Longitudinal measurement of subcutaneous and intratibial human prostate cancer xenograft growth and response to ionizing radiation by plasma Alu and LINE-1 ctDNA: A comparison to standard methods
Alok Mishra and Kenji Zennami contributed equally to this study.
Abstract
Background
Current preclinical models of metastatic prostate cancer (PCa) require sophisticated technologies and/or genetically engineered cells for the noninvasive monitoring of tumors in remote sites, such as bone. Recent developments in circulating tumor DNA (ctDNA) analysis provide an alternative method for noninvasive tumor monitoring at a low cost. Here, we sought to evaluate human Alu and LINE-1 ctDNA for the longitudinal measurement of subcutaneous and intratibial human PCa xenograft growth and response to ionizing radiation (IR) through comparison with standard slide caliper and bioluminescence measurements.
Material and Methods
Intratibial and subcutaneous xenografts were established in male athymic nude mice using LNCaP cells that stably express firefly luciferase. A subset of tumors was treated with a single dose of IR (CT-guided focal IR, 6 Gy). Tumor measurements were simultaneously taken by slide caliper (subcutaneous only), in vivo bioluminescence imaging, and quantitative real-time PCR (qPCR) of human-specific Alu and LINE-1 ctDNA for several weeks.
Results
Levels of ctDNA and bioluminescence increased concordantly with subcutaneous and intratibial tumor growth. A statistically significant correlation (Spearman) was observed between ctDNA and subcutaneous tumor volume (LINE-1, r = .94 and Alu, r = .95, p < .0001), ctDNA and bioluminescence (LINE-1, r = .66 and Alu, r = .60, p < .002), and bioluminescence and tumor volume (r = .66, p = .0003). Bioluminescence and ctDNA were also significantly correlated in intratibial tumors (LINE-1, r = .82 and Alu, r = .81, p < .0001). Following external beam IR, the tumor responses varied briefly by method of measurement, but followed a similar trend. Statistically significant correlations were maintained between ctDNA and slide caliper measurement in irradiated subcutaneous tumors (LINE-1, r = .64 and Alu, r = .44, p < .02), and ctDNA and bioluminescence in intratibial tumors (LINE-1, r = .55, p = .018).
Conclusions
Real-time qPCR of circulating human Alu and LINE-1 DNA provides an accurate measurement of subcutaneous and intratibial xenograft burden that is comparable with conventional bioluminescence imaging and slide caliper measurement. Transient differences in measurements were observed following tumor-targeted IR, but overall all measurements mirrored tumor growth and response.
Abbreviations
-
- ctDNA
-
- circulating tumor DNA
-
- IR
-
- ionizing radiation
-
- LINE-1
-
- long interspersed nuclear elements
-
- PCa
-
- prostate cancer
-
- qPCR
-
- quantitative real-time PCR
1 INTRODUCTION
Solid tumor malignancies readily shed cells and nucleic acid into the bloodstream. Circulating tumor DNA (ctDNA), harboring cancer-specific genomic and epigenomic alterations, is detectable in blood without the need for a tumor biopsy. These liquid biopsies are promising new tools in the treatment of early and late-stage malignancies because they can identify tumor subtypes, inform decisions for personalized medicine, and track therapeutic resistance.1 Circulating human-specific transposable element DNA is similarly useful for monitoring human tumor xenografts grown in mice.2 The high genomic copy number of human LINE-1 and Alu elements offer an abundance of tumor-specific signal for ctDNA detection and quantification. Most remarkably, human ctDNA monitoring does not require sophisticated imaging instruments or expensive materials yet still offers a noninvasive method to monitor indiscernible grafts inside animals.
Prostate cancer (PCa) is the most commonly diagnosed nonskin malignancy in American men and the second leading cause of male cancer deaths in the United States.3 Surgery or radiation therapy is curative for nearly all low-risk, localized PCa, while metastatic PCa remains incurable. The most frequent site for PCa metastasis is bone.4, 5 These metastases produce complex clinical phenotypes including bone pain, skeletal fractures, spinal cord compression, and hypercalcemia that impact the quality of life and overall survival of patients with PCa.6, 7 The complex interplay between bone marrow, osteoblast, osteoclast, stromal-derived chemokines, and growth factors generates a supportive microenvironment for the recruitment and growth of PCa metastases.8-11 It is crucial to study biology and therapeutic response of metastatic PCa in a comparably complex tumor microenvironment.
Preclinical animal models are essential for the analysis of prostate tumor biology and for the development and translation of new therapies.12, 13 Several genetically engineered mouse models (GEMMs) of PCa have emerged in recent decades, but none advance to significant bone metastases within the lifetime of the animal.14 To overcome this deficiency, intratibial bone metastatic models have been developed through the direct injection of PCa cells into the marrow space. This approach has been successful with a number of established human PCa cells lines and patient-derived xenografts (PDX) in athymic nude mouse and rat models.15, 16 One challenge with these metastatic models is the noninvasive and longitudinal measurement of tumor take, tumor growth, and therapeutic response. Small animal imaging approaches such as X-ray, magnetic resonance, computed tomography (CT), positron emission tomography (PET), and SPECT are useful in the study of these tumors, and their effects on the local bone microenvironment, but they require specialized and often expensive, equipment, materials, and expertise.17, 18 Bioluminescence imaging is another powerful technology to visualize metastases and quantify PCa tumor burden, although it requires genetically engineered cell lines and small animal imaging devices.19 Alternative methods for minimally invasive, longitudinal assessment of tumor burden would be beneficial to PCa research laboratories. The objective of this study is to investigate the accuracy of blood plasma Alu and LINE-1 ctDNA quantification as an approach to monitor subcutaneous and intratibial human PCa xenograft tumor growth and response to IR therapy in mice through comparison to two established methods, bioluminescent imaging and precision slide-caliper tumor measurement.
2 MATERIALS AND METHODS
2.1 Cell culture
LNCaP cells stably expressing firefly luciferase (LNCaP-CMV-Luc) were a generous gift from Ronald Rodriguez (Johns Hopkins University). Cells were maintained under G418 selection in RPMI-1640 (GIBCO, 11875-093) with 10% FCS at 37°C in 5% CO2. Cell line authentication and mycoplasma testing was completed through the Johns Hopkins Genetic Resources Core Facility JHU.
2.2 Subcutaneous tumor model
Animal studies were performed according to protocols approved by the Animal Care and Use Committee at Johns Hopkins University. Athymic (nu/nu) immunocompromised male mice were purchased commercially (Taconic Biosciences, NCRNU-M sp/sp). For subcutaneous tumor generation, 5 × 106 LNCaP-CMV-Luc cells were suspended in a 1:1 suspension with Matrigel (Corning, 354248) and inoculated subcutaneously in the right dorsal flank in a volume < 200 μl/animal. Tumor volume was monitored by slide caliper (Mituoyo) measurements of length, width, and height, and then using the equation: 0.52 × L × W × H (in mm3).
2.3 Intratibial tumor model
Animals were anesthetized with ketamine: xylazine: acepromazine (100:20:3 mg/kg). The knee was shaved and sterilized. A para-patellar incision (0.5 cm long) was created to expose the injection area. A 25G Hamilton syringe needle was inserted through the cortex of the anterior tuberosity of the tibia with a rotating “drill−like” movement to minimize cortical fracture. After the bone cortex was traversed, the needle was further inserted approximately 3 mm down to the diaphysis of the tibia to create a hollow core. 1 × 106 LNCaP-CMV-Luc cells were prepared in 10−15 µl of 1× phosphate-buffered saline (PBS) and injected slowly. Bone wax (Surgical Specialties Corporation, 903) was applied to seal the drilled area and prevent exodus of implanted cells. The incision was closed with sterile 4-0 chromic suture (Ethicon Inc., 6543478) on skin. Buprenorphine was administered for pain management.
2.4 Histology and microCT
Tibias were harvested and fixed in 10% formalin for 48 h. Fixed tibias were scanned using micro-CT (nanoScan PET/CT; Mediso, Budapest) and subsequently paraffin-embedded, sectioned (5 μm thick) and stained with hematoxylin and eosin (H&E) for histologic analyses.
2.5 Bioluminescent tumor imaging
Bioluminescence imaging was performed using the IVIS Spectrum imaging system (Xenogen, Caliper Sciences). Before imaging, each mouse was intraperitoneally injected with 3 mg of luciferin (Gold Biotechnology, 115144-35-9). Animals were anesthetized by the open-drop isoflurane exposure method and maintained at 1.5%–2% isoflurane anesthesia during imaging. All images were analyzed using Living Image 4.3 software under the same intensity scale to normalize across time. After normalization, a region of interest (ROI) was generated and applied to all tumors and average radiance (p/s/cm2/sr) calculated.
2.6 ctDNA extraction and quantification
Approximately 35 μl of tail vein blood was drawn into a marked heparinized micro-capillary tube (Thermo Fischer Scientific, 22-362-566) fitted with dropper cap (Drummond Microcaps, 101-000-0100). The collected blood was transferred to a 1.5 ml tube containing 200 μl of 4.5 mmol/L EDTA in PBS, centrifuged at 1500 × g for 15 min, and plasma supernatant was collected and stored at −80°C. DNA was extracted by QIAamp DNA Micro Kit (Qiagen, 56304) and eluted in 50 μl DNAse free water. Human LINE-1 and Alu ctDNA was quantified using iTaq™ Universal SYBR® Green Supermix (BioRad, cat no.1725121) and primers Human_LINE1_Fwd (TCACTCAAAGCCGCTCAACTAC) and Human_LINE1_Rev (TCTGCCTTCATTTCGTTATGTACC) as reported by Rago et al.,2 or primers Human_Alu_115_Fwd (GTCAGGAGATCGAGACCATCCT) and Human_Alu115_Rev (AGTGGCGCAATCTCGGC) as derived from Asangani et al.20 Samples with Threshold cycle (Ct) values above background controls were excluded. Serially diluted LNCaP-CMV-Luc DNA was spiked in the background of nonhuman DNA (salmon sperm, Thermo Fisher Scientific, cat no.15632011) and applied as a reference standard curve.
2.7 CT guided focal ionizing radiation (IR) treatment of animals
Ionizing radiation (IR) was delivered using the Small Animal Radiation Research Platform (SARRP) (Xstrahl). Mice were anesthetized using 1.5%–2% isoflurane gas and then immobilized on the SARRP's rotary stage. The SARRP was equipped with cone-beam computed tomography (CBCT) and a treatment planning system, which were used to image each animal to create a highly targeted and conformal IR delivery. For the subcutaneous tumor group, an AP (anterior to posterior) beam of 10 × 10 mm at a variable depth (due to tumor size) was used. For the tibia group, an AP beam of the same size with the radiation isocenter on the bone was used. The energy of the radiation beams used was 220 kVp and 13 mA. The slice gap was one degree and the resolution was 0.2 mm. Animals were treated with a nonablative single fraction of 6 Gy, 28 days postimplantation of LNCaP-CMV-Luc cells. Reconstruction of images based on data was done by software built into the machine. Tumors were identified as highly contrasted masses present in animals but absent in control (noninjected mice).
2.8 Statistical analysis
Each study cohort comprised N = 5 animals. Tumor growth and therapeutic response were recorded for animals that developed tumors, with 5/5 subcutaneous nonirradiated, 5/5 subcutaneous irradiated, 4/5 intratibial nonirradiated, and 3/5 intratibial irradiated cohorts developing tumors. Spearman correlation analysis was performed using GraphPad Prism 8.0 (GraphPad). p < .05 was considered statistically significant for all the interpretations. All tumor measurements are provided in Table S1 and Figures S1 and S2.
3 RESULTS
3.1 Models for comparative analysis of tumor burden by concurrent ctDNA, bioluminescence, and caliper measurement
LNCaP cells, engineered to stably express firefly luciferase, were used to generate an intratibial human tumor xenograft model in athymic nude mice. We applied X-ray, microCT, bioluminescence imaging, and tumor histology to optimize tumor engraftment and verify tumor retention within the marrow space (Figure 1A-C). We found that bone wax was important to seal the injection site and prevent tumor passage out of the bone. Once the model was optimized, we developed cohorts of mice bearing subcutaneous or intratibial xenograft tumors for comparative measurement of tumor growth by slide caliper measurement (subcutaneous only), bioluminescence imaging, and quantitative real-time PCR (qPCR) of circulating human tumor DNA (ctDNA) using LINE-1 or Alu transposable element amplicons. We completed all tumor measurements and plasma sampling within a single day to allow direct comparison of each measurement approach in the same animal (see Figure 1D for study schematic). A subset of animals received a single, non-curative dose of external beam IR (6 Gy) to the tumor region using the CT-guided SARRP to compare the different measurement approaches in the context of therapeutic response and tumor regrowth.
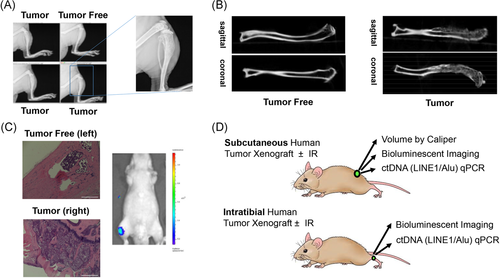
3.2 ctDNA level, bioluminescence, and caliper measurement are significantly correlated in subcutaneous prostate tumor xenografts
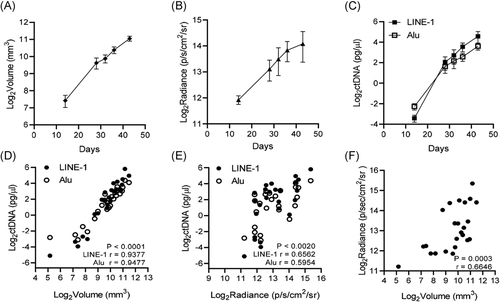
Following subcutaneous tumor engraftment, we concurrently measured tumor burden on multiple days by slide caliper measurement, bioluminescence, and LINE-1 and Alu ctDNA level. The data from each measurement was log2 transformed for comparative graphing analysis. All three measurements demonstrate a concordant increase in tumor size and signal over time (Figure 2A-C). We performed Spearman correlation analysis to compare the relationships between each measurement at every time point. Plasma concentrations of LINE-1 and Alu ctDNA were strongly and significantly correlated with tumor volume (Figure 2D), with Spearman r = .9377 for LINE-1 ctDNA with tumor volume, and Spearman r = .9477 for Alu with tumor volume (p < .0001 for both amplicons, independently). Levels of ctDNA and bioluminescent radiance were also significantly correlated (Figure 2E), with calculated Spearman r = .6562 for LINE-1 and Spearman r = .5954 for Alu (p < .002 for both amplicons, independently). Bioluminescent radiance was also significantly correlated with tumor volume (Figure 2F), with calculated Spearman r = .6646 (p = .0003). These results validate human LINE-1 and Alu ctDNA quantification as methods for assessing subcutaneous human PCa xenograft tumor burden in athymic nude mice.
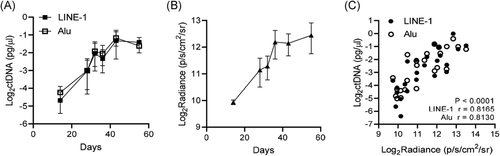
3.3 ctDNA level and bioluminescent radiance are significantly correlated in intratibial prostate tumor xenografts
To assess the accuracy of ctDNA in the measurement of inaccessible tumors in bone, we applied intratibial LNCaP-MLuc xenografts. Following engraftment, we measured ctDNA and bioluminescence for multiple days over a 2-month experiment. Both ctDNA level and bioluminescent radiance concurrently increased in signal over time (Figure 3A,B). Plasma concentrations of LINE-1 and Alu ctDNA were strongly and significantly correlated with tumor bioluminescence (Figure 3C), with a calculated Spearman r = .816 for LINE-1 ctDNA and bioluminescence, and Spearman r = .813 for Alu ctDNA and bioluminescence (p < .0001 for both amplicons, independently). These results further validate the use of LINE-1 and Alu ctDNA as noninvasive biomarkers for assessing human PCa xenograft tumor burden within the bone marrow of athymic nude mouse models.
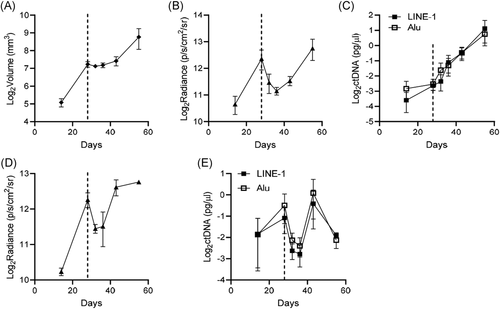
3.4 Evaluation of ctDNA, bioluminescence, and tumor volume in irradiated subcutaneous and intratibial prostate tumor xenografts
We then assessed the ability of ctDNA to measure tumor response to a single nonablative dose of IR therapy by comparison to bioluminescence and caliper (subcutaneous only) tumor measurement for several days over a 2-month experiment. We applied external beam radiation therapy because it is a standard treatment for the palliation of bone pain caused by PCa bone metastases and a developing approach for the treatment of oligometastatic PCa. For this model, LNCaP-MLuc cells were engrafted subcutaneously, or within the tibia, and tumor burden was concurrently measured by all methods before radiation, on the day of radiation (single dose, 6 Gy), and several days the following radiation. The average measurements at each time point are shown in Figure 4, and individual measurements of each tumor are provided in Figure S3. All three measurement approaches produced comparable growth and therapeutic response curves for subcutaneous tumors, although transient differences in signal were apparent for a few days following IR (Figure 4A-C). Caliper measurement reported stabilization of tumor volume in all animals for several days following IR (Figures 4A and S3A, Days 28–36), while bioluminescence signal markedly decreased and the recovered after IR (Figures 4B and S3B, Days 28–36). The ctDNA levels varied for a brief time following IR, with signal increasing in some animals and decreasing in others (Figures 4C and S3C, Days 28–36). In the intratibial model, both bioluminescence and ctDNA signals briefly decreased after radiation, and then recovered and increased after several days (Figures 4D,E and S3D,E).
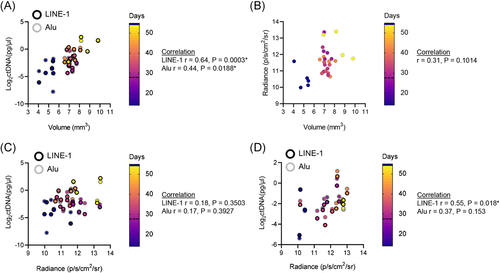
To compare the concordance of ctDNA, bioluminescence and tumor volume over the course of the irradiated tumor model experiments, we performed Spearman correlation analysis. The correlation between ctDNA and tumor volume was statistically significant in irradiated subcutaneous tumors (Figure 5A), with Spearman r = .6360 for LINE-1 and caliper measurement, and Spearman r = .4411 for Alu and caliper measurement (p < .02 for both amplicons, independently). However, the correlation between bioluminescence and caliper measurement (Figure 5B), and bioluminescence and ctDNA (Figure 5C), were not statistically significant in irradiated subcutaneous tumor models, possibly due to the transient differences in signal response following IR. The correlation between ctDNA and bioluminescence in intratibial tumors was statistically significant for LINE-1 analyses, with Spearman r = .55 (p = .018), but the correlation between Alu ctDNA and bioluminescence did not reach statistical significance (Figure 5D). The absence of a statistically significant correlation may be due to the transient, differential responses of the different measurement signals following radiation.
4 DISCUSSION
In the present report, we have demonstrated a strong and significant correlation between tumor burden measurement by human-specific ctDNA level and the conventional approaches of slide caliper measurement and firefly luciferase bioluminescence. These results validate human LINE-1 and Alu ctDNA as surrogate biomarkers of tumor burden in human PCa xenografts and demonstrate their utility in bone metastasis and tumor irradiation models. This quantitative method is inexpensive, simple, and does not require the use of labeled cells, imaging ligands, specialized imaging equipment, or imaging expertise. It is also notable that ctDNA measurement is irrespective of operator skill level, ROI drawing differences, tumor shape, or surface attenuation when compared to conventional tumor measurement approaches.
Human-specific transposable elements have been applied as ctDNA biomarkers for xenograft tumor burden in a number of tissue compartments including subcutaneous, cecum, and liver, as well as broad metastases established by intravenous inoculation.2 They have also been useful to detect and quantify dormant or micrometastatic tumor cells in mouse tissues, such as lung or bone, after necropsy.21-23 To our knowledge, our study is the first to demonstrate the utility of LINE-1 and Alu ctDNA in the measurement of metastatic xenograft tumor burden within the bone. Our results show an equally significant correlation between ctDNA and bioluminescence in subcutaneous and bone compartments, as well as ctDNA and slide caliper measurement in subcutaneous tumors (Figures 2, 3). Our study further validates the correlation between bioluminescence and slide caliper measurement for subcutaneous tumor xenografts (Figure 2F). We found LINE-1 and Alu elements to provide comparable sensitivity and linear dynamic range in our analyses, although Alu was consistently detectable at lower threshold cycles, possibly reflecting the higher genomic copy number when compared to LINE-1 elements. Because of this, circulating levels of Alu may provide greater sensitivity in models with lower tumor burden. Human-specific mitochondrial tumor DNA has also shown high sensitivity as a ctDNA biomarker for xenograft tumor burden.24
The results from our irradiation model demonstrate the utility of LINE-1 and Alu ctDNA quantification in the study of tumor response to radiation. We found subcutaneous tumor irradiation to produce a transient increase in ctDNA immediately following treatment in most animals (Figures 4C and S3C). This phenomenon has been previously reported in xenograft models, as well as cancer patients, following treatment with chemotherapy or radiation therapy.2, 25, 26 This increase in signal is likely due to increased cancer cell death and shedding of dead or dying cancer cells and free nucleic acids into the bloodstream. It is interesting that a similar increase in ctDNA was not observed after irradiation of animals bearing intratibial tumors (Figures 4C,D and S2C,D). These differences in response may be due to differences in experimental conditions, tumor burden, or vascular access for ctDNA clearance. Notably, in subcutaneous tumors, we also found transient differences between bioluminescence and slide caliper measurement following irradiation (Figures 4A,B and S3A,B). These results may reflect changes in tumor biology such as swelling, or increases or decreases in blood flow, that could differentially affect each measurement approach briefly after therapy. Further studies are needed to characterize the biologic mechanisms of these type of differential responses.
For unknown reasons, there has been a paucity of preclinical models capable of replicating the progression of primary PCa to bone-metastatic and castration-resistant PCa. Likewise, there are only a handful of human PCa cell lines. While these cell lines, tumor xenografts, and GEMMs replicate a significant proportion of PCa biology, and have been critical to our current understanding of the disease, they do not sufficiently replicate the heterogeneity or rapidly changing state of the disease. In recent years, the field has turned to patient-derived xenograft (PDX) models as a source to represent the current genomic and phenotypic landscape of the disease and to model its response to therapy.27-30 The majority of these models are serially transplantable tumors that are difficult to stably transfect or transduce with reporter gene vectors; therefore, human-specific Alu and LINE-1 ctDNA may be especially useful for the quantification of tumor burden for PCa PDX tumors grown in orthotopic or metastatic sites. Indeed, human-specific ctDNA assays have been applied to study disease burden in ovarian and glioblastoma PDX models, but have not yet been reported with prostate PDX models.24, 31
5 CONCLUSIONS
This study demonstrates the utility of human LINE-1 and Alu ctDNA in the noninvasive and nonterminal measurement of human PCa xenograft tumor burden through direct comparison to conventional measurements. Our results show that ctDNA accurately mirrors the growth and therapeutic response of prostate tumors to IR in soft tissue and in bone. This study supports the growing value and convenience of quantifying circulating and disseminated human-specific DNA sequences in xenograft tumor models of PCa.
ACKNOWLEDGMENTS
This study was supported in part by grants from the National Institutes of Health/National Cancer Institute grants 5P50CA058236 (William G Nelson, S.E.L, T.L.D, and S.Y.), P30CA006973 (William G Nelson), and the Patrick C. Walsh Prostate Cancer Research Fund. We thank Jonathan Coulter for helpful discussions of data and experimental design, and Yonggang Zhang and the Johns Hopkins Radiology and Radiological Sciences Imaging Cores for their assistance with small animal imaging.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
Data are available upon request.




