Improving early phase oncology clinical trial design: An opportunity for statisticians
Abstract
This short communication supports that rule-based study designs such as the ‘3 + 3’ study design are still being used in early phase oncology development programs despite their inferior performance to model-based and model-assisted designs. Statisticians have an opportunity to shape and improve early phase oncology drug development programs by introducing newer, more efficient study designs that estimate the Optimal Biological dose to their oncology trialist colleges.
1 INTRODUCTION
Traditionally First in Human dose escalation early phase oncology drugs have comprised rule-based study designs such as the standard or classical ‘3 + 3’ design to identify the maximum tolerated dose (MTD). Zhou et al.1 has shown model-based and model-assisted designs out-perform simple rule-based designs when estimating the MTD. Where limited information is available about the dose–response model, model-assisted designs are preferred. Phillips and Clark2 argue that newer Bayesian model-assisted designs, which are readily available via existing shiny apps should become the go to design for statisticians for estimating the MTD. This manuscript assesses how much progress has been made during the past 15 years in rolling out more efficient model-based and model-assisted designs in early phase oncology.
2 METHODOLOGY
A literature search was conducted using Web of Science on 1st June 2022 to identify all phase I oncology dose escalation studies published in the last 15 years. The search terms comprised TS = ((((phase I) OR (phase 1) OR (phase one)) SAME (study OR studies OR trial*)) AND cancer AND (patients OR subjects) AND (dose escalation) AND (toxicity OR efficacy) NOT ((phase III) OR (phase 3) OR (phase three))). The inclusion/exclusion criteria presented in Table 1 were subsequently applied to identify a short list of publications for analysis. The following data were extracted from publications meeting the selection criteria: Study Title, Author, Year of publication, Drug name, Drug Type (Traditional chemotherapy, Targeted Therapy, Biologic), Study sponsor, Type of dose escalation design used, Primary objective of study (MTD, OBD, Safety, PK), and Status of market authorization of drug.
| Inclusion criteria | 1 | Published between 1st January 2007 and 1st January 2022 |
| 2 | Language of publication must be English | |
| 3 | Original research article | |
| 4 | Study must be in Phase I or Phase I/II | |
| 5 | Study must assess dose escalation | |
| Exclusion criteria | 1 | Review articles/Reports/Case studies |
| 2 | Books/Documents | |
| 3 | Phase II/Phase III studies | |
| 4 | Paediatric studies | |
| 5 | Combination drug study in any form (e.g. two chemotherapy agents, one chemotherapy agent plus immunotherapy/targeted therapy, two immunotherapy agents, two targeted therapies or two small molecular drugs) |
3 RESULTS
Two hundred and seventy-six (276) English publications were retrieved from Web of Science using the specified search terms and applying the time filter 1st January 2007 to 1st January 2022. Out of these, one publication was a duplicate. Two hundred and thirty-three (233) of the remaining 275 publications were excluded based on the inclusion/exclusion criteria. The remaining 52 publications were subsequently included in the analysis. Some publications used individualised rule-based designs that were specific for their study. Since these designs did not fall under any recognised category, they were documented as ‘some form of rule-based design’. Figure 1 provides a scheme of the review process.
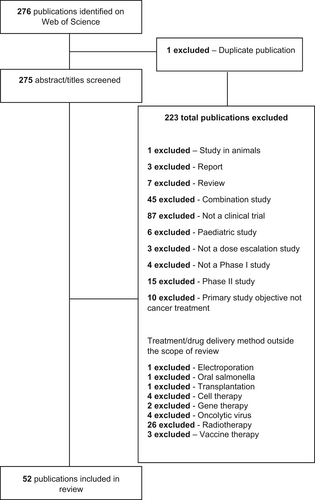
Around 40% of studies were excluded because they were theory-based publications instead of original research articles based on data from clinical trials. Twenty percent (20%) were excluded because they were combination type studies. This exclusion criterion was deemed necessary because of the complexities associated with designing combination studies which are well recognised within the industry, for example Paller et al.3 Nevertheless, publications were included in the analysis if they assessed combination as well as a monotherapy regime. Finally, case reports on individual patients and systematic reviews critiquing past literature accounted for 4% of all exclusions.
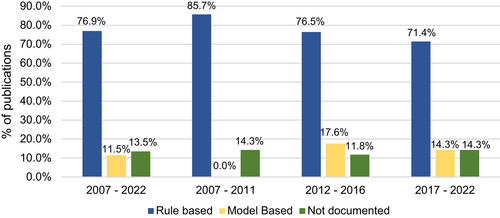
Between 2007 and 2022, rule-based designs were utilised by approximately 77% of all published literature. There has been a slow but steadily declining trend in the use of rule-based designs from 85.7% till 2011 to 76.5% till 2016, and ultimately, 71.4% till the start of 2022. These data are presented in Figure 2. On the other hand, model-based and model-assisted designs accounted for only 11.5% studies during the same period. The majority of model-based designs comprised the Continual Reassessment Method (CRM4) or some modified version of it. Other model-based designs used primarily included the Bayesian Logistic Regression Model (BLRM5) and modified Toxicity Probability design (mTPI6), in equal proportions. Given their fairly recent development, model assisted designs were not identified in any of the reviewed studies.
4 DISCUSSION
Rule based study designs such as the ‘3 + 3’ design are still very prevalent in early phase oncology development programs. Based on the literature review conducted over 71.4% of studies use rule-based designs. The first reported study that used a model based design was Roberts et al.7 This Phase I dose escalation study used the Continual Reassessment Method design to determine the MTD of the study drug, now called Navitoclax. However, since 2012 there has not been any rapid uptake in model-based or model-assisted designs, despite published evidence that such designs are more efficient in identifying the MTD. Phillips and Clark2 proposal that model-assisted designs should be the go-to design for statisticians is timely. Statisticians have an opportunity to shape and improve early phase oncology drug development programs by introducing newer, more efficient model-based and model-assisted designs when estimating the MTD.
In addition, historically oncology drugs have been developed based on the belief that ‘more is better’ as supported by the frequent use of ‘3 + 3’ designs. However, this presumption is not true for many modern targeted drugs, immunotherapies, cell therapies and vaccines. Designs that focus on estimating the MTD alone can identify incorrect doses for future research. Not only does the early phase oncology research paradigm need to change to embrace more efficient designs, a refocusing to include study designs that estimate the Optimal Biological Dose (OBD), as appropriate, is needed; for example, the BOIN128 or TITE-BOIN129 study designs. This premise also aligns with regulators who are encouraging sponsors to change. Project Optimus is a FDA OCE regulatory initiative encouraging sponsors to identify the OBD.10 Statisticians have an opportunity to be at the forefront of these changes.
CONFLICT OF INTEREST
There is no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.




