Hypoxia-induced pulmonary hypertension upregulates eNOS and TGF-β contributing to sex-linked differences in BMPR2+/R899X mutant mice
Disclosures: This work was in part presented at 2022 Vascular Biology: From Genes to Medicine (Seattle, WA); at the 2021 University of Illinois College of Medicine (UIC COM) Research Day; at the 2021 UIC Cardiovascular Research Forum (CCVR; Honorable mention by Ejehi O. Erewele); and at the 2022 UIC CCVR (Omar Loya).
Abstract
Dysfunctional bone morphogenetic protein receptor 2 (BMPR2) and endothelial nitric oxide synthase (eNOS) have been largely implicated in the pathogenesis of pulmonary arterial hypertension (PAH); a life-threatening cardiopulmonary disease. Although the incident of PAH is about three times higher in females, males with PAH usually have a worse prognosis, which seems to be dependent on estrogen-associated cardiac and vascular protection. Here, we evaluated whether hypoxia-induced pulmonary hypertension (PH) in humanized BMPR2+/R899X loss-of-function mutant mice contributes to sex-associated differences observed in PAH by altering eNOS expression and inducing expansion of hyperactivated TGF-β-producing pulmonary myofibroblasts. To test this hypothesis, male and female wild-type (WT) and BMPR2+/R899X mutant mice were kept under hypoxic or normoxic conditions for 4 weeks, and then right ventricular systolic pressure (RVSP) and right ventricular hypertrophy (RVH) were measured. Chronic hypoxia exposure elevated RVSP, inducing RVH in both groups, with a greater effect in BMPR2+/R899X female mice. Lung histology revealed no differences in vessel thickness/area between sexes, suggesting RVSP differences in this model are unlikely to be in response to sex-dependent vascular narrowing. On the other hand, hypoxia exposure increased vascular collagen deposition, the number of TGF-β-associated α-SMA-positive microvessels, and eNOS expression, whereas it also reduced caveolin-1 expression in the lungs of BMPR2+/R899X females compared to males. Taken together, this brief report reveals elevated myofibroblast-derived TGF-β and eNOS-derived oxidants contribute to pulmonary microvascular muscularization and sex-linked differences in incidence, severity, and outcome of PAH.
INTRODUCTION
Mutations or reduced expression of bone morphogenetic protein receptor 2 (BMPR2) is a hallmark in the onset and development of different subgroups of pulmonary arterial hypertension (PAH), accounting for up to 75% of inherited PAH cases and 11%–40% of idiopathic PAH patients.1 Although PAH has a clear multifactorial component, lung endothelial cell (EC) injury and expansion of pathogenic cell populations within the pulmonary vascular wall are major triggers of progressive remodeling and narrowing of small pulmonary arteries, characteristic of the disease.2, 3 In response to increased pulmonary vascular resistance, the pressure inside the right ventricle increases, which often results in right ventricular hypertrophy (RVH) and premature death by heart failure.4, 5 Despite extensive efforts to alleviate PAH morbidity and reduce its mortality, the disease remains incurable.
Reduced BMPR2-mediated signaling in pulmonary vascular cells is known to contribute to hyperactivation of the TGF-β pathway by shifting endothelial protective P-SMAD1/5/8 signaling to the pathological P-SMAD2/3 pathway. This switch has been reported to significantly contribute to smooth muscle cell, endothelial cell, and fibroblast migratory and proliferative changes characteristic of the disease.6, 7 It is evident that TGF-β is the major promoter of endothelial-to-mesenchymal transition (EndoMT) in human and animal models, meaning TGF-β leads to the expansion of abnormal EC-derived populations with myofibroblast-like characteristics.8 Moreover, loss of endothelial BMPR2 expression is associated with dysfunction of the endothelial protective enzyme, endothelial nitric oxide synthase (eNOS).9 Dysfunction or “uncoupling” of eNOS leads to formation of free radical oxidants that perpetuate EC injury as well as contribute to elevated vasoconstriction.9, 10 Thus, it is evident that balance between BMPR2 and TGF-β-mediated signaling is critical for vascular homeostasis and injury repair. Imbalance can lead to enhanced TGF-β control over cell phenotype as well as survival, proliferation, and migration mechanisms of the various cell types in the pulmonary vascular wall, including pulmonary ECs.
Of note, over 300 BMPR2 mutations have been identified1, 11, 12 with the heterozygous nonsense mutation Bmpr2R899X in exon 12, inducing age-associated pulmonary hypertension (PH) in mice.13 Importantly, amongst the population of heritable PAH patients carrying the heterozygous BMPR2 mutation, males often have a worse prognosis, despite the fact that females have nearly three times higher incidence of this disease.14 In line with these observations, previous studies indicated that estrogen could reduce BMPR2 expression, thus contributing to the higher prevalence of PAH in females carrying the BMPR2 mutation.15-18 Although previous studies have demonstrated that sex plays a role in the prevalence and mortality associated with PAH, the specific mechanism and a selective molecular target behind these sex-associated differences remain under investigation. Together, these observations prompted us to investigate the contribution of lung EC BMPR2 expression and mutation in PAH-associated sexual disparity. In particular, we evaluated whether chronic hypoxia-induced PH in the humanized mouse model carrying the BMPR2+/R899X loss-of-function mutation would play a role in the onset of endothelial dysfunction, EndoMT, and pulmonary microvascular muscularization that leads to elevated right ventricular systolic pressure (RVSP) and PAH.
MATERIALS AND METHODS
Mouse model and genotyping
Male and female wildtype (WT) mice and a mutant strain carrying the heterogenous human knock-in mutation BMPR2+/R899X were genotyped using amplification refractory mutation system PCR (ARMS-modified PCR) and the following primers: GAGGGAACGG CCATTAGAAGGTGGAT (ARMS_mBMPR2_inFw); AGAAGCCACAATGTTAATTCCCATGCTG (ARMS_mBMPR2_outFw) and; ATACTGCTGCCATCCAGGATAT TTGTGG (ARMS_mBMPR2_outrv), as previously described.19 WT and BMPR2+/R899X mutants mice were randomized by sex for subsequent experiments. In all cases, strain- and age-matched mice were used as approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee.
Hypoxia-induced pulmonary hypertension and hemodynamics assessment
WT and BMPR2+/R899X mutant mice (12-month-old) were randomized and kept under normoxia (21% O2) or hypoxia chamber (10% O2) for 4 weeks with water and food access ad libitum. Animals were then weighted and anesthetized using an intraperitoneal injection of Ketamine/Xylazine (100 and 10 mg/kg, respectively). After animals undergo general anesthesia, the surgical area was disinfected using 70% alcohol and a small skin incision was performed to access the jugular vein. As described previously,20 a Millar Mikro-Tip catheter transducer (model PVR-1030) was carefully inserted into the right ventricle (RV) via the right jugular vein for measurement of RVSP, calculated using an MPVS-300 system connected to a Powerlab A/D converter (AD Instruments, Colorado Springs, CO). After recordings were completed, the animals were ventilated, and about 1 ml of blood was collected using 3.8% sodium citrate-treated syringes via vena cava puncture. Then, animals were exsanguinated and lungs were cleared by perfusion of 5 ml of cold PBS via cannula placed in the RV. After complete lung perfusion, the lung lobes were either removed and snap-frozen in liquid nitrogen for Western blot analysis analysis, freshly removed for fibroblast isolation, or carefully inflated with 4% paraformaldehyde (PFA) solution for posterior paraffinization, sectioning, histological and immunohistochemistry analysis. Finally, freshly isolated hearts were dissected for evaluation of RV hypertrophy using the Fulton index (RV/left ventricle + septum weight ratio).20
Lung sample preparation and Western blot analysis
Frozen lung tissue was fully homogenized using cold radiommunoprecipitation assay (RIPA) buffer containing 1% protease and 0.1% phosphatase inhibitor cocktail. After 20 min incubation at 4oC, homogenates were centrifuged at 12,000×g (20 min at 4oC) and supernatants collected for protein measurement by bicinchoninic acid (BCA) protein assay by colorimetry using a microplate reader (Benchmark Scientific MR9600-T SmartReader™ 96 Plate). Then, 10–20 μg of lung tissue lysates diluted in Laemmli Sample Buffer (4×) with β-mercaptoethanol, boiled by 10 min at 95oC or kept unboiled for native protein analysis to assess eNOS uncoupling by increase in monomers. Before loading the samples on gradient SDS-PAGE gels (8%–12%), tubes were centrifuged for 2.5 min at 16,200×g. After running the samples, separated proteins were transferred to nitrocellulose membranes as described.21 Protein transfer to the membranes was assessed by absence of ladder markers in the gel simultaneously with detection of a continuous gradient of Ponceau Rouge staining over the membranes. Membranes were then washed twice with TBS-Tween 1X for 5 min and blocked using 5% milk or BSA (according to antibody datasheet instructions) for 1 h at room temperature, followed by primary antibody incubation overnight at 4oC or 2–3 h at 37oC. After washing (2 × 5 min; 1 × 15 min), the membranes were incubated for 1 h with the specific secondary HRP-conjugated antibody, washed again, and then, proteins were detected using an ECL kit (Amersham). Finally, membranes were scanned with Li-Cor Odyssey CLx (Lincoln, NE). Data were normalized to β-actin or GAPDH loading controls and analyzed using ImageJ software (https://imagej.nih.gov/ij/).
Fibroblast isolation, immunostaining, and proliferation assay
Freshly isolated, and perfused lungs from anesthetized mice were excised, and finely minced into 2- to 5-mm fragments, rinsed with sterile PBS, and incubated in 2 mg/ml type I collagenase in DMEM (60 min at 37°C under agitation [≈80 rpm]). After fragments were dissociated and the resulting suspension was filtered through a 40 μm cell strainer, cell suspension was centrifugated (300×g; 8 min), resuspended, and assayed for viability using Trypan blue reagent (2–4 × 106 cells per adult lung). Then, cells were allowed to attach at 37°C on 10 cm tissue culture dishes. After 1 h, nonadherent cells were removed by a gentle washing and remaining attached cells were further cultured in DMEM+ 10% FBS. WT and mutant-derived fibroblasts were cultured under the same conditions. Cell growth was evaluated daily by brightfield microscopy for 7 days, when cells were fixed in 4% PFA solution for 15 min at room temperature.22 After permeabilization, cells were blocked with 10% donkey or goat serum diluted in PBS for 1 h (room temperature), followed by overnight incubation with primary antibody against Ki67 at 4oC (in a humidified chamber). After washing, slides were incubated with Alexa 488 and Alexa 555 secondary antibodies (excitation/emission: 499/519 and 553/568 nm, respectively) for 1 h, washed again, and mounted on Prolong antifade mounting media containing DAPI (4′,6-diamidino-2-phenylindole) for nuclear staining. Cell culture purity was assessed by HSP47 and prolyl 4-hydroxylase staining. Ki67 and DAPI were used to quantify proliferation. Fluorescent images were collected using a confocal microscope (Zeiss LSM810).
Immunohistochemistry and quantification of vascular remodeling
Formalin-fixed, paraffin-embedded lung sections were used to evaluate protein expression (primary antibodies: α-SMA, vWF, and FSP-1) and for histological analysis of vessel area, thickness, and collagen deposition. Samples were deparaffinized by serial exposure to xylene and ethanol and then to high temperature, used to promote antigen retrieval (5–10 min under pressurized container; sodium citrate buffer). For immunohistochemistry, lung sections were blocked using 10% donkey or goat serum diluted in PBS for 1 h (room temperature) followed by overnight incubation with the primary antibody at 4oC (in a humidified chamber). After washing, slides were incubated with antibodies Alexa fluor-labeled secondary antibodies, washed again and mounted and imaged as above. Then, fluorescent images were collected using a LSM880 confocal microscope from Carl Zeiss MicroImaging, Inc. Four fluorescent images were taken from different randomized peripheral lung segments in each sample for manual quantification of alpha-smooth muscle actin (α-SMA) and DAPI expressing muscularized vessels in WT and BMPR2+/R899X mutant males compared to females. The number of vessels were normalized by the total area. In addition, histological analysis of collagen deposition was performed using picrosirius FAST red stained lung sections. After staining, individual slides containing 2–3 lung sections of 5–10 μm each, were scanned using an Aperio brightfield automated microscope slide scanner (Leica Aperio AT2) at 20x magnification. Digitilized images were used to determine the pulmonary microvessel area and thickness (μm) in 10 microvessels per animal, that is, vessels with a diameter smaller than 100 μm, specifically varying between 30 and 70 μm, using the ImageScope software 12.4.6 (Leica Biosystems). Briefly, microvessel wall thickness was obtained via utilization of the ImageScope ruler tool to measure the length across the thickest part of the arterial segment defined as the space between the peripheral outer wall of the vasculature and luminal boundary. Microvessel wall area was subsequently collected by manually tracing the contours of the peripheral outer wall and luminal boundary to obtain the total microvessel area and luminal area respectively and calculated by subtracting the luminal area from the total area of the microvessel as previously reported.20 Mean microvessel wall thickness and area were calculated for each sample using Excel software.
Enzyme-linked immunosorbent assay (ELISA)
Frozen lung tissue was fully homogenized using cold RIPA buffer containing 1% protease and 0.1% phosphatase inhibitor cocktail. After 20 min incubation at 4oC, tissue homogenates were centrifuged at 12,000×g (20 min at 4oC), and the supernatant collected for measurement of interleukin-6 (IL-6) and transforming growth factor-β1 (TGF-β1) in male and female WT and BMPR2+/R899X mutant mice and isolated fibroblasts according to manufacturer instructions (R&D ELISA kits).
Reagents and antibodies
Rabbit polyclonal anti-α-SMA was acquired from Abcam. Rabbit polyclonal anti-GAPDH, anti-von Willebrand Factor (vWF), and anti-fibroblast-specific protein 1 (FSP1) were acquired from Santa Cruz Biotechnology. Rabbit Polyclonal Endothelin-1 was purchased from Fisher Scientific. Rabbit polyclonal anti-BMPRII and anti-P-SMAD1/5/8 were purchased from Cell Signaling Technology. Mouse monoclonal anti-eNOS, anti-β-actin and rabbit polyclonal anti-Cav-1 were purchased from BD PharMingen. Alexa-Fluor 488 and 555-conjugated goat and donkey antimouse or antirabbit IgG were purchased from Life Technologies. Antimouse and antirabbit HRP-conjugated IgG were purchased from Cell Signaling Technology or Kierkegaard and Perry Laboratories (Supporting Information 1). RIPA buffer, protease, and phosphatase inhibitor cocktail, collagenase type I, paraformaldehyde, heparin, and sucrose were purchased from SIGMA Chemical, Co. Mounting media containing DAPI (VectaShield) was obtained from Vector. Stock solutions were prepared in 100% dimethylsulfoxide (DMSO), buffered physiological solution or sterile phosphate-buffered saline (PBS) and diluted daily in sterile PBS or DMEM. The highest final concentration of the solvent was 0.1% (v/v) and did not affect the experiments. PCR primers were purchased from Integrated DNA Technologies, Inc.
Statistics and scientific rigor
Data were analyzed using GraphPad Prism v9 (GraphPad). Normally distributed data are presented as arithmetic mean ± standard error of the mean (SEM). Shapiro–Wilk test was used to determine the normality of data and the Brown–Forsythe or F-test used to assess equality of variances. Normally distributed data were analyzed using a parametric unpaired Student t-test between two groups and parametric Two-way analysis of variance (ANOVA) followed by post hoc analysis (Bonferroni or Tukey Multiple Comparison test) to assess differences between more than two groups with two variables. All tests were performed as two-sided, and p < 0.05 was considered statistically significant.
RESULTS
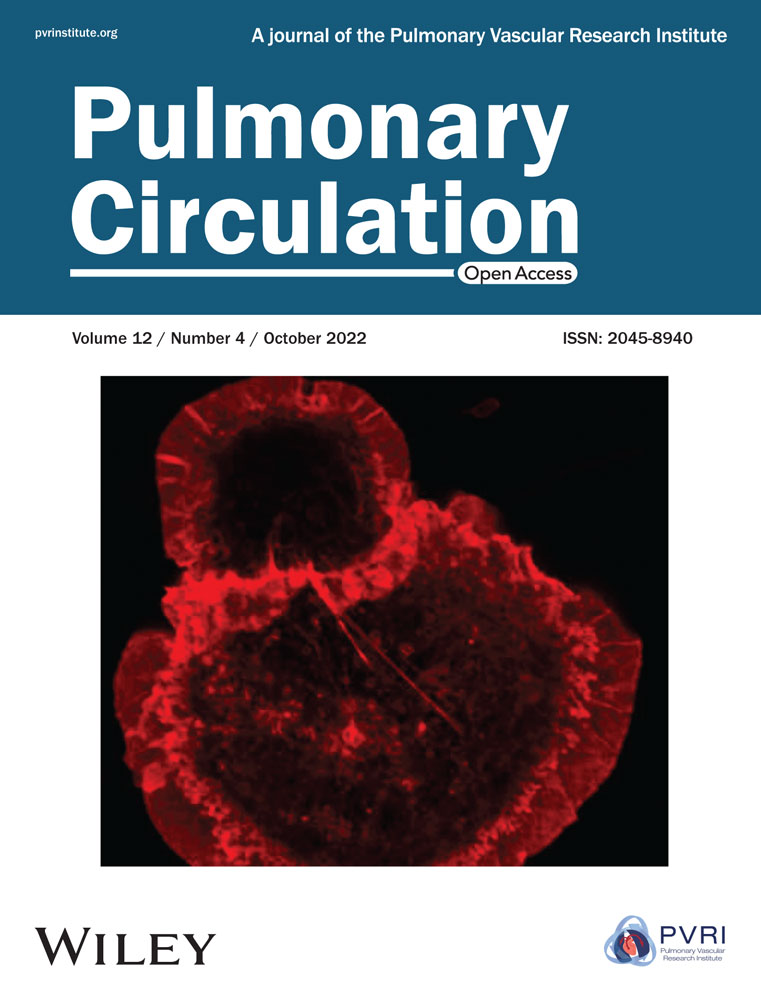
BMPR2/R899X mutation leads to microvessel muscularization independent of Cav-1 expression
Silencing or mutation of BMPR2 promotes cell death and abnormal proliferation of both pulmonary vascular ECs and smooth muscle cells, leading to vascular remodeling and PAH.13, 23 Similarly, reduced, mutated or absent expression of the anti-inflammatory protein Cav-1 within the lungs contribute to severe vascular remodeling and PAH via elevated eNOS-derived oxidants, depletion of physiologic pulmonary BMPR2 expression, and disrupted canonical BMPR2/phosphorylated-SMAD1/5/8 (P-SMAD1/5/8)-mediated signaling.6 Interestingly, these studies revealed that heterozygous mice carrying familial genetic mutation in exon 12 of BMPR2 (R899X), reduce BMPR2-long form (LF) expression and lead to vascular remodeling (Figure 1a,b), consistent with a previous report.24 BMPR2+/R899X mutant mice also showed low levels of BMPR2-short fragment (SF) and reduced expression of P-SMAD1/5/8 relative to controls, with no signs of altered pulmonary Cav-1 expression in normoxia exposed mice (Figure 1a,c). Moreover, although the heterozygous knock-in R899X mutation did not increase the overall microvessel area in 12-month-old mice, it was associated with significant accumulation of pulmonary collagen and expression of α-SMA which were associated with muscularization of pulmonary microvessels (Figure 1b,d).
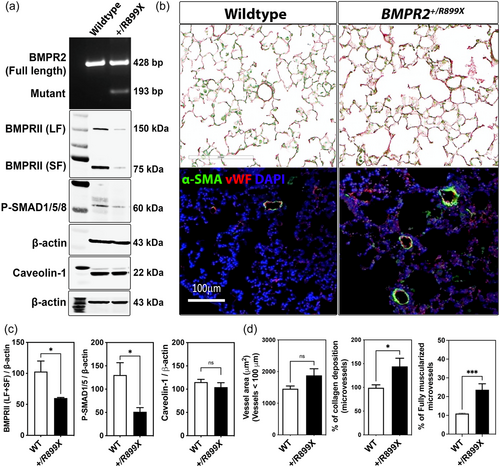
BMPR2 loss in R899X mutant mice contribute to sex-linked differences in hypoxia-induced PH
Previously, we observed that hypoxia-induced TGF-β secretion promoted PH in mouse models with reduced BMPR2 expression.6 Thus, to further investigate the role of reduced expression of BMPR2 in heterozygous knock-in R899X mutant mice in the onset and progression of PH, WT, and BMPR2+/R899X mutant mice were exposed for 1 month to hypoxia (10% O2) or kept under normoxic conditions (21% O2). In normoxic conditions, RVSP was significantly higher in mutant animals compared to WT mice group (Figure 2a). However, there was no statistically significant difference in RVH between the groups (Figure 2d). Chronic hypoxia exposure elevated RVSP and induced RVH in both WT and BMPR2+/R899X mutant groups, with a greater effect observed in the mutant mice (Figure 2a,d). Moreover, higher RVSP but not RVH was significantly dependent on sex in animals carrying the heterozygous knock-in R899X mutation, as female mice primarily accounted for the elevated RVSP both at normoxia and hypoxia (Figure 2c–f). On the other hand, neither normoxia or hypoxia-mediated increase in RVSP or RVH were sex-linked in the WT group (Figure 2b–e). Lung histological analysis revealed no difference in the overall microvessel thickness and area between sexes in the WT and BMPR2+/R899X mutant groups (Figure 2g–i), indicating the increase in fully muscularized vessel number or the degree of pulmonary vascular contractility may account for the elevated RVSP observed in the female BMPR2+/R899X mutant animals. Taken together, these data imply elevated RVSP in mice carrying the human R899X mutation is sex-dependent but unlikely dependent on hypoxia-associated microvascular narrowing within the lungs.
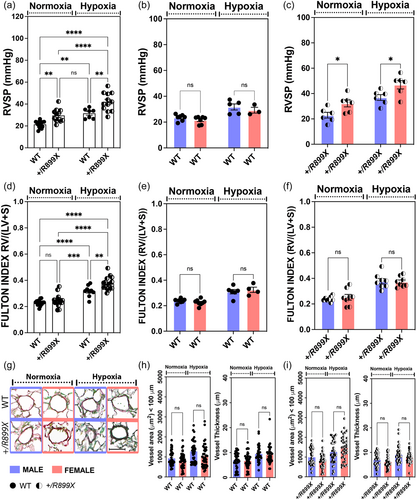
Hypoxia-induced RVSP in BMPR2+/R899X females is associated with eNOS-Cav-1 dichotomy
Prolonged exposure to hypoxia induces structural and functional alterations in the pulmonary vasculature, which strongly varies depending on sex.24 Elevated expression of α-SMA in the pulmonary microcirculation has been extensively used as indicative of microvessel muscularization, and is often associated with increased pulmonary vasoconstriction and vascular resistance, leading to pathological elevation in RVSP that characterizes PAH. The increase in microvascular α-SMA expression occurs in response to different processes, including the expansion and hyperactivation of fibroblasts, myofibroblasts, or muscle-like cells within the vasculature, as observed in response to EndoMT. In line with these observations, our data indicate that reduced expression of BMPRII protein in the lungs of heterozygous knock-in R899X mutant mice increased the number of fully muscularized microvessels, an effect significantly elevated in female animals upon chronic hypoxia exposure (Figure 3a,b). Moreover, our data also showed that chronic hypoxia exposure increased eNOS expression, whereas it reduced the expression of Cav-1, in the lungs of the BMPR2+/R899X females but not males (Figure 3c,d). In opposition to the expected increase in eNOS monomers accumulation in unboiled samples, indicative of Cav-1 depletion-dependent eNOS uncoupling, we detected no difference among the groups (Figure 3e). Since eNOS dysfunction often results in vasoconstrictor endothelin-1 (ET-1) production by ECs, we next assessed ET-1 levels in the lungs of all mutant groups. BMPR2+/R899X females exhibited a significant increase in ET-1 levels under normoxic conditions, an effect not sustained under hypoxia exposure (Figure 3f). Altogether, these data suggest BMPR2+/R899X mutation has a sex bias, contributing to elevate expression of pulmonary vasoconstrictor ET-1 in female animals at normoxia, and under hypoxia, reducing vasoprotective levels of Cav-1, increasing expression of pulmonary eNOS and the number of muscularized vessels, which seems to align at least in part with the vasoconstrictive/proliferative phases during the course of the disease.
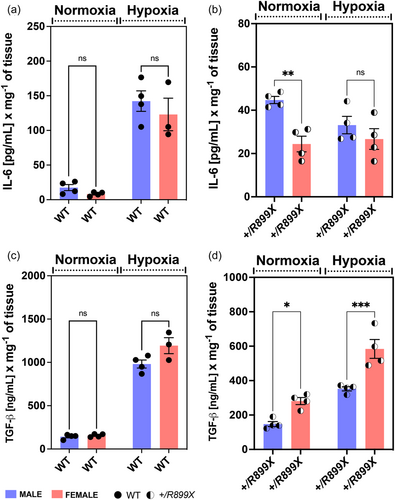
Hypoxia reduced IL-6 but induced TGF-β overexpression in the lungs of female BMPR2+/R899X mice
The IL-6/IL-6R-signaling pathway induces Cav-1 depletion and eNOS uncoupling in human pulmonary artery ECs, leading to TGF-β-mediated EndoMT and inflammatory vascular remodeling.7 Overexpression of IL-6 per se is also known to increase TGF-β-mediated signaling by reducing TGF-RI association with Cav-1,25 thereby promoting vascular remodeling and PH in mice.26 Thus, to evaluate whether hypoxia-induced PH in BMPR2+/R899X female mice is associated with an inflammatory component, especially related to pro-fibrotic signaling via IL-6 and TGF-β, we quantified these inflammatory signals in the lungs of WT and mutant animals at normoxia and following chronic hypoxia exposure. Although hypoxia significantly increased IL-6 levels in the lungs of WT animals, no significant differences were observed between sexes (Figure 4a). Interestingly, the lungs of female BMPR2+/R899X mutant mice showed lower levels of IL-6 compared to male animals under normoxia or after chronic hypoxia exposure, indicating IL-6-mediated signaling may not account for sex differences observed in BMPR2+/R899X mutant mice (Figure 4b). Similarly, pulmonary TGF-β levels increased upon hypoxia exposure in WT male and female animals (Figure 4c). Measurements of pulmonary TGF-β levels in BMPR2+/R899X mutant mice revealed that chronic hypoxia enhances TGF-β secretion in both sexes, however, in opposition to that observed in the WT group, females displayed higher levels even in normoxic conditions (Figure 4d). These results suggest enhanced TGF-β-mediated pro-fibrotic signaling may contribute to increased incidence of microvascular muscularization in female BMPR2+/R899X mutant mice.
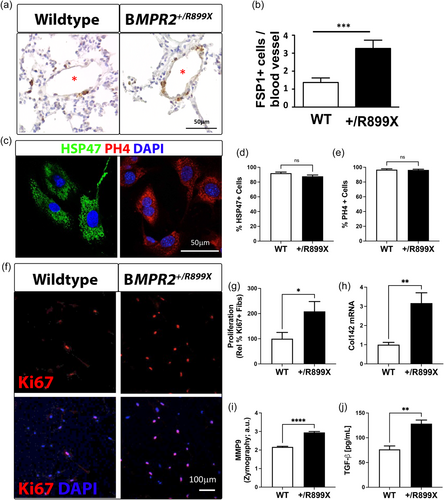
BMPR2+/R899X mutation expands TGF-β-producing myofibroblasts within the lung microvasculature
Increased α-SMA expression and collagen production, which are characteristics of fibroblasts and myofibroblasts derived from several cellular processes, including EndoMT, contribute to vascular remodeling. In fact, studies herein revealed that besides elevated collagen deposition and an increase in the number of α-SMA + microvessels, as shown in Figures 1, 3, accumulation of fibroblast-specific protein-1 (FSP-1) was also observed in pulmonary precapillary resistance vessels of BMPR2+/R899X mutant animals as opposed to WT controls (Figure 5a,b). Moreover, when isolated, the proliferation rate of BMPR2+/R899X-derived pulmonary fibroblasts22 (characterized by expression of HSP4727 and PHD428) was twofold higher than WT mouse-derived cells (Figure 5c–f), which may also contribute to the increased number of fully muscularized microvessels observed in BMPR2+/R899X mutants. Finally, BMPR2+/R899X-derived pulmonary fibroblasts expressed higher levels of collagen and matrix metalloproteinase 9 (MMP9) and secreted more TGF-β than WT fibroblasts (Figure 5g,h), indicating BMPR2+/R899X-induced loss-of-function increases the percentage of activated myofibroblasts leading to microvascular muscularization, elevated pulmonary pressure, and PAH.
DISCUSSION
Substantial evidence indicates that progression of pulmonary vascular remodeling during PAH occurs as a dual-phase process. First, there is an apoptotic phase in which cells within the pulmonary vascular wall are damaged, and second, in an abnormal proliferative phase, the vascular cells that were able to survive the apoptotic stimuli undergo epigenetic reprogramming, proliferate and alter the structure of the pulmonary vasculature, leading to the formation of chronic inflammatory vascular lesions.2, 3, 6, 29, 30 Identification of the molecular mechanisms that trigger pulmonary vascular injury and promote vascular remodeling remains an important goal to advance the field and shed light on new therapeutic approaches and molecular targets.30 Accordingly, several EC-associated biological processes, including apoptosis-resistant cell growth and EndoMT, have been implicated in the onset and severity of cardiopulmonary diseases, including PAH.29-31 Most interestingly, all of these processes seem to convey the idea that chronically-injured pulmonary endothelium attempts but is unable to heal itself, and thus, affected lung vessels become remodeled and occluded over time leading to PAH.
In support of these well-accepted findings, despite the fact that initiating events that promote alterations in the healthy endothelium remain not fully clear, reduced expression and mutations of the antiproliferative and anti-inflammatory proteins Cav-1 and BMPR2 as a result of lung vascular injury, have been shown to significantly contribute for the progression of PAH.6, 32-35 Moreover, loss of pulmonary endothelial Cav-1 expression plays a key role in impaired BMPR2-mediated signaling and increased TGF-β production by macrophages, which in turn promotes the expansion of abnormal Cav1-depleted ECs.6 Of note, both BMPR2-LF and SF have been found associated with Cav-1,36, 37 while endocytosis of BMPR2-SF appears to be uniquely dependent on caveolae.38 Although poorly described, increased expression of the BMPR2-SF splice variant on the cell surface correlates with increased P-SMAD1/5/8 signaling, suggesting BMPR2-SF depletion may be a major player in impaired SMAD1/5/8 phosphorylation and signaling. Here, we observed that BMPR2+/R899X mutant mice showed a reduction of both fragments in their lungs, and as consequence, lower phosphorylation of pulmonary SMAD1/5/8. Despite strong evidence in support of the role of reduced BMPR2 expression and signaling in the onset and progression of PAH, as well as ongoing efforts to target BMPR2-associated signaling to reduce PAH morbidity, how lung ECs with reduced BMPR2 expression contribute to pulmonary vascular remodeling remain under extensive investigation.
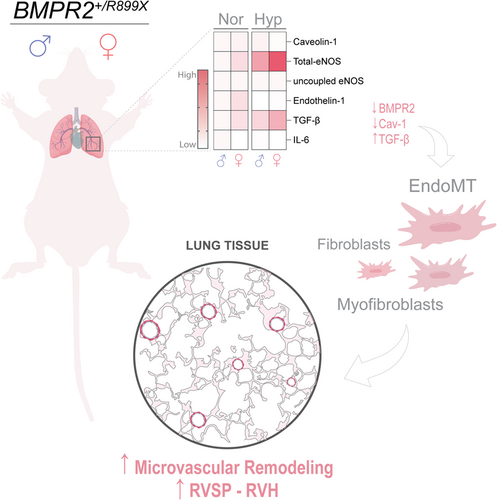
Chronic hypoxia induces structural and functional alterations in the pulmonary vasculature, which strongly varies depending on species, developmental stage, and sex.24 In fact, the studies presented here indicate that hypoxia-induced PH in BMPR2+/R899X mutant mice is sex-linked, since it recapitulates at the least in part, the clinical pattern of increased incidence of PAH in females. It is well-known that hypoxia induces vasoconstriction, EndoMT, and remodeling of the pulmonary vasculature which varies in magnitude depending on sex.24 Recent data by Frump et al.18 revealed that BMPR2 expression is in fact required for 17β-estradiol (E2) and apelin-mediated a cardioprotective effects on the RV of mice and rats, which addresses a potentially important mechanism by which females have a better PAH prognosis than their male counterparts despite higher disease incidence in females. In line with these observations, Umar and colleagues. also showed that estrogen has been shown to reverse severe PH in rats via cardiopulmonary neoangiogenesis and suppression of inflammation, fibrosis, and RV hypertrophy.39 Indeed, E2 directly affects vascular ECs, at least in part, by stimulating the expression and function of the eNOS.40-42 Here, data indicate that although the 1-month hypoxia exposure did not significantly change the degree of RVH in male or female BMPR2+/R899X mutant as compared to normoxia exposed mice, it increased RVSP in females despite higher level of eNOS expression. Although these data suggest oxidant-associated eNOS monomers do not accumulate in the lungs of BMPR2+/R899X female mice, elevated ET-1 levels at normoxia may contribute to the spontaneous increase in RVSP, followed by a hyperproliferative phase. Previous studies have reported that elevated level of ET-1 and eNOS-associated oxidative stress have potent vasoconstrictor effects during the course of PH.43-46 Moreover, inflammatory cytokines such as IL-6 and TGF-β are known to promote eNOS dysfunction and oxidant generation leading to EndoMT and PAH.7, 26 Thus, we evaluated whether hypoxia-induced PH in BMPR2+/R899X mice is associated with a significant inflammatory component. Results indicate that hypoxia-induced TGF-β upregulation varies with sex in BMPR2+/R899X mutant mice. Moreover, our studies also indicated that a spontaneous increase in pulmonary TGF-β level occurs in BMPR2+/R899X female mice. Besides the strong role of TGF-β in PAH, this fibrosis-associated cytokine has also been implicated as a potent stimulus for ET-1 via ALK-5/SMAD2/3 activation in chronic thromboembolic PH.46 Indeed, TGF-β-mediated ALK-5/SMAD2/3 exacerbates PH in mice lacking EC-Cav-1 expression and contributes to EndoMT.6, 7 Also, IL-6 was associated with poor survival in PAH patients,47 and was elevated in human PAH male patiens as compared to females.48 Thus, reduced IL-6 in the lungs of female BMPR2+/R899X animals might contribute to improved disease prognosis. Of note, although BMPR2+/R899X mutant animals exposed to hypoxia appear to have reduced IL-6 and TGF-β lung tissue level when compared to WT animals, measurements were performed in separate experiments, and thus sex comparisons herein are only made within the same genotype. Altogether, these findings indicate that BMPR2+/R899X mutation in female mice substantially contribute to increase pro-fibrotic TGF-β and vasoactive ET-1 levels which may contribute to lung EC activation and de-differentiation into hyperproliferative myofibroblast-like phenotype that contribute to microvascular muscularization, remodeling, and PAH (Figure 6).
EndoMT, myofibroblasts, or activated fibroblasts - despite the terminology, it is unmistakably evident that all those processes culminate in the generation of a proliferative collagen-producing phenotype with high expression of muscle markers such as α-SMA, which in turn contributes to increased vascular tone in the normally low resistance arteries within the lungs.47, 48 The specific molecular mechanisms that give rise to these cell phenotypes require further investigation. Nonetheless, a substantial amount of evidence implicates elevated TGF-β as the major determinant of the transformation of vascular cells into abnormal apoptosis-resistant hyperproliferative cell types.6, 49, 50 Furthermore, it is unclear how defective BMPR2 and hyperactivated TGF-β-mediated signaling first emerges, or how sex-associated differences contribute to elevated RVSP in females. Here, our data indicate that the density of proliferative fibroblast-like cells is spontaneously elevated within the vascular wall of small pulmonary resistance vessels in BMPR2+/R899X mutant mice. Moreover, we identified that this cell population is hyperactivated and contributes to elevated collagen deposition in the lung vasculature of BMPR2+/R899X mutants, as well as the elevated number of pulmonary α-SMA+ microvessels in the hypoxia-exposed BMPR2+/R899X female animals. Since dysfunctional ECs also produce increased levels of pro-proliferative factors such as ET-1,51, 52 perhaps it may be an alternative mechanism for stimulating the proliferation of fibroblast-like cells and increase RVSP in females. Finally, we observed that BMPR2+/R899X-derived fibroblast-like cells produced high levels of TGF-β and expressed higher levels of activated MMP9, an enzyme previously reported to be important in the pathogenesis of PAH. This reinforces the significant role of BMPR2+/R899X mutation and loss of functional BMPR2 expression within the lung vasculature in the development of PAH, especially in terms of the sexual dimorphism-associated with the disease.
AUTHOR CONTRIBUTIONS
Suellen D. Oliveira, Richard D. Minshall, and Glenn Marshboom conceived and planned the experiments. Suellen D. Oliveira, Ejehi O. Erewele, Maricela Castellon, Omar Loya, Glenn Marshboom, Andrew Schwartz, Kayla Yerlioglu, Christopher Callahan, and Jiwang Chen contributed to sample preparation. Suellen D. Oliveira, Glenn Marshboom, Ejehi O. Erewele, Omar Loya, and Jiwang Chen contributed to graph preparation, statistical analysis, and interpretation of results. Suellen D. Oliveira, Ejehi O. Erewele, and Omar Loya carried out the histological analysis, western blots, and ELISA measurements. Glenn Marshboom conceived, carried out, and analyzed the fibroblast-related experiments. Maricela Castellon and Kayla Yerlioglu performed strain genotyping, sex randomization, and hypoxia exposure. Jiwang Chen collected data of the right ventricle systolic pressure and right ventricular hypertrophy.
ACKNOWLEDGMENTS
We thank Dr. Nicholas W. Morrell (Professor of Medicine; University of Cambridge - United Kingdom) for providing the BMPR2+/R899X mutant mice and for critically reading the manuscript. We also thank the University of Illinois at Chicago Research Resources Cores facilities for assistance with Histology and Imaging and the Graphic Designer Pedro C. H. Simões for support in generating Figure 6. This work was supported in part by a Catalyst Award from the American Lung Association and a K01 award from the National Institutes of Health/National Heart, Lung, and Blood Institute (ALA 697907; NIH HL159037, respectively—Oliveira, SDSD), National Institutes of Health (NIH HL142636—Minshall, RD), a scholarship from the College of Medicine Urban Health Research Program (COM-UHP—Erewele, EO), and a scholarship from UIC Latin@s Gaining Access to Networks for Advancement in Science (L@s GANAS - Omar Loya).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
All animal experiments in this study were performed as approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee.