Hormone Therapy Usage Is Associated With Adverse Cardiovascular Events in Prostate Cancer Patients of the All of Us Research Program Cohort
ABSTRACT
Background
Hormone therapy (HT) has greatly improved overall survival for prostate cancer patients, but may also influence cardiovascular health in an already high-risk population.
Methods
This retrospective cohort study examined participants in the All of Us Research Program with prostate cancer, had no prior history of adverse cardiovascular events, and were either treated or not treated with HT. HT was defined as GnRH agonists, GnRH antagonists, abiraterone, androgen antagonists, or androgen receptor pathway inhibitors. We defined adverse cardiovascular event as myocardial infarctions, heart failure, or strokes. Time to adverse cardiovascular event was defined using longitudinal electronic health record data. We evaluated whether HT use affected the risk of adverse cardiovascular events using a Cox regression model adjusted for established cardiovascular risk factors.
Results
The final cohort included 5156 participants. After adjustment for cardiovascular risk covariates, HT treatment was associated with increased risk of adverse cardiovascular event (HR: 1.22; 95% CI: 1.01–1.48; p = 0.03). In participants with pre-treatment dyslipidemia, HT usage was associated with increased risk of adverse cardiovascular events (HR: 1.52; 95% CI: 1.19–1.95; p < 0.001). In participants without pre-treatment dyslipidemia, no association was found (HR: 0.96; 95% CI: 0.71–1.30; p = 0.81).
Conclusions
Our results show that HT-associated cardiovascular risk may be synergistically amplified by dyslipidemia. These results suggest that risk stratification by dyslipidemia status may improve cardiovascular outcomes for prostate cancer survivors.
1 Introduction
Prostate cancer (PCa) is the most common non-cutaneous malignancy in men in the United States [1], with over 200,000 new cases and 30,000 deaths annually [2]. Androgens drive PCa progression [3], making hormone therapy (HT) a cornerstone of treatment for both localized and advanced disease [4, 5]. However, HT has been associated with lipid dysregulation, insulin resistance, and increased blood pressure [6-10]. Given the role of androgens in cardiac myocyte physiology, HT has also been associated with QTc interval prolongation and QRS interval shortening [11, 12].
Since 2006, the link between HT and adverse cardiovascular events (ACE) has been supported by observational studies [13-16]. However, randomized controlled trials (RCTs) have shown conflicting results. For example, while the phase III RTOG 85-31 trial found no increased cardiovascular (CV) mortality with HT [17], a meta-analysis of five RCTs showed a significant increase in CV events [18]. Evidence also suggests that CV risk varies by HT type. For example, gonadotropin-releasing hormone (GnRH) antagonists seem to pose a lower risk than GnRH agonists [19] and adding androgen receptor pathway inhibitors (ARPI) to a HT regimen further increases CV risk [20].
CV disease is the most common non-cancer cause of death for PCa patients [21, 22]. As both life expectancy and HT duration continue to increase, CV risk has become more prominent in PCa patients. This risk has in turn prompted caution from the American Heart Association, American Cancer Society, and American Urological Association on the usage of HT [23]. Currently, no standardized guidelines exist for assessing the ACE risk associated with HT. Additionally, the role of common CV risk factors like dyslipidemia, hypertension, and Type 2 diabetes in HT-induced ACE remains unclear. The finite follow up period of RCTs [24, 25] further complicates the application of these findings to the general population, highlighting the need for better risk stratification.
The All of Us Research Program is an actively enrolling, national cohort study. Currently, over 400,000 participants are enrolled [26]. To better account for real-world patient treatment patterns, we sought to further understand the risk of ACE with HT usage in All of Us.
2 Materials/Subjects and Methods
2.1 Study Design
This retrospective cohort study utilized the Controlled Tier Dataset v7. Data analysis was conducted from February 10, 2024 to August 17, 2024.
2.2 Study Population
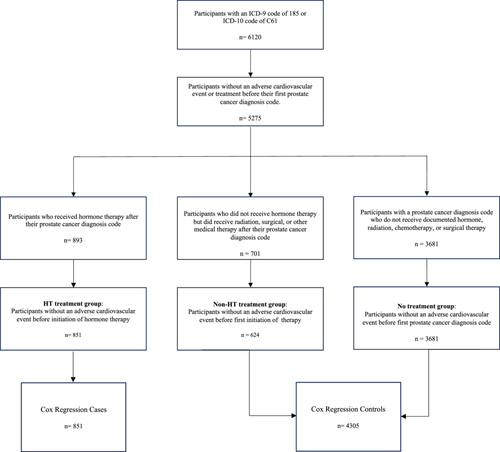
We identified 6120 participants with an International Classification of Diseases (ICD) Ninth Revision (ICD-9) code of 185 or an ICD-10 code of C61, which represents a diagnosis of “Malignant Neoplasm of the Prostate.” These participants were then categorized into three groups: HT treatment, non-HT treatment, and no treatment. Participants in the HT treatment group had their first exposure to a HT after the appearance of a PCa ICD code. Participants in the non-HT treatment group were not exposed to HT, but received either surgical therapy, radiation therapy, or non-HT medical therapy after their first documented PCa diagnosis (Supporting Information S1: Table S1). Participants in the no treatment group did not have any previously defined PCa therapies after their first PCa diagnosis date, which was suggestive of patients who were under active surveillance for their PCa. Index date for participants in the HT treatment group and non-HT treatment group were defined as the earliest date of HT exposure and non-HT treatment, respectively. Participants in the no treatment group had their index date set to the first date of their PCa diagnosis. We excluded from analysis 845 participants with PCa who had their first ACE before or on their index date.
2.3 Exposures
The primary exposure of our study was HT. We additionally stratified HT treatment by regimens. A HT drug was included in a regimen if a participant had exposure to the drug after PCa diagnosis (Full list of HT drugs available in Supporting Information S1). HT regimens included in our analysis were: GnRH agonist only, GnRH antagonist only, GnRH agonist and antagonist, combined androgen blockade (CAB), and anti-androgen only. Participants in the GnRH agonist only and GnRH antagonist only regimens received only those HT. Participants in the GnRH agonist and antagonist group have a history of receiving both GnRH agonists and GnRH antagonists, but no anti-androgen drugs. Participants in the CAB regimen received a GnRH agonist and/or a GnRH antagonist, in addition to an anti-androgen. Participants in the anti-androgen only group received exclusively anti-androgens as part of their treatment regimen. Duration of HT exposure was defined as the first known date of exposure to the most recent of either the last known date of exposure or the known end date.
Our non-HT treatment control group participants were exposed to non-HT medical therapy, surgery, and/or radiation for their PCa. Within this group, 30 participants had non-HT medical therapy, 262 had surgery, and 336 had radiation as their first treatment.
2.4 Outcomes
The primary outcome measured was ACE time to event, which was defined as the interval from the index date until the date of first ACE. We defined ACE as the appearance of an ICD-9 or ICD-10 diagnosis code for myocardial infarction, stroke, or heart failure (Supporting Information S1: Table S2). Participants who did not develop an ACE were right censored at their last visit to a healthcare provider. We compared the time-to-event between the HT, non-HT, and no treatment groups.
Secondary outcomes measured were changes in QRS, QTc, and PR intervals. Within our final cohort, we utilized measured electrocardiogram (ECG) intervals before and after a participant's index date. To be most representative of a patient's baseline physiology, we removed interval values that indicated acute pathologies (QTc > 500 ms, QRS > 120 ms, PR > 220 ms). Following this, we calculated the difference between the most recent interval after a participant's index date to that of the most recent ECG reading before. We then compared the intervals between the HT treatment group with the combined non-HT treatment and no treatment groups.
2.5 Covariate Measurement
Covariates included in the primary Cox regression model were pre-selected based on known cardiac risk. Covariates used in our primary analysis were age, dyslipidemia, Type 2 diabetes, hypertension, chronic kidney disease, peripheral vascular disease, statin usage, self-reported race/ethnicity, and smoking history. Dyslipidemia, Type 2 diabetes, hypertension, chronic kidney disease, and peripheral vascular disease were defined as the presence of an ICD-9 or ICD-10 diagnosis code on or before a participant's index date (Supporting Information S1: Table S3). Statin usage was defined as the presence of a statin drug exposure on or before a participant's index date Self-reported race, ethnicity, and smoking history were ascertained through the All of Us survey data.
We also included metastasis status as a covariate in a subgroup analysis of the HT and non-HT treatment groups. Metastasis status was predicted as the documentation of ICD-9/ICD-10 diagnosis code of “secondary malignancy” (Supporting Information S1: Table S3) after the first ICD-9/ICD-10 PCa diagnosis code and before a participant's index date. This approach is similar to a previous method for identifying metastatic PCa in structured electronic health record (EHR) data [27].
2.6 Statistical Analyses
Kaplan–Meier curves were utilized to show the cumulative occurrence of ACE events over time. Results between treatment groups were compared using log-rank tests. To evaluate the association of potential risk factors, we utilized a Cox proportional hazards model adjusting for covariates. Pre-planned interactions terms analyzed corresponded to the effects of HT on baseline physiology, and were HT × Type 2 Diabetes, HT × Hypertension, and HT × Dyslipidemia.
Statistical comparisons between ECG interval changes across treatment groups were performed using unpaired t-tests. Interaction p-values were calculated using likelihood ratio tests comparing Cox models with and without interactions. We generated survival curves with ggsurvplot/ggplot2 and conducted Cox regression analyses with the survival package in R (version 4.4.0). All p-values were two-sided with significance set at 0.05. All data analyses were conducted using R in the All of Us Researcher Workbench.
3 Results
3.1 Baseline Characteristics
Our final study cohort included 5156 participants: 851 participants in the HT treatment group, 624 participants in the non-HT treatment group, and 3681 participants in the no treatment group (Figure 1). Baseline characteristics and demographic information for the cohort are shown in Table 1. In the HT treatment group, 136 (16.0%) participants developed an ACE after their index date. In the non-HT treatment group and the no treatment group, 128 (20.5%) and 581 (15.8%) participants, respectively, developed an ACE. Mean EHR follow up after index date was 4.2 years for the HT treatment group, 7.3 years for the non-HT treatment group, and 6.1 years for the no treatment group. A total of 719 (13.9%) of our cohort self-identified as Black/African-American and 383 (7.4%) self-identified as Hispanic/Latino.
| Cohort (n = 5156) | HT treatment (n = 851) | Non-HT treatment (n = 624) | No treatment (n = 3681) | |
|---|---|---|---|---|
| Age at index date, mean (SD) | 66.2 years (8.5) | 68.5 years (8.2) | 65.0 years (8.3) | 65.9 years (8.5) |
| EHR follow-up time after index datea, mean (SD) | 5.9 years (5.3) | 4.2 years (3.8) | 7.4 years (6.1) | 6.1 years (5.4) |
| Self-reported race or ethnicityb, No. (%) | ||||
| Asian | 42 (0.8%) | ≤ 20 (≤ 2.3%) | ≤ 20 (≤ 3.2%) | 28 (0.8%) |
| Black/African-American | 719 (14.0%) | 135 (16.0%) | 67 (10.7%) | 517 (14.0%) |
| Hispanic/Latino | 383 (7.4%) | 67 (7.9%) | 44 (7.1%) | 272 (7.4%) |
| White | 3716 (72.1%) | 590 (69.3%) | 470 (75.3%) | 2656 (72.2%) |
| Pre-existing cardiovascular risk distribution, No. (%) | ||||
| Dyslipidemia | 2373 (46.1%) | 451 (53.0%) | 339 (54.3%) | 1587 (43.1%) |
| Type 2 diabetes | 726 (14.1%) | 164 (19.3%) | 88 (14.1%) | 474 (12.9%) |
| Hypertension | 2334 (45.3%) | 467 (54.9%) | 303 (48.6%) | 1564 (42.4%) |
| Chronic kidney disease | 354 (6.8%) | 67 (7.9%) | 59 (9.5%) | 228 (6.2%) |
| Peripheral vascular disease | 234 (4.5%) | 64 (7.5%) | 30 (4.8%) | 140 (3.8%) |
| Statin usage | 1766 (34.3%) | 378 (44.4%) | 248 (39.7%) | 1140 (31.0%) |
| Smoking history | 2433 (47.2%) | 430 (50.5%) | 299 (47.9%) | 1704 (46.3%) |
| Adverse cardiovascular events outcomes, No. (%) | 848 (16.4%) | 136 (16.0%) | 128 (20.5%) | 584 (15.9%) |
| Heart failure | 361 (7.0%) | 57 (6.7%) | 51 (8.2%) | 253 (6.9%) |
| Myocardial infarction | 198 (3.8%) | 33 (3.9%) | 32 (5.1%) | 133 (3.6%) |
| Stroke | 289 (5.6%) | 46 (5.4%) | 45 (7.2%) | 198 (5.4%) |
- Abbreviations: EHR, electronic health record; HT, hormone therapy.
- a EHR follow-up time is defined as the amount of time after a participant's index date until either right censoring or adverse cardiovascular event.
- b All of Us prohibits reporting participant cohorts that have equal to or less than 20 participants. All categories with equal to or less than 20 participants are thus reported as ≤ 20. We additionally did not report participants who self-identified as “Middle Eastern or North African,” “Native Hawaiian or Other Pacific Islander,” or responded “none of these,” “more than one population,” “prefer not to answer,” or skipped the question due to risk of re-identification.
3.2 ACE Risk After HT Treatment
Unadjusted Kaplan–Meier curves (Figure 2) showed that participants in the HT treatment group had lower time-to-event than either the non-HT treatment group (log rank test; p = 0.004) or the non-treatment group (log rank test; p < 0.001). There was no difference between the non-HT treatment group and the no treatment group (log rank test; p = 0.57). Because of this, we combined the non-HT treatment group and no treatment groups into our reference group for our Cox proportional hazard models (Supporting Information S1: Table S4). In an unadjusted, univariate Cox regression, HT treatment was associated with decreased ACE time-to-event (HR: 1.45; 95% CI: 1.20–1.75; p < 0.001). After adjustment for baseline cardiovascular covariates in our multivariate analysis, HT treatment remained associated with lower ACE time-to-event (HR: 1.22; 95% CI: 1.01–1.48; p = 0.03). Independent risk factors for ACE time-to-event were pre-existing Type 2 diabetes, hypertension, chronic kidney disease, age, and smoking history. Statin usage was associated with a protective effect. Self-identification as Black/African-American (HR: 1.29; 95% CI: 1.04–1.58; p = 0.02) and as Hispanic/Latino (HR: 1.51; 95% CI: 1.16–1.96; p = 0.002) was found to be associated with ACE time-to-event (Supporting Information S1: Table S4). Multivariable Cox models of the Black/African-American or Hispanic/Latino participant subgroups are shown in Supporting Information S1: Table S7.

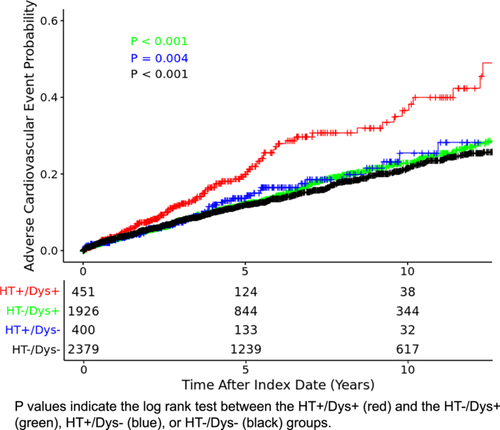
3.3 HT Interaction With Dyslipidemia
We found that the HT and dyslipidemia interaction term was significant in our multivariate analysis (HR: 1.57; 95% CI: 1.03–2.34; p = 0.03), which suggested a synergistic effect on ACE risk between HT and pre-treatment dyslipidemia. No other pre-determined interaction terms were found to be significant (Supporting Information S1: Table S5). As a result, we both created a Kaplan–Meier curve (Figure 3) and stratified our Cox model based on participant baseline dyslipidemia status (Table 2). Our Kaplan–Meier curve (Figure 3) showed that participants on HT and with dyslipidemia had lower time-to-event than the participants on HT without dyslipidemia (log rank test; p = 0.004), non-HT group with dyslipidemia (log rank test; p < 0.001) and the non-HT group without dyslipidemia (log rank test; p < 0.001). In the Cox model, HT treatment was associated with ACE in participants with pre-existing dyslipidemia (HR: 1.52; 95% CI: 1.19–1.95; p < 0.001). In participants without pre-existing dyslipidemia, HT had no association with ACE (HR: 0.96; 95% CI: 0.71–1.30; p = 0.81). Similarly, statin usage was associated with a reduced risk for cardiac events in participants with dyslipidemia (HR: 0.72; 95% CI: 0.59–0.89; p = 0.002). In participants without dyslipidemia, no significant association was found between statin usage and cardiac events (HR: 0.90; 95% CI: 0.65–1.23; p = 0.50).
| Pre-treatment Dyslipidemia (n = 2377) | No pre-treatment Dyslipidemia (n = 2779) | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Hormone therapy | 1.52 (1.19–1.95) | < 0.001 | 0.96 (0.71–1.30) | 0.81 |
| Type 2 diabetes | 1.34 (1.07–1.70) | 0.01 | 1.18 (0.79–1.76) | 0.41 |
| Hypertension | 1.68 (1.31–2.15) | < 0.001 | 1.43 (1.15–1.78) | 0.001 |
| Chronic kidney disease | 1.96 (1.46–2.62) | < 0.001 | 1.45 (0.85–2.47) | 0.18 |
| Peripheral vascular disease | 1.47 (1.02–2.01) | 0.04 | 1.10 (0.54–2.26) | 0.79 |
| Age | 1.05 (1.03–1.06) | < 0.001 | 1.04 (1.03–1.05) | < 0.001 |
| Statin usage | 0.72 (0.59–0.89) | 0.002 | 0.90 (0.65–1.23) | 0.50 |
| Smoking history | 1.18 (0.96–1.45) | 0.11 | 1.20 (1.00–1.44) | 0.05 |
3.4 Stratification by HT Regimen
Next, we included HT regimen as a covariate in our analysis. In an adjusted Cox regression model, the CAB regimen was associated with decreased ACE time-to-event in the overall cohort (HR: 1.31; 95% CI: 1.03–1.68; p = 0.03). No other treatment regimen was found to be associated with ACE time-to-event. In participants with pre-existing dyslipidemia (Table 3), the CAB regimen showed increased risk of ACE (HR: 1.58; 95% CI: 1.14–2.19; p = 0.006). No association was appreciated between the CAB regimen and ACE time-to-event in participants without pre-existing dyslipidemia (HR: 1.08; 95% CI: 0.74–1.58; p = 0.70). Similar associations were seen when anti-androgen drugs in the CAB regimen were limited to abiraterone and ARPI (Supporting Information S1: Table S6).
| Pre-treatment Dyslipidemia (n = 2377) | No pre-treatment Dyslipidemia (n = 2779) | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| GnRH agonist only (n ≤ 20) | 1.51 (0.21–10.80) | 0.68 | 1.46 (N/A) | 0.99 |
| GnRH antagonist only (n = 260) | 1.51 (1.02–2.22) | 0.038 | 0.71 (0.38–1.34) | 0.27 |
| GnRH agonist and antagonist (n ≤ 20)a | 4.39 (0.61–31.6) | 0.14 | 1.45 (0.20–10.4) | 0.72 |
| Combined androgen blockade (n = 409)b | 1.58 (1.14–2.19) | 0.006 | 1.08 (0.74–1.58) | 0.70 |
| Anti-androgen only (n = 152) | 1.34 (0.77–2.35) | 0.31 | 1.09 (0.57–2.05) | 0.84 |
| No hormone therapy (n = 4305) | Reference | Reference | Reference | Reference |
- Note: Additional covariates utilized in this model were age, dyslipidemia, Type 2 diabetes, hypertension, chronic kidney disease, peripheral vascular disease, statin usage, and smoking history.
- Abbreviations: HR, hazard ratio; N/A, not available due to small sample size.
- a Participants in the GnRH agonist and antagonist group had both exposures to GnRH agonists and GnRH antagonists after their first prostate cancer ICD code. This is likely due the result of the participant switching between hormone therapy regimens.
- b Participants in the combined androgen blockage group received either a GnRH agonist or GnRH antagonist, in addition to an anti-androgen after their first prostate cancer ICD code.
3.5 Pre-Treatment Metastasis in HT and Non-HT Treatment Groups
Additionally, we assessed the effects of pre-treatment metastatic disease in a Cox model of the combined HT and non-HT treatment groups (Supporting Information S1: Table S8). In the HT treatment group, 158 participants had metastatic disease before their index date. In the non-HT treatment group, 32 had metastatic disease before their index date. The presence of metastatic disease was not associated with decreased ACE time-to-event (HR: 1.33; 95% CI: 0.93–1.88; p = 0.11). The HT and dyslipidemia interaction continued to be significant in this sub-group analysis (HR: 1.82; 95% CI: 1.07–3.10; p = 0.03).
3.6 Androgen Receptor Pathway Inhibitors in HT Treatment Groups
We also individually examined the ARPIs enzalutamide, darolutamide, and apalutamide. In total, 106 participants in our cohort were exposed to an ARPI. A total of 86 of these participants were exposed to enzalutamide, ≤ 20 were exposed to darolutamide, and ≤ 20 were exposed to apalutamide. We then created a Cox proportional hazards model of the HT treated cohort with the addition of individual APRI associations. Exposure to enzalutamide was associated with increased cardiac risk (HR: 2.20; 95% CI: 1.42–3.41; p = < 0.001). Exposure to apalutamide (HR: 1.11; 95% CI: 0.35–3.55; p = 0.86) or darolutamide (HR: 1.71; 95% CI: 0.41–7.03; p = 0.46) was not associated with increased cardiac risk. These results have been included as Supporting Information S1: Table S9.
3.7 Duration of Exposure in HT Treatment Group
We additionally stratified the HT treatment group into subgroups based on duration of HT exposure: 0–6 months, 6–36 months, and 36+ months. These intervals were selected to align with clinical guidelines and prior literature for short term, long term, and continuous HT treatment, respectively [28-30]. In the subsequent analysis, we substituted the binary HT treatment group variable with these stratified exposure intervals in a Cox proportional hazards model. Out of the 851 participants who received HT, 315 were exposed for 0–6 months, 345 for 6–36 months, and 191 for 36+ months. Our results show stronger associations in the previously noted HT × Dyslipidemia interaction term with progressive HT exposure intervals (Supporting Information S1: Table S10), suggesting dose-dependency of the relationship between HT × Dyslipidemia and ACE risk.
3.8 ECG Intervals
There were 127 participants with measured QTc intervals in the cohort, 122 participants with measured QRS intervals, and 108 participants with measured PR intervals both before and after their index date (Supporting Information S1: Table S11). We observed a significant increase in measured QTc intervals after index date when comparing the HT treatment participants to the combined non-HT treatment and no treatment participants (p = 0.03). No differences were observed in the QRS and PR intervals. No participant in our cohort had an ICD-9/ICD-10 diagnosis code for torsades de pointes (TdP).
4 Discussion
Currently, there exists no standardized guidelines in assessing ACE risk for patients who start HT treatment. Furthermore, because of the complexity of CV disease drivers and the large number of PCa therapies available, there is no clear consensus about how to implement changes from observed associations into the real-world clinical setting. Previous studies have demonstrated that HT use is associated with an increased mortality risk in patients with high CV disease risk, but not among low risk [19]. Our findings corroborate this study but uniquely identify dyslipidemia as a modifiable risk factor, while also observing that this interactive risk may progressively increase with longer durations of HT. To our knowledge, this is the first documentation in the literature of an interactive effect for HT-associated CV risk. Previous mechanistic studies suggest that HT promotes endothelial dysfunction and inflammation [31-33], and we hypothesize that HT synergizes with abnormal lipid metabolism to accelerate atherosclerotic plaque formation and rupture. In other words, our results imply that HT exacerbates plaque instability specifically in dyslipidemic environments.
In our cohort, we also observed that CAB usage was associated with greater ACE risk. The increased cardiotoxicity of CAB has been previously noted in randomized controlled trials [34]. One posited explanation is that CAB regimens have greater potency when compared to single HT agent regimens and thus lead to increased downstream toxicities associated with androgen deprivation. After limiting the anti-androgen drugs in the CAB regimen to only ARPIs and abiraterone, our results remain congruent with recent studies detailing the CV risk posed by these specific drugs (Supporting Information S1: Table S6).
ARPIs are advancements that have revolutionized metastatic and non-metastatic castration-resistant PCa treatment. However, their cardiovascular risk profiles have not been completely defined. Our results suggest that enzalutamide usage may be associated with increased cardiac risk. A limitation of this in our cohort is that ARPI usage was often in the context of the CAB regimen, as 86 out of the 106 participants who received an ARPI also received other HT. Future ARPI monotherapy studies may be required to thoroughly evaluate CV risk.
Black/African-American men are known to have a higher incidence of PCa compared to White men [35]. Furthermore, Black/African-American individuals suffer from greater cancer-related and CV mortality in the setting of a PCa diagnosis [36, 37]. On the other hand, Hispanic/Latino individuals with PCa are suggested to be at decreased risk of CV mortality compared to other race/ethnicities [38, 39]. After adjusting for baseline CV covariates, our analysis indicates that both self-identified Black/African-American and self-identified Hispanic/Latino participants in our cohort were at independently elevated risk of ACE.
The effects of HT on QTc interval have been previously documented in both prospective clinical studies and in in-vitro experiments but is not currently recognized in clinical practice. Uniquely, our study replicates these findings of QTc prolongation in a real-world cohort. A prolonged QTc interval is associated with the lethal cardiac arrhythmia TdP. However, no TdP events were documented in our cohort and cardiac specific death was not collected by the All of Us Research Program. As such, our results suggest that additional research may be needed to determine clinical significance in this patient population.
Cancer staging and Gleason score are predictors of all-cause and cancer-specific mortality in PCa patients [40-43]. It remains unknown if these factors directly affect risk of cardiac diseases due to the presence of multiple confounding variables that include HT usage [44]. After adjusting for metastasis status in our HT and non-HT treatment groups, our study supports a possibility of no association. Future studies may be warranted to explore the complex interplay between Gleason scores/cancer staging, treatment regimen, and CV outcomes in PCa patients.
4.1 Limitations
Limitations of this study include its retrospective nature, exclusion of surgical castration, low power to meaningfully evaluate mortality, and the lack of available cancer staging or Gleason score data. In addition, we were unable to completely exclude the possibility of residual confounding due to treatment indications.
5 Conclusion
In conclusion, ACE are the most common cause of non-cancer mortality in patients with PCa. Leveraging data from the All of Us cohort, we showed that treatment with HT is associated with a higher risk of ACE in PCa patients with pre-existing dyslipidemia. Our data also suggests that the usage of CAB may have higher CV risk than other HT regimens, especially in patients with dyslipidemia. These findings suggest areas for future research, including optimal approaches for monitoring lipid levels during HT treatment.
Author Contributions
Yuanchu J. Yang and Lincoln A. Brown initiated the main conceptual ideas behind the project. Yuanchu J. Yang, Kerry R. Schaffer, and Chenjie Zeng wrote the manuscript. Yuanchu J. Yang, Chenjie Zeng, Kerry R. Schaffer, Tam C. Tran, Peter J. Sauer, Lincoln A. Brown, Ben H. Park, and Joshua C. Denny designed the research. Yuanchu J. Yang, Chenjie Zeng, and Joshua C. Denny performed the research and analyzed the data. All authors provided critical feedback that shaped the research, analysis, and manuscript.
Acknowledgments
We thank all participants enrolled in the All of Us Research Program for their trust and partnership, without which this study would not have been possible. This study was supported by the National Human Genome Research Program Intramural Research Program (Grant Number: ZIA HG200417).
Ethics Statement
This study was performed in accordance with the Declaration of Helsinki.
Consent
Participants enrolled in the All of Us Research Program provided consent for their deidentified electronic health record to be used for research and usage was determined by the All of Us Research Program institutional review board to be non-human subject research.
Conflicts of Interest
The authors declare the following competing interests: B.H.P. is a paid consultant for Jackson Labs, Jansen, Hologics, EQRx, Guardant Health, Caris and is a paid scientific advisory board member for Celcuity Inc. B.H.P. is an unpaid consultant for Tempus Inc. Under separate licensing agreements between Horizon Discovery, LTD, and The Johns Hopkins University, B.H.P. is entitled to a share of royalties received by the University on sales of products. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflicts of interest policies. The other authors declare no conflicts of interest.
Open Research
Data Availability Statement
The dataset analyzed in the current study, as well as further information on the All of Us Research Program cohort, is available at: https://allofus.nih.gov/ and https://workbench.researchallofus.org/.