Immune checkpoint B7-H3 protein expression is associated with poor outcome and androgen receptor status in prostate cancer
Abstract
Background
Novel immune checkpoint-based immunotherapies may benefit specific groups of prostate cancer patients who are resistant to other treatments.
Methods
We analyzed by immunohistochemistry the expression of B7-H3, PD-L1/B7-H1, and androgen receptor (AR) in tissue samples from 120 prostate adenocarcinoma patients treated with radical prostatectomy in Spain, and from 206 prostate adenocarcinoma patients treated with radical prostatectomy in Norway.
Results
B7-H3 expression correlated positively with AR expression and was associated with biochemical recurrence in the Spanish cohort, but PD-L1 expression correlated with neither of them. Findings for B7-H3 were validated in the Norwegian cohort, where B7-H3 expression correlated positively with Gleason grade, surgical margins, seminal vesicle invasion, and CAPRA-S risk group, and was associated with clinical recurrence. High B7-H3 expression in the Norwegian cohort was also consistent with positive AR expression.
Conclusion
These results suggest distinct clinical relevance of the two immune checkpoint proteins PD-L1 and B7-H3 in prostate cancer. Our findings highlight B7-H3 as an actionable novel immune checkpoint protein in prostate cancer.
Abbreviations
-
- AR
-
- androgen receptor
-
- BR
-
- biochemical recurrence
-
- CAPRA-S
-
- cancer of the prostate risk assessment postsurgical
-
- DAB – 3′
-
- diaminobenzidine
-
- FFPE
-
- formalin fixated paraffin embedded
-
- HE
-
- hematoxyiln and eosin
-
- HR
-
- hazard ratio
-
- HRP
-
- horseradish peroxidase
-
- ICI
-
- immune checkpoint inhibitor
-
- IHC
-
- immunohistochemistry
-
- NK
-
- natural killer
-
- PD-1
-
- programmed cell death protein 1
-
- PD-L1
-
- programmed death ligand 1
-
- PSA
-
- prostate-specific antigen
-
- TIL
-
- tumor-infiltrating lymphocyte
-
- TMA
-
- tissue microarray
1 INTRODUCTION
Prostate cancer is the most common cancer type in men, and it is estimated that one in nine men will develop prostate cancer during their lives.1 Prostate cancer accounts for 11.3% of total cancer deaths in men in Europe.2 Whereas the reported prostate cancer incidence rates vary substantially between different countries, the mortality rates vary less geographically.3 The most common treatments for prostate cancer patients are surgery, radiation, and hormone deprivation therapy.4 When hormone deprivation therapies fail, docetaxel combination chemotherapies are often used for patients with metastatic disease,5-8 although resistance is frequent and the overall survival is 2–3 years.9 This highlights the necessity for novel and more efficient therapies.
Immunotherapy enhancing the antitumor response is a successful approach in some cancer types. Immunotherapy can be categorized in three types: therapeutic cancer vaccines, cytokines, and checkpoint inhibitor-based therapies.10 To date, cancer vaccines are approved as an immunotherapeutic approach option for treatment of patients with hormone-refractory prostate cancer,11 while immune checkpoint inhibitors (ICIs) are approved for any cancer with high tumor mutational burden and defective mismatch repair, including prostate cancer. The therapeutic potential of cytokine and further use of checkpoint inhibitor-based immunotherapies in prostate cancer are under investigation.10 Until now, studies with ICIs targeting CTLA-4, PD-1, and PD-L1 in prostate cancer therapy have shown limited benefits for most of the patients. Some patients have been observed to benefit from ipilimumab and nivolumab, which enhance antitumor immunity through inhibiting CTLA-4 and PD-1/PD-L1.12, 13 In addition, PD-1/PD-L1 inhibitors (PD-1: pembrolizumab, nivolumab; PD-L1: atezolizumab, avelumab, and durvalumab) as single agents or in combination with current treatments are under scrutiny, with several clinical trials ongoing.12, 14-16 Interim results with pembrolizumab monotherapy showed encouraging overall survival estimates, and observed responses seem to be durable in a subset of patients.17 Thus, a more precise stratification of patients, as well as novel alternative immunotherapies, might be necessary to improve the treatment of advanced prostate cancer.
PD-L1 (also known as B7-H1) belongs to the B7 family of immunoreceptors, whose members are considered essential in the regulation of the adaptive immune system and are emerging as important players in antitumor immunity.18-20 PD-L1 expression in prostate cancer is highly heterogeneous both in tumor cells and tumor-infiltrating lymphocytes (TILs), with expression rates ranging from 7% to 80%.21 However, an overall higher PD-L1 expression has been found in tumor cells in advanced or metastatic prostate cancer compared to the low expression level found in localized prostate cancer.22, 23 While some reports have found positive association between PD-L1 expression and biochemical recurrence (BR),24 others have not observed significant associations between PD-L1 expression and recurrence in prostate cancer.25, 26
The B7 family member B7-H3 is upregulated in a variety of human cancers and constitutes, together with PD-L1, an important immune checkpoint protein involved in the inhibition of T-cell activation.27-29 In addition to its effect on anticancer immunity, B7-H3 favors tumor cell proliferation, migration, and invasion as well as the glycolytic and metastatic capacity of the tumor.28 As a consequence, the attention on B7-H3 as a novel cancer biomarker and therapeutic target in cancer is increasing.27 The aim of this study was to evaluate the association between B7-H3, PD-L1, and androgen receptor (AR) protein expression and clinicopathologic parameters, and to validate the findings in a separate prostate cancer cohort.
2 MATERIALS AND METHODS
2.1 In silico expression analysis
In silico mRNA expression analysis of B7-family proteins: normal prostate tissue expression was from publicly available data sets at NCBI Gene Resource30 (https://www.ncbi.nlm.nih.gov/gene/); and prostate cancer expression was from publicly available TCGA data set retrieved from The Protein Atlas31 (https://www.proteinatlas.org/).
2.2 Immunohistochemical staining and scoring
The antibodies used for immunohistochemistry were: PD-L1 (SP263 ready to use, Ventana, Roche), PD-L1 (22C3 pharmaDx, Agilent), B7-H3 (AF1027 [R&D], 1:2000 in antibody diluent [Dako]), AR (SP107 ready to use, Ventana, Roche), AR (AR441 [Abcam], 1:120 in antibody diluent), and PTEN (6H2.1 [Merck], 1:50 in antibody diluent). Immunostaining was performed in fully automated immunostainers following routine methods. Antigen retrieval was performed at pH 9 using PT link system (Agilent Technologies). B7-H3, PD-L1 (22C3), AR (AR441), and PTEN immunostaining were performed with EnVision FLEX and Dako Autostainer Link 48 (Agilent). PD-L1 (SP263) and AR (SP107) immunostaining were performed with BenchMark Ultra, Ventana (Roche). B7-H3 antibody was incubated for 30 min, followed by secondary antibody incubation for 15 min using Secondary polyclonal rabbit anti-goat Ig/HRP (Dako), FLEX/HPR for 20 min, FLEX DAB/Sub Chromo for 10 min, and finally counterstaining with hematoxylin. Secondary goat antibody (Dako) was used at 1:200 (in the Spanish cohort) and 1:100 (in the Norwegian cohort) dilution in Dako antibody diluent. Slides were dehydrated through incubations with sequentially increasing alcohol concentrations, before xylene incubated and cover-slipped. TMAs and tissue slides were evaluated manually by an experienced uropathologist (José I. López). B7-H3 and AR immunostaining of whole tumor (focal and diffuse) tissue were considered, whereas in the case of PD-L1 only immunostaining of the inflammatory mononuclear TILs presented in the tumor was considered because we observed very weak PD-L1 immunostaining in tumor cells (as reported by others32) and therefore considered them not suitable for reliable analysis. PD-L1 expression was scored as low if very focal or no staining in TILs/tumor cells, and as high if the TILs/tumor cells staining was more frequently stained. B7-H3 and AR expression was scored as low if low staining intensity or no staining in tumor cells was detected, and as high if the tumor cells staining had moderate to strong intensity.
2.3 Clinical samples
All methods were carried out in accordance with relevant guidelines and regulations, and all experimental protocols were approved by a named institutional and/or licensing committee. Two separate cohorts with different routine clinical follow-up were used (Figure S1). The Spanish study cohort consisted of 120 prostate cancer patients treated with radical prostatectomy at Cruces University Hospital in Spain between 2000 and 2005. An experienced pathologist (José I. López) selected tumor areas with well-preserved tissue representative of the whole tumor from formalin-fixed and paraffin-embedded (FFPE) tissue blocks from these patients, and TMA blocks were made from these areas. 4 µm sections were made from the TMA blocks, one of which was stained with hematoxylin and eosin to verify the presence of tumor content. Differential adjacent staining of consecutive sections is shown for B7-H3 and PTEN staining to illustrate consistency on the fixation of samples (Figure S2). The Norwegian study cohort consisted of 253 prostate cancer patients treated with radical prostatectomy at Oslo University Hospital in Norway between 1987 and 2005. For each patient, a 3 µm whole tissue section was made from an FFPE block with well-preserved tissue representative of the worst Gleason grade. Whole tissue sections were evaluated for B7-H3 and AR expression. In addition, TMA blocks were made from tumor areas from 163 patients and used for evaluation of PD-L1 expression. Pathology of the Spanish and Norwegian cohorts was centrally reviewed by an experienced uropathologist (José I. López and Ljiljana Vlatkovic, respectively), who were blinded with respect to patient outcome, using the 2005 ISUP consensus and the ADASP practice guidelines.33 Both prostate cancer cohorts have been previously described.34, 35 Ethical approvals, including informed consent from all included patients, have been obtained for both cohorts (Clinical research ethical committee [CEIC] number E16/51 from Spain and Regional Committees for Medical Research Ethics [REK] number S-07443a from Norway). Cancer of the Prostate Risk Assessment Postsurgical (CAPRA-S) score was calculated according to its definition,36 that is, by combining preoperative PSA, Gleason grade, surgical margins, extracapsular extension, seminal vesicle invasion, and lymph node invasion.
2.4 Statistical analysis
Follow-up has been recorded until October 1, 2016, for patients in the Spanish cohort and December 31, 2008, for patients in the Norwegian cohort. BR in the Spanish cohort was defined as a PSA measurement equal to or greater than 0.4 ng/ml after surgery. Clinical recurrence in the Norwegian cohort was assessed with biopsy, digital rectal examination, or imaging modalities, and time to recurrence was analyzed according to the definition suggested by Punt et al.37 Statistical analyses were performed using the statistical software Stata/SE 15.1 (StataCorp) and SPSS Statistics V.23 (IBM). Spearman rho (ρ) test was used to correlate B7-H3, PD-L1, and AR expression to clinicopathologic parameters. Associations with recurrence were evaluated using Fisher's exact test for categorical variables and Mann–Whitney U test for continuous variables. The estimated survival curves were compared using the log-rank test. Univariable and multivariable survival analyses were performed using Cox's proportional hazards regression model. A two-sided p value of less than 0.05 was considered significant. All statistical analyses were performed using only patients with non-missing values.
3 RESULTS
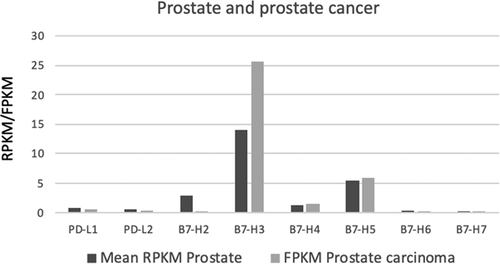
As illustrated in Figure 1, B7-H3 is amongst the most abundant B7-family immune checkpoint genes expressed in prostate carcinoma and normal prostate, suggesting that B7-H3 could play an important role in prostate cancer progression.
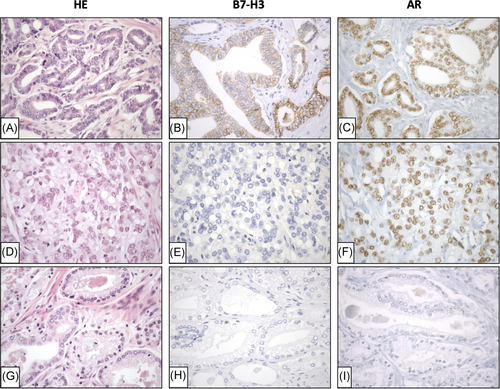
In the Spanish prostate cancer cohort, immunoexpression of B7-H3, PD-L1, and AR was analyzed in radical prostatectomy specimens from 120 prostate cancer patients. PD-L1 was expressed in 11% (12 out of 105), B7-H3 in 15% (18 out of 119), and AR in 80% (94 out of 117) of the cases. No significant co-expression of B7-H3 and PD-L1 was observed (p = .37). However, significant co-expression between B7-H3 and AR was found (p = .016), where all tumors with high B7-H3 protein expression were also positive for high AR protein expression (Table 1). Examples of B7-H3 and AR low and high cases are shown in Figure 2. B7-H3 expression correlated negatively with age (p = .024), and the positive correlation coefficients to Gleason grade and CAPRA-S did not reach significant values (p = .074 and p = .063, respectively). PD-L1 expression in TILs correlated positively with seminal vesicle invasion (p = .012), and the negative correlation coefficient to age and the positive correlation coefficient to surgical margins were not significant (p = .055 and p = .067, respectively). As for B7-H3, all positive PD-L1 cases were also positive for high AR protein expression (Table 1), but their correlation was not statistically significant (p = .067). AR expression correlated positively with the CAPRA-S risk group (p = .023), and the positive correlation coefficients to preoperative PSA level and Gleason grade were not significant (p = .063 and p = .052, respectively).
| Characteristic - Spanish cohort | B7-H3 low | B7-H3 high | PD-L1 low | PD-L1 high | AR low | AR high | |
|---|---|---|---|---|---|---|---|
| Patients – No. | N = 120 | (N = 101) | (N = 18) | (N = 95) | (N = 12) | (N = 25) | (N = 92) |
| Median follow-up time (IQR) – years | ρ = −.10/p = .27 | ρ = .03/p = .97 | ρ = −.086/p = .36 | ||||
| 10.5 (9.8–12.4) | 10.7 (9.8–12.6) | 10.6 (9.6–12.4) | 10.7 (9.7–12.5) | 10.5 (9.7–12.4) | 11.0 (10.1–13.5) | 10.6 (9.6–12.4) | |
| Median age at surgery (IQR) – years | ρ = −.12/p = .19 | ρ = .17/p = .072 | ρ = .17/p = .063 | ||||
| 63 (59–68) | 62 (59–68) | 61 (57–65) | 62 (58–66) | 65 (62–68) | 63 (59–68.5) | 62 (59–66) | |
| Age at surgery – No. (%) | ρ = −.21/p = .024 | ρ = .19/p = .055 | ρ = −.15/p = .10 | ||||
| ≤65 years | 79 (65) | 62 (61) | 16 (89) | 66 (69) | 5 (42) | 13 (52) | 64 (70) |
| >65 years | 43 (35) | 39 (39) | 2 (11) | 29 (31) | 7 (58) | 12 (48) | 28 (30) |
| Preoperative PSA – No. (%) | ρ = .018/p = .85 | ρ = −.030/p = .76 | ρ = .045/p = .063 | ||||
| ≤6 ng/ml | 36 (30) | 33 (33) | 3 (17) | 31 (32) | 3 (25) | 11 (46) | 25 (28) |
| >6 ng/ml and ≤10 ng/ml | 42 (35) | 31 (30) | 11 (61) | 33 (36) | 6 (50) | 4 (17) | 37 (40) |
| >10 ng/ml and ≤20 ng/ml | 33 (28) | 29 (29) | 4 (22) | 25 (26) | 2 (17) | 7 (29) | 26 (28) |
| >20 ng/ml | 4 (3) | 4 (4) | 0 (0) | 4 (4) | 0 (0) | 2 (8) | 2 (2) |
| Missing | 5 (2) | 4 (4) | 0 (0) | 2 (2) | 1 (8) | 1 (4) | 2 (2) |
| Gleason grade – No. (%) | ρ = .16/p = .074 | ρ = .077/p = .43 | ρ = .18/p = .052 | ||||
| ≤6 | 72 (60) | 63 (62) | 8 (44) | 58 (61) | 6 (49) | 19 (76) | 52 (57) |
| 3+4 | 22 (18) | 19 (19) | 3 (17) | 19 (20) | 2 (17) | 4 (16) | 18 (19) |
| 4+3 | 7 (6) | 6 (6) | 1 (6) | 3 (3) | 2 (17) | 1 (4) | 6 (7) |
| ≥8 | 19 (16) | 13 (13) | 6 (33) | 15 (16) | 2 (17) | 1 (4) | 16 (17) |
| Surgical margins – No. (%) | ρ = −.010/p = .91 | ρ = .17/p = .067 | ρ = −.20/p = .83 | ||||
| Negative | 78 (65) | 66 (65) | 12 (67) | 65 (68) | 5 (42) | 16 (64) | 61 (66) |
| Positive | 42 (35) | 35 (35) | 6 (33) | 30 (32) | 7 (58) | 9 (36) | 31 (34) |
| Extracapsular extension– No. (%) | ρ = .12/p = .18 | ρ = .089/p = .36 | ρ = .060/p = .52 | ||||
| Absent | 102 (84) | 86 (85) | 13 (72) | 81 (85) | 9 (75) | 22 (88) | 76 (82) |
| Present | 20 (16) | 15 (15) | 5 (28) | 14 (15) | 3 (5) | 3 (12) | 16 (1) |
| Seminal vesicle invasion – No. (%) | ρ = .028/p = .76 | ρ = .24/p = .012 | ρ = .11/p = .24 | ||||
| Absent | 117 (96) | 97 (96) | 17 (94) | 93 (98) | 10 (83) | 25 (100) | 87 (95) |
| Present | 5 (4) | 4 (4) | 1 (6) | 2 (2) | 2 (17) | 0 (0) | 5 (5) |
| Pathologic node (N) – No. (%) | n/a | n/a | n/a | ||||
| N0/x | 120 (100) | 101 (100) | 18 (100) | 97 (100) | 12 (100) | 25 (100) | 92 (100) |
| N1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| CAPRA-S risk group – No. (%)a | ρ = .19/p = .063 | ρ = .15/p = .14 | ρ = .23/p = .023 | ||||
| Low | 48 (40) | 43 (43) | 5 (29) | 37 (39) | 4 (40) | 16 (64) | 32 (35) |
| Intermediate | 45 (37) | 34 (34) | 9 (53) | 40 (42) | 2 (20) | 6 (24) | 36 (39) |
| High | 9 (8) | 6 (6) | 3 (18) | 3 (3) | 4 (80) | 1 (4) | 8 (9) |
| Missing | 18 (15) | 18 (18) | 0 (0) | 15 (16) | 2 (17) | 2 (8) | 16 (17) |
| Androgen receptor – No. (%) | ρ = .22/p = .016 | ρ = .18/p = .067 | |||||
| AR low | 25 (20) | 25 (25) | 0 (0) | 21 (22) | 0 (0) | ||
| AR high | 92 (77) | 74 (73) | 18 (100) | 72 (76) | 12 (100) | ||
| Missing | 3 (3) | 2 (2) | 0 (0) | 2 (2) | 0 (0) | ||
| PD-L1 – No. (%) | ρ = .087/p = .37 | ||||||
| Low | 94 (78) | 80 (79) | 14 (78) | ||||
| High | 12 (10) | 9 (9) | 3 (17) | ||||
| Missing | 14 (12) | 12 (12) | 1 (5) | ||||
- Note: Spearsman's correlation ρ/p value.
- Abbreviations: AR, androgen receptor; CAPRA-S, Cancer of the Prostate Risk Assessment Postsurgical; IQR, interquartile range; n/a, not applicable; PSA, prostate-specific antigen.
- a The CAPRA-S score was categorized to give three CAPRA-S risk groups: Low risk if score 0–2; Intermediate risk if score 3–5; High risk if score 6–12
We validated our findings for B7-H3 in a Norwegian cohort consisting of patients that had underwent radical prostatectomy, and identified B7-H3 positivity in 38% (78 out of 206) of the prostate cancer patients (Table 2). PD-L1 positivity was only found in 4 samples of 120 evaluable samples, and did not reach statistical significance in correlation to B7-H3 staining (p = .68). Since PD-L1 positivity was only seen in four samples, we did not include them in further statistical analysis. B7-H3 positivity correlated with higher Gleason grade (p = .024), positive surgical margins (p = .023), presence of seminal vesicle invasion (p = .001), and higher CAPRA-S risk group (p = .034). The negative correlation coefficient between B7-H3 expression and age was not significant (p = .086), and there were no significant correlations between B7-H3 expression and preoperative PSA, extracapsular extension, or pathologic node stage (p = .65, p = .11, and p = .60, respectively). All evaluable whole tissue sections were found positive for AR immunostaining (a representative example for B7-H3 and AR expression from the same whole tissue section is shown in Figure S3). Importantly, all areas with B7-H3 staining were also AR positive (Figure S3), although the lack of AR negative samples precluded statistical analysis.
| Characteristic – Norwegian cohort | B7-H3 low | B7-H3 high | Spearman's correlation | |
|---|---|---|---|---|
| (N = 128) | (N = 78) | ρ (95% CI) | p | |
| Median follow-up time (IQR) – years | 10.9 (7.5–14.3) | 11.7 (9.0–14.0) | 0.04 (−0.10 to 0.18) | .56 |
| Median age at surgery (IQR) – years | 63 (58–67) | 62 (57–66) | −0.09 (−0.23 to 0.04) | .18 |
| Age at surgery – No. (%) | −0.12 (−0.25 to 0.02) | .086 | ||
| ≤65 years | 75 (59) | 55 (71) | ||
| >65 years | 53 (41) | 23 (29) | ||
| Preoperative PSA – No. (%) | 0.03 (−0.11 to 0.17) | .65 | ||
| ≤6 ng/ml | 27 (21) | 19 (25) | ||
| >6 ng/ml and ≤10 ng/ml | 29 (23) | 9 (12) | ||
| >10 ng/ml and ≤20 ng/ml | 43 (34) | 31 (40) | ||
| >20 ng/ml | 28 (22) | 18 (23) | ||
| Gleason grade – No. (%) | 0.16 (0.02–0.29) | .024 | ||
| ≤6 | 4 (3) | 1 (1) | ||
| 3+4 | 55 (43) | 24 (31) | ||
| 4+3 | 40 (31) | 26 (33) | ||
| ≥8 | 29 (23) | 27 (35) | ||
| Surgical margins – No. (%) | 0.16 (0.02–0.29) | .023 | ||
| Negative | 51 (40) | 19 (24) | ||
| Positive | 77 (60) | 59 (76) | ||
| Extracapsular extension – No. (%) | 0.11 (−0.02 to 0.25) | .11 | ||
| Absent | 32 (25) | 12 (16) | ||
| Present | 95 (75) | 65 (84) | ||
| Seminal vesicle invasion – No. (%) | 0.23 (0.09–0.35) | .001 | ||
| Absent | 105 (82) | 48 (62) | ||
| Present | 23 (18) | 30 (38) | ||
| Pathologic node (N) stage – No. (%) | 0.04 (−0.10 to 0.17) | .60 | ||
| N0/x | 122 (95) | 73 (94) | ||
| N1 | 6 (5) | 5 (6) | ||
| CAPRA-S risk group – No. (%)a | 0.15 (0.01–0.28) | .034 | ||
| Low | 20 (16) | 4 (5) | ||
| Intermediate | 47 (37) | 27 (36) | ||
| High | 59 (47) | 45 (59) | ||
| PD-L1 – No. (%) | 0.04 (−0.14 to 0.22) | .68 | ||
| Negative | 70 (97) | 46 (96) | ||
| Positive | 2 (3) | 2 (4) | ||
- Abbreviations: CAPRA-S, Cancer of the Prostate Risk Assessment Postsurgical; IQR, interquartile range; PSA, prostate-specific antigen.
- a The CAPRA-S score was categorized to give three CAPRA-S risk groups: Low risk if score 0–2; Intermediate risk if score 3–5; High risk if score 6–12
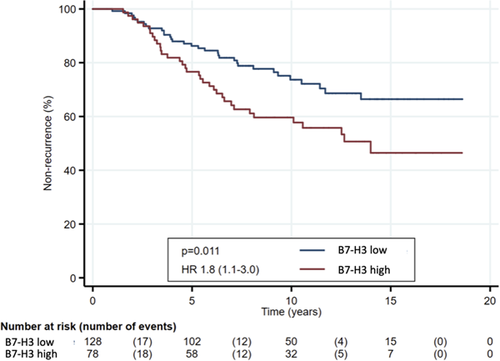
B7-H3 expression was significantly associated with BR in the Spanish cohort and clinical recurrence in the Norwegian cohort (p = .003 and p = .005, respectively), while PD-L1 expression and AR expression was not significantly associated with BR in the two cohorts (Table 3). In univariable survival analyses of time to recurrence, statistical significance was observed for B7-H3 expression (p = .011), preoperative PSA (p = .001), Gleason grade (p < .001), surgical margins (p = .020), extracapsular extension (p = .002), seminal vesicle invasion (p < .001), and pathological node stage (p = .004) (Table S1). Kaplan–Meier plot shows the significant association between B7-H3 expression and time to recurrence (Figure 3). Only Gleason grade (p = .002) and seminal vesicle invasion (p = .010) were significant in multivariable analysis of time to recurrence (Table S1).
| Spanish cohort | Norwegian cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N (%) | No biochemical recurrence, n (%) | Biochemical recurrence, n (%) | p | N (%) | No recurrence, n (%) | Recurrence, n (%) | p |
| Patients – No. | 120 | 78 | 42 | 206 | 138 | 68 | ||
| Median follow-up time (IQR) – years | 10.54 (9.8–12.4) | 10.6 (9.7–12.7) | 10.8 (9.8–11.9) | .41 | 11.3 (7.8–14.3) | 10.4 (7.5–14.3) | 12.3 (9.3–14.2) | .21 |
| Median age at surgery (IQR) – years | 63 (59–68) | 63 (59–68) | 61 (58–65) | .76 | 62 (57–67) | 62 (57–67) | 62 (57–68) | .64 |
| Age at surgery – No. (%) | .027 | .78 | ||||||
| ≤65 years | 42 (35) | 33 (42) | 9 (21) | 130 (63) | 88 (64) | 42 (62) | ||
| >65 years | 78 (65) | 45 (58) | 33 (79) | 76 (37) | 50 (36) | 26 (38) | ||
| Preoperative PSA – No. (%) | .034 | <.001 | ||||||
| ≤6 ng/ml | 36 (30) | 29 (37) | 7 (17) | 46 (23) | 40 (29) | 6 (9) | ||
| >6 ng/ml and ≤10 ng/ml | 43 (36) | 25 (32) | 18 (43) | 38 (19) | 33 (24) | 5 (8) | ||
| >10 ng/ml and ≤20 ng/ml | 33 (28) | 19 (24) | 14 (33) | 74 (36) | 41 (30) | 33 (50) | ||
| >20 ng/ml | 4 (3) | 1 (1) | 3 (7) | 46 (23) | 24 (17) | 22 (33) | ||
| Missing | 4 (3) | 4 (6) | 0 (0) | 2 (1) | 0 | 2 (3) | ||
| Gleason grade – No. (%) | .038 | <.001 | ||||||
| ≤6 | 72 (60) | 52 (67) | 20 (47) | 5 (2) | 5 (4) | 0 | ||
| 3+4 | 22 (18) | 15 (19) | 7 (17) | 79 (38) | 71 (51) | 8 (12) | ||
| 4+3 | 7 (6) | 2 (3) | 5 (12) | 66 (32) | 42 (30) | 24 (35) | ||
| ≥8 | 19 (16) | 9 (11) | 10 (24) | 56 (27) | 20 (14) | 36 (53) | ||
| Surgical margins – No. (%) | .43 | .011 | ||||||
| Negative | 78 (65) | 53 (68) | 25 (60) | 70 (34) | 55 (40) | 15 (22) | ||
| Positive | 42 (35) | 25 (32) | 17 (40) | 136 (66) | 83 (60) | 53 (78) | ||
| Extracapsular extension – No. (%) | .019 | <.001 | ||||||
| Absent | 100 (83) | 70 (90) | 30 (71) | 44 (22) | 39 (29) | 5 (7) | ||
| Present | 20 (17) | 8 (10) | 12 (29) | 160 (78) | 97 (71) | 63 (93) | ||
| Missing | 2 (1) | 2 (1) | 0 | |||||
| Seminal vesicle invasion – No. (%) | .34 | <.001 | ||||||
| Absent | 115 (96) | 76 (97) | 39 (93) | 153 (74) | 119 (86) | 34 (50) | ||
| Present | 5 (4) | 2 (3) | 3 (7) | 53 (26) | 19 (14) | 34 (50) | ||
| Pathologic node (N) stage – No. (%) | n/a | .026 | ||||||
| N0/x | 120 (100) | 78 (100) | 42 (100) | 195 (95) | 134 (97) | 61 (90) | ||
| N1 | 0 (0) | 0 (0) | 0 (0) | 11 (5) | 4 (3) | 7 (10) | ||
| CAPRA-S risk group – No. (%)a | .002 | <.001 | ||||||
| Low | 48 (39) | 37 (47) | 11 (26) | 24 (12) | 24 (18) | 0 | ||
| Intermediate | 44 (36) | 24 (29) | 20 (48) | 74 (37) | 62 (46) | 12 (18) | ||
| High | 9 (7) | 2 (3) | 7 (16) | 104 (51) | 50 (37) | 54 (82) | ||
| Missing | 19 (18) | 15 (19) | 4 (10) | 4 (2) | 2 (1) | 2 (3) | ||
| B7-H3 – No. (%) | .003 | .005 | ||||||
| Low | 101 (84) | 72 (91) | 29 (69) | 128 (62) | 95 (69) | 33 (49) | ||
| High | 18 (15) | 6 (8) | 12 (29) | 78 (38) | 43 (31) | 35 (51) | ||
| Missing | 1 (1) | 0 (0) | 1 (2) | |||||
| PD-L1 – No. (%) | .33 | .400 | ||||||
| Low | 95 (80) | 64 (82) | 31 (74) | 116 (56) | 62 (45) | 54 (80) | ||
| High | 12 (10) | 6 (8) | 6 (14) | 4 (2) | 3 (2) | 1 (1) | ||
| Missing | 13 (10) | 8 (10) | 5 (12) | 86 (42) | 73 (53) | 13 (19) | ||
| AR – No. (%) | .099 | n/a | ||||||
| Low | 25 (20) | 20 (26) | 5 (12) | 0 (0) | 0 (0) | 0 (0) | ||
| High | 92 (77) | 56 (72) | 36 (88) | 196 (95) | 135 (98) | 61 (90) | ||
| Missing | 3 (3) | 2 (8) | 1 (2) | 10 (5) | 3 (2) | 7 (10) | ||
- Abbreviations: AR, androgen receptor; CAPRA-S, Cancer of the Prostate Risk Assessment Postsurgical; IQR, interquartile range; n/a, not applicable; PSA, prostate-specific antigen.
- a CAPRA-S score was categorized to give three CAPRA-S risk groups: Low risk if score 0–2; Intermediate risk if score 3–5; High risk if score 6–12.
4 DISCUSSION
B7-H3 is frequently overexpressed in cancer and is thus considered a pan-cancer antigen displaying immune-related and non-related oncogenic functions.27, 28, 38-40 This makes B7-H3 an actionable target for immunotherapy, and phase 1 and phase 2 clinical trials with Enoblituzumab, an anti-B7-H3 inhibitory antibody, are ongoing in various types of cancer, including prostate carcinoma (NCT01391143, NCT02923180). In prostate cancer, B7-H3 is upregulated in malignant compared to benign tissues, and a positive correlation has been observed between B7-H3 expression and clinical recurrence, BR, and BR after salvage radiation therapy.41-45 Opposite functional effects of B7-H3 have been described in mice, where ablation of B7-H3 resulted in increased tumor burden in a model of spontaneous prostate cancer.46 Downregulation of B7-H3 expression in PC-3 human prostate cancer cell line has been reported to diminish cell adhesion properties, but did not affect cell proliferation.44 Our in silico analysis of prostate tissue and prostate carcinoma showed high expression of B7-H3, and our analyses of two distinct prostate cancer cohorts disclosed correlation between B7-H3 positivity and worse prostate cancer patient outcome, specifically clinical recurrence and BR. These findings support the hypothesis that B7-H3 may play a pro-oncogenic role in prostate cancer.
Our studies also unveiled a positive correlation between B7-H3 and AR protein expression. All B7-H3-high tumors were found to have AR expression high by immunostaining, while some AR-high tumors were low for B7-H3 protein expression. This is consistent with the finding of positive correlation between B7-H3 and AR expression at the mRNA level.47 Chromatin immunoprecipitation analysis has revealed an AR binding site upstream of the CD276/B7-H3 gene coding region, and androgens decreased B7-H3 mRNA expression in LNCaP prostate cancer cells.47 Chavin et al.48 reported an increase in B7-H3 expression in prostate cancer bone metastasis, but not in the primary tumors, after hormone-ablation therapy, in comparison to untreated lesions. It is possible that the increased B7-H3 expression observed in these patients is due to an increase in therapy resistance. Together, these observations suggest a direct relation between B7-H3 expression and AR signaling. Whether B7-H3 expression is altered after anti-hormonal therapy requires further studies, and additional analysis is needed to unveil the precise role of a potential B7-H3/AR axis in the proliferation and viability of prostate cancer cells.
We analyzed B7-H3 protein expression in two prostate cancer cohorts to determine the validity of our findings in two distinct retrospective cohorts. The two cohorts represent slightly different groups of prostate cancer patients as the Spanish cohort includes mainly lower grade prostate cancer patients, while the Norwegian cohort includes more patients with aggressive prostate cancers. TMAs were used for the Spanish cohort, while whole tissue sections were used for the Norwegian cohort. Together with intratumoral heterogeneity, these differences between the two cohorts could explain the lower proportion of B7-H3 positive patients in the Spanish cohort compared to the Norwegian cohort (15% and 38%, respectively). However, despite these differences, we found B7-H3 immunostaining to have clinical relevance in both cohorts.
PD-1/PD-L1 immune checkpoint-based therapies have been shown to be highly effective in several types of cancer. However, in prostate cancer patients, the antitumor activity of PD-1/PD-L1 ICIs is only observed in a subset of patients with treatment-refractory metastatic castration-resistant prostate cancer.49 Biomarkers are needed to better identify which patients respond to PD-1/PD-L1 ICIs. Major biomarkers under clinical evaluation include PD-L1 expression on tumor or immune infiltrating cells, tumor mutational load, and amount and function of infiltrating T or NK cells.12, 50 Whether B7-H3 expression could be informative for stratification of prostate cancer patients to receive PD-1/PD-L1 ICIs treatment remains to be tested. It is also unclear whether B7-H3 expression can provide prognostic information complementing established prognostic factors, even though it appeared to be a stronger indicator of recurrence than PD-L1 expression. However, most likely B7-H3 expression may be used to identify patients that could benefit from anti-B7-H3 inhibition therapy, and could potentially be used as an additional therapeutic intervention to anti-hormonal therapy in prostate cancer.
Our results suggest that inhibition of the immune checkpoint protein B7-H3 could have therapeutic benefits in advanced prostate cancer and deserves further investigation. In addition, our comparative immunohistochemical analyses indicate that B7-H3 could be a better prognostic indicator than PD-L1 in prostate cancer. We also observed a positive correlation between protein levels of B7-H3 and AR. Our findings highlight B7-H3 as an actionable novel immune checkpoint protein in prostate cancer.
ACKNOWLEDGMENTS
The authors would like to thank Ljiljana Vlatkovic (LV) (Department of Pathology, Oslo University Hospital) for pathological evaluation of the Norwegian cohort, Nick Leslie (Heriot-Watt University, Edinburgh, Scotland) for his support, and Arantza Perez Dobaran (University of the Basque Country, Leioa, Bizkaia, Spain) for expert technical support. This study was funded by The Research Council of Norway (Grant number 239813) and Marie Skłodowska-Curie Actions, UNIFOR-FRIMED Legacy (2020, Norway), and Radium Hospital Legacy (Radiumhospitalets legater 2020, Norway) to Caroline E. Nunes-Xavier; and by Ministerio de Economía y Competitividad (Spain and The European Regional Development Fund; Grant number SAF2016-79847-R) to Rafael Pulido and José I. López. Caroline E. Nunes-Xavier is the recipient of a Miguel Servet Research Contract from Instituto de Salud Carlos III (ISCIII; Spain and The European Social Fund+; Grant number CP20/00008).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conceptualization: Caroline E. Nunes-Xavier, Øystein Fodstad, Rafael Pulido, and José I. López. Investigation: Caroline E. Nunes-Xavier, Wanja Kildal, Andreas Kleppe, Håvard E. Danielsen, Håkon Wæhre, Roberto Llarena, Gunhild M. Mælandsmo, Øystein Fodstad, Rafael Pulido, and José I. López. Caroline E. Nunes-Xavier, Wanja Kildal, Andreas Kleppe, Rafael Pulido, and José I. López wrote the manuscript. All authors read and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
All data are included in the manuscript.




