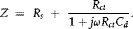On the Electropolishing Mechanism of Nickel Titanium in Methanolic Sulfuric acid − An Electrochemical Impedance Study
Abstract
Electropolishing of NiTi shape memory alloys is possible in methanolic 3 m H2SO4. The electro-dissolution behavior of NiTi in methanolic 3 m H2SO4 is ascertained in terms of Nyquist plots using electrochemical impedance spectroscopy (EIS) under limiting current flow (mass transfer control) condition. The electro-dissolution behavior is studied under convective conditions using a rotating disc electrode. The influence of changes in rotation rate, applied potential, and temperature are determined. This study demonstrates that electro-dissolution under mass transfer condition follows a compact salt-film mechanism. In order to quantitatively characterize the salt film formed during electropolishing, EIS is performed under stationary conditions. The increase in applied voltage causes an increase in polarization resistance and decrease in capacitance of the interface film.
1 Introduction
Equiatomic NiTi alloys are outstanding materials, owing to the fact that they show special thermo-mechanical properties such as shape memory effect and pseudoelasticity. These special properties make them a widely preferred material in biomedical applications.1 Apart from the mechanical properties, the material chemistry especially, the surface stability of the alloys has been revealed as an impacting factor for the usage as a bio-implant. Among various methods to achieve surface stability, electropolishing is one of the basic methods for the preparation of well-defined surfaces. This method is advantageous especially for shape memory alloys, as the surface undergoes neither thermal nor mechanical damage. Irrespective of whatever process an implant may undergo, the electropolishing is an inevitable process.2 Electropolishing improves the fatigue life by removing the residual stresses, smoothing stress risers, removing micro-cracks, and rounding corners.3 Thus, brightening or micromachining of NiTi becomes very important to be investigated.4-6 Another positive side effect of electropolishing resulting from the smooth and defect free surface is the reduction of susceptibility against corrosion.7, 8
The discussion on the electropolishing highlights three possible mass transport mechanisms namely, i) where the anodically generated metallic ion species (i.e., dissolving ion as the rate limiting species, salt-film mechanism); ii) acceptor anion (anions in electrolyte as rate controlling species, acceptor mechanism); and iii) water as transport limiting species.9 In a previous paper, voltammetric studies with a macroscopic stationary electrode revealed that anodic dissolution of binary NiTi in methanolic 3 m H2SO4 at 263 K provides a smooth surface and is the optimal condition for NiTi electropolishing and thereby electrochemical fabrication.10 Voltammetry utilizing a rotating disk electrode (RDE) elucidated that the dissolution limiting current is controlled by mass transport and gave hints that the dissolved species are the rate determining species.11 Similar behavior was found for a ternary shape memory alloys of NiTiCu type and for NbTi.12, 13 However, in the case of binary NiTi still a clear picture on the electro-dissolution mechanism is elusive. For example, it has not been yet known which of the electropolishing mechanisms (salt film mechanism or acceptor mechanism) holds good for binary NiTi and which of the elements is the rate determining species (either Ni/Ti or both).
- The measured ohmic resistance varies with applied potential or rotation speed;
- The effective capacitance changes with applied potential or rotation speed; or
- The polarization resistance changes with applied potential.
For a porous salt film in which ionic transport occurs in the electrolyte filling the pores of the film, condition (i) is fulfilled, while for a thin, compact film in which a solid-state conduction process occurs in parallel to capacitive charging of the film, conditions (ii) and (iii) are satisfied. Grimm et al.16 studied dissolution of iron in concentrated chloride media and found that all three conditions were fulfilled suggesting existence of an anodic salt film with a duplex structure that consists of an outer porous and an inner compact layer. For an acceptor mechanism, none of these conditions should apply. However, the system is expected to exhibit a Warburg − Nernst impedance indicating the mass transport behavior.
This study presents independent experiments carried out using a rotating disc electrode for a better understanding and stationary electrode measurements to quantitatively describe the mechanism.
2 Experimental Section
To understand the electro-dissolution kinetics of NiTi under convective conditions, electrochemical impedance spectroscopy (EIS) was carried out under limiting current conditions using a rotating disc electrode set up. NiTi (Ni55 at% − Ti45 at%) wires of 0.8 mm diameter were inserted in PTFE holders and exposed as rotating disc electrodes (A = 5.03 × 10−3 cm2). The discs were mechanically polished up to 4000 grit emery paper, ultrasonicated in ethanol, rinsed with distilled water and dried. The details of the rotating disc electrode and its controller were described earlier.11 The experiments under stationary conditions were performed using NiTi (50.62 at% – Ni 49.38 at%–Ti) alloy discs with 10 mm diameter and 2 mm thickness as specimens. After mechanical surface finishing with 1 μm diamond slurry, the specimen was introduced to an electrochemical cell with an O-ring limiting the exposed electrode area to 0.385 cm2.
The electrolyte used was methanolic 3 m H2SO4, which was prepared from analytical-grade reagent (Merck). Gold plates or wires were used as counter electrodes. The electrode potential was measured using an Ag/AgCl electrode at 3 m KCl solution with a Haber–Luggin capillary as reference electrode. In this paper, all potentials are expressed with the standard hydrogen electrode (SHE) potential at 298 K, though the experimental temperatures of 263 and 273 K were maintained using a cryostat (Lauda RC20-CP).
A computer controlled combination of a potentiostat and a frequency response analyzer (EG&G 273 and 1255B) was employed. To study the effect of rotation, EIS was carried out during anodic polarisation at constant potential E, which corresponds to the limiting current flow conditions and the rotation rate was varied. To study the effect of applied potential, the rotation rate was maintained constant at 400 rpm and E was varied, from 2.2 to 8.2 V in steps of 1 V. The frequency was swept down from 106 to 10 Hz for experiments with rotating disc and 105 to 10 Hz for experiments under stationary conditions. The amplitude of AC perturbation signal was 10 mV. It should be noted that there are differences in cell geometries and in operating conditions between RDE and stationary experiments.
3 Results and Discussion
Matlosz14, 15 brought out the differences in the impedance spectra that can be observed for different mechanisms of electropolishing namely, the salt film mechanism and the acceptor mechanism. Following the arguments put forward by Matlosz et al., it is possible to differentiate between the two mechanisms. For the NiTi system, here, the impedance behaviour under limiting current flow conditions is shown in Figure 1 and 2. Both figures have in common, that the spectra at high frequencies show semicircles corresponding to a charge transfer behaviour, whereas at low frequencies a line (transforms to a bow in certain cases) corresponding to a diffusion process.
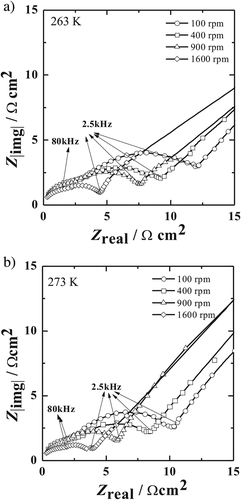
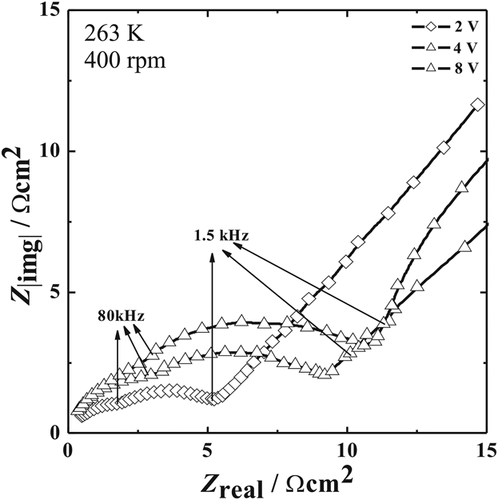
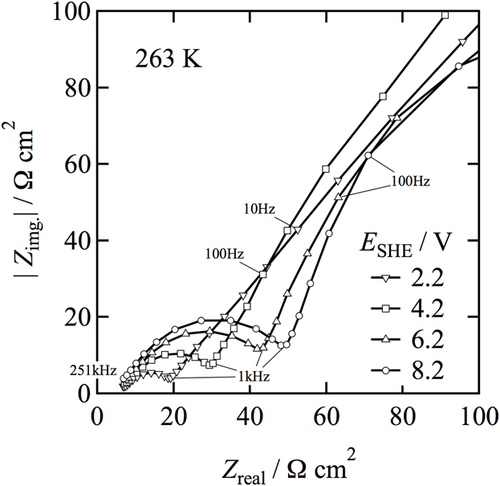
Figure 1a,b show the effect of rotation rate on the impedance behavior at two different temperatures (263 and 273 K). Qualitative analyses of the data reveals that an increase in rotation rate leads to a decrease in diameter of the semicircle with an only negligible change in the left intercept to the real part axis. As described by Matlosz,15 the left intercept to the real part is denoted as the high frequency impedance limit (Ro/Ω cm2). In the case of porous salt film model, this is related to the Ohmic resistance for a uniform current distribution (RΩ/Ω cm2) and the resistance of the porous film (per unit length)/Ω cm and the thickness of the porous film (λp/cm). This corresponds to Ohmic resistance for primary current distribution, RΩ, primary for a compact salt film model. A similar behaviour was reported by other authors.17, 18 It is well recorded that for a salt film model consisting of compact dielectric layers, the capacitance would vary with rotation rate, since the effective capacity is a function of the thickness of the dielectric film. It should be noted that an increased rotation rate leads to thinner films due to enhanced ion transport in the electrolyte resulting in film dissolution. Increase in the rotation rate leads to a decrease in resistance and an increase in effective capacitance.14-18 Figure 2 shows the effect of applied potential on the impedance behaviour recorded at 263 K and at constant rotation rate of 400 rpm. It can be seen that the diameter of the semicircle arc observed at high frequencies increases with increasing potential E, while no significant change occurs in the left intercept to the real part axis.
Careful examination of the high frequency regime in Figures 1 and 2 shows two overlapping semicircles. The presence of a smaller semicircle in the frequency range 106 to 8 × 104 Hz and a larger one beyond 8 × 104 down to 2.5 × 103 Hz indicates two distinct time dependent processes. The reason for the observation of the smaller loop at high frequencies (106 to 8 × 104 Hz) could be associated to the finite length of the diffusion layer under specific hydrodynamic conditions.19 Additionally the high frequency regime does not show a perfect semicircle that is typical for an ideal capacitive behavior but one with a certain depression. The depression in loop could be due to the overlap of the two semicircle arc and/or the non-uniform current distribution the surface of the disc electrode.20, 21 Moreover, it is common practice to measure impedance spectra starting from frequencies 105 Hz10, 16 or even if measured from 106 Hz onward, data fitting is performed only from 105 Hz.15 The reason is that access to the high frequency range in electrochemical impedance spectroscopy is sensitive to artefacts such as a phase shift in the current amplifier of the frequency response analyzer or the potentiostat. There are measures to reduce this effect by using electrolytes of higher conductance, the use of reference electrodes with a low inner resistance or even the use of two different reference electrodes with a frequency separating filter.
Due to the discrepancies seen in impedance spectra obtained from rotating disc electrode experiments, these results were used only for a qualitative description of the mechanism.
Hence, a quantitative discussion is carried out based on the results obtained from stationary experiments. The impedance measurement carried out under stationary conditions is given in Figure 3. This show only a single semicircle, which rules out the two distinct time dependant processes as recorded with rotating disc electrode experiments. Figure 3 shows a Nyquist plot when NiTi is polarized at E, at which the electro-dissolution of NiTi is under limiting current flowing condition. It demonstrates the Randles type in which a semicircle at high frequencies corresponding to the Faradaic charge transfer behavior and a line at low frequencies corresponding to the linear diffusion process of electro-active species. The diameter of the semicircle arc observed at frequencies higher than 1 × 103 Hz increases with increase in E while no change in the left intercept to the real part axis is found. The line observed at frequencies lower than 1 × 103 Hz is straight with a slope of unity at E = 2.2–4.2 V but might transform to a part of an arc of a second larger semicircle at E = 5.2–8.2 V. This means that the equivalent circuit model of the interface at higher potentials would need to be extended. However, at low frequencies, it is difficult to obtain reproducible data in the polishing system undergoing high-rate dissolution. Therefore it is of interest to discuss the more reproducible data that is obtained at high frequencies.

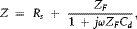 (1)
(1) is the angular frequency,
is the angular frequency, 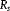 is the resistance of electrolyte solution, and
is the resistance of electrolyte solution, and 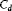 is the capacitance of the electrode/electrolyte interface. The reaction kinetics and diffusion are characterized by the Faradaic impedance
is the capacitance of the electrode/electrolyte interface. The reaction kinetics and diffusion are characterized by the Faradaic impedance 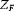 that is composed of the resistance of charge transfer
that is composed of the resistance of charge transfer 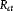 in series with Warburg impedance
in series with Warburg impedance  describing the diffusion behavior.
describing the diffusion behavior.
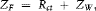 (2)
(2)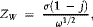 (3)
(3) is the Warburg coefficient related to concentration
is the Warburg coefficient related to concentration 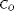 and diffusion coefficients
and diffusion coefficients 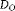 of oxidized and reduced species as the following:
of oxidized and reduced species as the following:
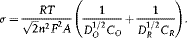 (4)
(4)
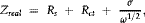 (6a)
(6a)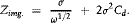 (6b)
(6b)The value of  can be deduced from the so-called Randles plot, a plot of
can be deduced from the so-called Randles plot, a plot of 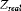 and
and 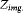 against
against 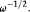
The values of Rs, Rct, Cd, and σ were derived by fitting to the model of Figure 4b as shown in Figure 5. The values of Rct and 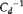 depend on E in the potential range of 2.2–8.2 V which allows to flow a limiting current.
depend on E in the potential range of 2.2–8.2 V which allows to flow a limiting current.  and
and 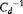 increase linearly with increase in E, indicating that a layer on the electrode surface thickens to compensate the potential drop along the limiting current plateau. The value of σ corresponding to the concentration of dissolving species at the electrolyte/electrode interface is almost independent of E, suggesting that the dissolution is under diffusion control. It is in full agreement with the compact salt-film model, which was successfully applied to the electro-dissolution kinetics for titanium17 or tantalum18 in methanol concentrated with H2SO4.
increase linearly with increase in E, indicating that a layer on the electrode surface thickens to compensate the potential drop along the limiting current plateau. The value of σ corresponding to the concentration of dissolving species at the electrolyte/electrode interface is almost independent of E, suggesting that the dissolution is under diffusion control. It is in full agreement with the compact salt-film model, which was successfully applied to the electro-dissolution kinetics for titanium17 or tantalum18 in methanol concentrated with H2SO4.

Figure 6 shows a comparison of Nyquist plots for pure nickel, pure titanium and NiTi. The semicircle arcs are also obtained at frequencies higher than 1 × 103 Hz in the plots of both nickel and titanium. Both diameters of the semicircles increased with increasing E while there were no changes in the left intercept to the real part axis, indicating that the compact salt-film model is also applicable to the dissolution of pure nickel. The straight lines are also obtained at frequencies lower than 1 × 103 Hz in the plots of both nickel and titanium. However, the shape of the NiTi locus is close to that of titanium rather than nickel whose slope is steeper. Obviously the diffusion control mechanism for NiTi coincides with that for titanium and the diffusion of dissolved Ti species from the NiTi anode to the solution is identified as the rate-determining step in the electro-dissolution.

A better understanding of the electropolishing mechanism of NiTi is important not only for this material alone but also similar binary alloys such as NiW,22 TiMo,23 or ternary. Nevertheless it should be emphasized that electropolishing is not the only step or only way to achieve better NiTi surfaces. There are various methods such as electrochemical etching in neutral fluoride solutions,24 passivation,5 plasma treatment,25 selective surface oxidation and nitridation,26 or oxochloridation.27
4 Conclusions
- The qualitative study using RDE allowed to identify the effect of rotation rate and applied voltage. The increase in rotation rate leads to a decrease in polarization resistance and variation in capacitance. On the contrary, the increase in applied potential revealed an increase in polarization resistance and decrease in capacitance.
- The study under stationary conditions allowed quantitative characterisation of the salt film. It corroborates the results obtained using the rotating disc electrode. As an influence of applied potential,
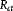 and
and 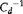 increase linearly with increase in E, indicating that a layer on the electrode surface thickens to compensate the potential drop along the limiting current plateau.
increase linearly with increase in E, indicating that a layer on the electrode surface thickens to compensate the potential drop along the limiting current plateau. - This study confirms that the electropolishing of NiTi follows a compact salt film mechanism.
- The EIS behavior exhibited by Ni, Ti, and NiTi show that diffusion of dissolving Ti species is most likely the mass transfer limiting species.
Acknowledgements
Financial support from the FFG through the Competence Center for Electrochemical Surface Technology in the strategic project UPPER is gratefully acknowledged. The authors thank the Sonderforschungs Bereich 459 of the Deutsche Forschungemeinschaft. Financial assistance of the Max-Planck-Gesellschaft through a Max-Planck fellowship is gratefully acknowledged (K.F.). L.N. thanks the IMPRS-SurMat for the financial support. Some parts of the experiment were conducted at the Max Planck Institut für Eisenforschung GmbH, Düsseldorf, Germany which is gratefully acknowledged.
Conflict of Interest
The authors declare no conflict of interest.



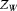 becomes negligible and eq.
becomes negligible and eq.