveform was averaged to get the mPAP. At the end of the procedure, the animal was electively euthanized under anesthesia. A paired, single tailed T-test was used to calculate statistically significant differences.

2.3.2 Deflated Device Safety Testing
To evaluate the safety of the device when the Aria CV balloon was deflated, which is the worst-case scenario for thrombus formation, a chronic animal study was conducted in a healthy ovine animal model (n = 9, 5 females, 4 males). The animal was anticoagulated using heparin to maintain an ACT of 180–400 s during implant procedure. Vascular access was through the right internal jugular and the device was implanted in the PA. The catheter was tunneled to the reservoir that was placed subcutaneously on the dorsal flank of the animal. The gas mixture was evacuated from the reservoir to a pressure of − 200 mmHg, ensuring the balloon was maximally deflated, resulting in the worst-case for sharp creases and thrombosis. The animals were anticoagulated with enoxaparin (2 mg/kg, twice daily), and monitored for clinically significant adverse events during the study period. Bloodwork (CBC, serum chemistry, and coagulation) was collected for clinical pathology assessments at baseline (before treatment) as well as on the day of termination. Animal weights and body condition scores (BCS) were obtained at baseline (before treatment) as well as on the day of termination. At the end of the study period, the animals were heparinized (20,000 IU), euthanized, and gross necropsy was performed by a board-certified veterinary pathologist. The entire device pathway was examined, including the subcutaneous pocket, subcutaneous connective tissue, right jugular vein, superior vena cava, right heart, tricuspid and pulmonic valves, pulmonary trunk and the left PA. The device, including anchor, balloon, and the catheter was examined for thrombus formation and assigned a quantitative clot score. End organs, including lungs, were examined for thrombus and lesions.
2.3.3 Gas Safety Testing
To evaluate the safety of the Aria CV device in the unlikely event that the gas from the device is released into the blood due to balloon rupture, 24 ml (representative of full balloon volume) of the proprietary gas was injected into the left PA in a sheep model (n = 5). Hemodynamics (pulmonary and systemic blood pressures and CO), blood oxygenation and visual observation of the gas embolus and blood flow on contrast fluoroscopy were monitored until the gas embolus was completely reabsorbed and the affected lung returned to baseline. Specifically, measurements were performed at baseline, immediately after gas bolus injection, 10 min after bolus, and every 30 min after the bolus injection until the emboli were no longer visible and blood flow was fully restored as observed on contrast fluoroscopy. Visual analysis of the angiograms and comparison to baseline were made to check for (a) visibly blocked vessels (vessels visible at baseline that don't fully fill with contrast after gas embolism) and (b) contrast blush of tissue at the margins of the lung downstream of the gas emboli. After euthanasia, a targeted necropsy with histopathology of the lungs was performed to assess for evidence of acute lung injury.
2.3.4 Chronic Animal Testing
The chronic safety of the Aria CV device was evaluated in a 60-day animal study in a healthy ovine model (n = 8). During device implantation, the delivery system and placement of the device was evaluated per ISO 10993-4. The reservoir was implanted at the base of the neck. After device and reservoir implant, each animal was recovered and monitored for adverse events. After elective euthanasia, gross necropsy was performed and the device, device pathway, and end-organs were examined for thrombus and structural integrity. Histopathological analyzes were performed per ISO 10993-11 for chronic and sub-chronic toxicity.
3 Results
Anatomic fit, durability, and procedural and device safety and efficacy were successfully demonstrated. Details of these results are presented below:
3.1 In Silico Anatomic Fit and Cadaver Testing
In the 46 PH patient CT scans reviewed, the diameters of the left branch and main PA were characterized. These studies demonstrated that the Aria CV device can be anchored in a target landing zone diameter in the left main branch of the PA. The implant procedure and tunneling of the catheter to the reservoir were optimized in the cadaver study. Cadaver study results demonstrated that the forces due to arm and torso movements were less than 1 pound-force (lbf) and within the device specifications (3.4 lbf).
3.1.1 In Vitro Testing
The simulated use test passed all specifications with successful delivery, recapture and redeployment of all 6 devices. The pulse duplicator compliance tests demonstrated that the Aria CV device activation increased PA compliance by 0.43 ± 0.01 ml/mmHg. The anchoring force for the nitinol anchor was 8.43 ± 0.11 N (8.23–8.59 N) at 16 mm diameter and 8.00 ± 0.29 N (7.60–8.45 N) at a diameter of 22 mm. All Aria CV device components passed durability testing. The balloon could be disconnected from the anchor at the end of the durability test, demonstrating the ability to explant the balloon, if needed, after chronic implant. After the durability test, the balloon increased pulmonary compliance by 0.43 ± 0.01 ml/mmHg (range = 0.40 – 0.44 ml/mmHg). The helium permeance rate after durability testing was 1.33 × 10−4 ± 9.36 × 10−6 ml/s (Range = 1.19 – 1.45 × 10−4 ml/s), passing the helium permeance rate specifications.
3.2 Animal Studies
3.2.1 Hypoxic Model Efficacy Testing
Eight out of nine animals exhibited progressive development of PH and RHF with graded exposure to normobaric hypoxia. One animal died during model development and one animal had a cardiac arrest during induction of anesthesia, before device placement. The Aria CV device was successfully implanted in the six remaining animals. Three of the six animals developed other physiologic complications due to model development (tricuspid regurgitation, hemodynamic instability, normalization of pulmonary pressure and LV function) that did not allow complete collection of efficacy data.
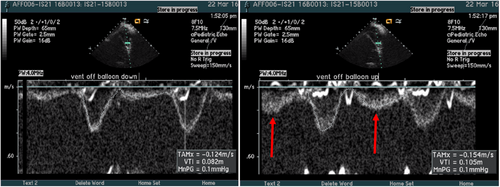
Paired hemodynamic data including right heart catheter and wedge pressure were measured at baseline and device activation were obtained in the three remaining calves with PAH. Despite the tested calves having a higher PA compliance than humans with PH, all three animals demonstrated an increase in pulmonary compliance with device activation. Improvement in CO and RV stroke volume suggest an improvement in RV function upon device activation, resulting in an increase in mean and systolic PAP. Despite an increase in CO, there was a reduction in PA pulse pressure (Table 1). Significantly, echocardiography data demonstrated PA compliance enhancement due to Aria CV device activation, augmented diastolic PA flow and preserved systolic flow, resulting in a higher stroke volume and CO (Figure 5).
| Calf 1 | Calf 2 | Calf 3 | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| Device status | Off | On | Off | On | Off | On | ||
| sPAP | 38.2 | 38.5 | 39.3 | 40.7 | 44.5 | 44.2 | 0.224 | |
| dPAP | 20.9 | 23.9 | 19.0 | 21.3 | 29.7 | 31.5 | 0.010 | |
| mPAP | 27.6 | 29.9 | 27.8 | 30.6 | 35.2 | 35.5 | 0.071 | |
| PVR | 2.5 | 2.4 | 2.0 | 2.2 | 2.8 | 2.8 | 0.371 | |
| PA PP | 17.5 | 14.7 | 20.3 | 19.4 | 14.8 | 12.7 | 0.037 | |
| CO | 6.3 | 7.5 | 7.7 | 8.3 | 7.5 | 7.8 | 0.059 | |
| HR | 72 | 72 | 89 | 90 | 87 | 86 | 0.500 | |
| PAC | 5.0 | 7.0 | 4.3 | 4.8 | 5.9 | 7.1 | 0.052 | Average |
| PAC change | +2.1 | +0.5 | +1.2 | +1.3 | ||||
| CO change | +1.2 | +0.6 | +0.3 | +0.7 | ||||
| PP change | −2.8 | −0.9 | −2.1 | −1.9 | ||||
- Abbreviations: CO, cardiac output; dPAP, diastolic pulmonary artery pressure; HR, heart rate; mPAP, mean pulmonary artery pressure; PA PP, pulmonary artery pulse pressure; PAC, pulmonary artery compliance; PVR, pulmonary vascular resistance; sPAP, systolic pulmonary artery pressure.
3.2.2 Deflated Device Thrombogenicity Testing
Seven out of nine animals were successfully implanted with the Aria CV device. Two animals were excluded from the study due to non-device related adverse events (cardiac arrhythmia and anesthesia intolerance). Animals were electively euthanized at a planned endpoint of 15 days (n = 3) and 30-days (n = 4). No clinically significant adverse events such as thrombosis, pulmonary embolism/infarction, or death related to the Aria CV device were observed.
3.2.3 Gas Safety Testing
The gas was absorbed in all 5 animals within 150 min. The blood oxygen saturation SPO2 measurements were 96% or higher in all animals throughout the study. The CO increased briefly after gas bolus injection and returned to baseline values within 60 min and remained stable until euthanasia. There were no gross or microscopic pathology findings consistent with lung injury, suggesting that all the gas was absorbed and perfusion to the tissues was not affected by the injection of gas. These results demonstrate the safety of the Aria CV device even during a simulated balloon failure.
3.2.4 Chronic Animal Testing
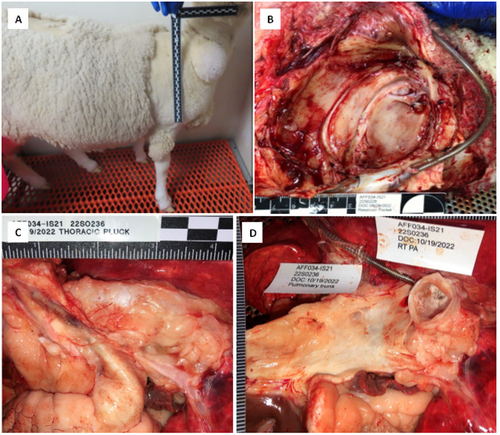
The Aria CV device was successfully implanted in all 8 GLP ovine animals. The system passed the ISO 10993 tests for implantation, thrombogenicity, and toxicity. Necropsy findings were unremarkable (Figure 6) and no device related adverse events were observed in any of the animals after 60-days of implant.
4 Discussion
The in silico, cadaver, bench, animal, and clinical studies consistently demonstrated the ability of the Aria CV device to increase pulmonary vascular compliance by approximately 0.4 mmHg/ml, augment cardiac output and stroke volume, and narrow PA pulse pressure as designed and expected.
In a seminal manuscript examining the correlation of varying degrees of baseline compliance in a series of PAH patients in the lowest pulmonary vascular capacitance quartile reported a 4-year mortality of 61% compared to 15% mortality in the next lowest (second) quartile [53]. Comparing the results in compliance changes obtained with the Aria CV device activation, the 0.4 mmHg/ml change could theoretically shift patients from the lowest compliance quartile to the second quartile.
Higher vascular compliance is strongly related to lower mortality risk [53]. Thus, the long-term improvement in pulmonary compliance due to Aria CV implantation, though mechanical, is hypothesized to improve RV energetics by reducing peak pulmonary pressures. The energy expended by the right ventricle to eject blood is inversely proportional to pulmonary vascular compliance [54]. During ejection, while some of the ejected stroke volume flows through the vasculature, a large portion of the ejected blood is temporarily stored in the pulmonary vasculature during the ejection phase. Significantly, the stored blood, enabled by vessel elasticity, results in diastolic PA flow, distributing the ventricular outflow over the entire cardiac cycle and lowering ventricular energy expenditure [55].
Aria CV device implantation in calves resulted in a reduction of PA systolic and pulse pressures, RV systolic pressures, and an improvement in PAC and CO. These hemodynamic benefits were observed clinically in humans with a short-term Aria CV device that had a non-anchored 20 ml balloon, and an external reservoir. Briefly, the short-term Aria CV device was implanted in Group 1 (n = 10), Group 2 (n = 8), and Group 3 (n = 10) PH patients [56, 57]. Device activation in Group 1 patients resulted in reduction of pulse pressures (47.6 ± 12.1 mmHg to 44.1 ± 11.6 mmHg), augmentation of cardiac output (6.8 ± 1.7 l/min to 7.5 ± 2.2 l/min), PAC (2.1 ± 1.0 to 2.5 ± 1.2), and end-systolic elastance (Ees)/effective arterial elastance (Ea; 1.01 ± 0.25 to 1.27 ± 0.21). Device activation in Group 2 patients resulted in statistically significant reductions in PA pulse pressure (5.6 ± 4.2 mmHg), augmentation of PAC (0.4 ± 0.2 ml/mmHg), and increased right ventricular-to-pulmonary vascular (RV-PV) coupling by 0.24 ± 0.18. In Group 3 patients, device activation resulted statistically significant reduction of PA pulse pressures (4.2 ± 2.2 mmHg), compliance (0.4 ± 0.3 ml/mmHg), and increased RV-PV coupling by 0.11 ± 0.07. These results demonstrate the clinical efficacy of the Aria CV device. Increased pulmonary vascular compliance due to device implant may delay wave reflections, lower ejection pressures and afterload, further improving RV energetics and protecting the RV from the deleterious effects of higher pulsatile load in PH.
Aria CV device activation and the concomitant increase in pulmonary vascular compliance resulted in an immediate improvement in stroke volume dependent cardiac output and function (without increasing left ventricular end diastolic pressures), demonstrating that the Aria CV device is non-obstructive in the main PA. The Aria CV device augments PAC using mechanical means, and while the mechanisms for the immediate hemodynamic improvements are not fully known in this study, hypothesized factors potentially associated with these immediate hemodynamic improvements may include—(1) augmentation of diastolic PA flows (Figure 5), (2) improving the timing and magnitude of RV ejection flow, contraction and reversing the ventricular dyssynchony, (3) improved RV-vascular coupling, (4) reduction of systemic and pulmonary venous congestion, (5) restoration of interventricular septal geometry and improved septal contribution to ejection, (6) improved systolic RV coronary perfusion and lower ventricular energy expenditure and (7) improvement in intra- and interventricular dyssynchrony.
Significantly, the improvement in CO was dynamic, with more pronounced effects during exercise with the Aria CV device, potentially increasing exercise tolerance and quality of life in patients with PH [56]. The magnitude of CO improvement with Aria CV device implant was similar in magnitude to the CO changes observed in intra-aortic balloon pumping and bi-ventricular pacing in patients with left ventricular failure (Table 2) [58-65]. Significantly, in a small clinical trial for patients with PH, biventricular pacing reduced cardiac output in 4 out of 6 patients [65].
The loss of compliance, and increase in vascular resistance, ventricular and vascular pressures, and ventricular afterload resulting in RV failure and mortality is a common feature in PH. During PH, mechanosensation and mechanotransduction of the non-physiologic pulmonary arterial pulse pressures, arterial shear, and ventricular wall stress have been implicated in triggering the remodeling of the pulmonary vasculature and right ventricle, which contributes to the progression of disease in a positive feedback loop [5, 15, 66-69]. Mechanotransduction, sensed by cell surface, cytoplasmic and extracellular matrix (ECM) receptors induce gene transcription and biological signals that drive vascular remodeling, including hypertrophy, hyperplasia, apoptosis, and ECM synthesis and degradation in attempts to maintain vascular and regional pulmonary homeostasis. The large proximal arteries and the small distal arteries and capillaries are in a dynamic, feed forward dialog that result in continuing loss of vascular compliance and pathologic molecular activation resulting in continuing adverse ventriculo-vascular remodeling. Compliance alteration in the proximal pulmonary vasculature amplifies pulse-wave transmission to the distal pulmonary arteries, and can result in inflammation, pathologic shear stress, endothelial injury, and smooth muscle remodeling. Distal pulmonary vascular compliance changes associated with muscularization increase the mean vascular pressures and result in continuing large vessel remodeling including wall thickening, dilation, and alterations in ECM [70-72]. The addition of compliance due to the Aria CV device implant, and the resulting reduction of these abnormal biomechanical signals may potentially induce reverse remodeling [73] by favorably altering gene activation and transcription in myocardial and vascular cells. RV and vascular reverse remodeling attributed to reduced systemic and pulmonary congestion and normalization of vascular pressures has been previously reported with lung transplantation [74, 75] and left sided mechanical support in patients with biventricular failure [76-78].
In summary, the Aria CV device is designed to be a chronic intravascular device to enhance pulmonary vascular compliance. Compliance enhancement with Aria CV device therapy is synergistic with drug therapy that focuses on PVR. In vitro and animal studies have demonstrated the safety of chronic device implantation and the feasibility of the device to augment the pulmonary vascular compliance, reduce RV afterload and pulmonary pressures chronically. These studies have resulted in the Food and Drug Administration (FDA) approval of a 30-subject clinical trial for Groups 1, 2 and 3 PH with RV dysfunction.
5 Limitations
There was variability in the development of PH in the hypoxia calf study and mortality was high. The pulmonary compliance in calves is higher than in humans with PH. Device implant was through the right jugular vein in the animal models to accommodate the anatomy of the animal. In vitro testing is not capable of replicating all physiological responses. Despite these limitations, extensive testing of the Aria CV device through multiple models per FDA and ISO standards successfully demonstrated safety and efficacy, resulting in FDA approval of a clinical trial.
Author Contributions
Karl Vollmers: data generation, data analysis, writing – review and editing. John Scandurra: funding acquisition, writing – original draft. Joshua R. Woolley: conceptualization, writing – original draft Guruprasad A. Giridharan: conceptualization, and writing – original draft. Alexander M.K. Rothman: data acquisition, review, editing. Marc Pritzker: writing – review and editing. E. Kenneth Weir: writing – review and editing. Christian Gerges: writing – review and editing. Irene Lang: conceptualization, writing – review and editing.
Acknowledgments
The authors would like to thank the Sheffield ASPIRE consortium for providing access to the registry data for virtual anatomic fit studies. ASPIRE registry is supported by the National Institute for Health and Care Research (NIHR) Sheffield Biomedical Research Center (NIHR203321). Funding for the studies was provided by Aria CV.
Ethics Statement
All animal studies were approved by the appropriate Institutional Animal Care and Use Committees (IACUC). Ethical approval for the ASPIRE registry for human imaging data was granted by the Institutional Review Board and approved by the National Research Ethics Service (16/YH/0352 subsequently 22/EE/0011).
Conflicts of Interest
Karl Vollmers: Employee of Aria CV. John Scandurra: Employee of Aria CV. Joshua R. Woolley: Employee of Aria CV. Guruprasad A. Giridharan: Employee of the University of Louisville, Consultant. Alexander Rothman: Employee of University of Sheffield. Marc Pritzker: Employee of the University of Minnesota, Consultant. E. Kenneth Weir: Employee of the University of Minnesota, Consultant. Christian Gerges: Employee of Medical University of Vienna. Irene Lang: Employee of Medical University of Vienna.




