Clinical outcomes, management, and treatment patterns in patients with metastatic castration-resistant prostate cancer treated with radium-223 in community compared to academic settings
Abstract
Background
The most common site of disease in metastatic castration-resistant prostate cancer (mCRPC) is the bone. The ALSYMPCA study demonstrated that radium-223 significantly improved overall survival (OS) in mCRPC patients with symptomatic bone metastases and without visceral metastases. However, administration requires a multidisciplinary approach and an infrastructure that supports coordination of care, which may differ by practice site. We aimed to evaluate practice patterns and treatment outcomes in patients with mCRPC treated at a community practice (CP) compared with those treated at an academic center (AC).
Methods
This retrospective review included 200 adult mCRPC patients receiving radium-223 between January 2014 and June 2017. The primary endpoint, OS, was estimated from the date of radium-223 initiation. Secondary outcomes included a comparison of baseline characteristics, reasons for initiation and discontinuation of radium-223, and treatment sequencing. A subset analysis of OS based on the number of radium-223 doses and on sequencing of radium-223 either before or after chemotherapy was also conducted.
Results
Most patients were treated at a CP (57%). Patients treated at CP sites were significantly older (74.9 vs. 71.9 years; p = .031) and had more comorbidities (Klabunde score 1.1 vs. 0.7; p = .020) than those in an AC but initiated treatment within a shorter period of time from diagnosis of mCRPC (1.3 vs. 1.9 years; p < .001) and received a greater mean number of radium-223 doses (5.4 vs. 4.8; p = .001). There were no observed differences in OS between CPs versus ACs (21.6 vs. 20.7 months; p = .306). Overall, patients who received 5–6 doses versus 1–4 doses of radium-223 had a longer median OS (23.3 vs. 6.4 months; p < .001). The most common reason for discontinuation in patients who did not complete treatment was disease progression. Overall, 43% of patients received radium-223 monotherapy and 57% concurrently with other agents.
Conclusions
Most patients received radium-223 concurrently with abiraterone acetate or enzalutamide and were able to complete 5–6 doses of radium-223. Despite differences in the populations and treatment patterns, no survival differences between patients treated in ACs versus CPs were observed. Additional real-world data are needed to validate these findings.
1 INTRODUCTION
Prostate cancer is the most common noncutaneous cancer in men in the United States and is the second most common cause of cancer-related deaths in men.1 Cancer mortality is generally due to metastatic castration-resistant prostate cancer (mCRPC), an advanced clinical phenotype characterized by progressive disease despite a castrate level of serum testosterone.2, 3
Approximately 90% of patients with metastatic disease have bone metastases, which are independent and reliable predictors of both morbidity and mortality.4 Radium-223 dichloride (radium-223) is the only alpha particle-emitting radioactive therapeutic approved by the Food and Drug Administration as monotherapy for the treatment of patients with mCRPC who have symptomatic bone metastases and no known visceral metastatic disease.5, 6 It is administered intravenously at a dose of 55 kilobecquerel (1.49 microcurie) per kilogram of body weight once every 4 weeks for up to 6 injections.6 Radium-223 was studied in an international, prospective, randomized, double-blind, placebo-controlled Phase 3 trial, ALSYMPCA. A total of 921 patients were randomized 2:1 to receive either radium-223 or placebo, in addition to standard of care (defined as routine care such as radiation, steroids, antiandrogens, etc.). The trial was terminated after the prespecified interim analysis revealed the study reached its primary endpoint, with a 30% reduction in the risk of death with radium-223 compared to placebo.3 Furthermore, radium-223 treatment demonstrated a delay to symptomatic skeletal events (SSEs) and improvement in pain control and quality of life compared with placebo.3, 7-10 Additional reports have shown similar results, with improvement in pain seen in 50%–60% of patients treated with radium-223.11-13
In addition to radium-223, several other treatments with varying mechanisms of action have demonstrated improved overall survival (OS) in the treatment of patients with mCRPC.14 As these drugs were developed mainly in parallel, treatment choices are currently based on provider preference, available guidelines, and patient- or disease-specific factors, such as symptomatic disease, pain, performance status, and disease burden.14 The optimal sequence of these agents and the place of radium-223 in the treatment paradigm remains undefined, highlighting the need for real-world evidence to characterize utilization patterns and outcomes.
The administration of radium-223, as a radiopharmaceutical, requires a high level of care coordination and infrastructure that may differ by site of care. Critical issues surrounding radium-223 administration include ensuring administration of standard of care concomitant medications, such as bone antiresorptive agents or pain medications, evaluating pertinent laboratory results before each dose, storing and administering the radiopharmaceutical appropriately, and providing extensive education to patients, caregivers, and providers who may be exposed to radiation either directly (i.e., exposure to radium-223) or indirectly through patient excretions. These issues are frequently addressed through the development of a multidisciplinary team that may include urology, medical oncology, radiation oncology, and nuclear medicine teams, among others.4, 15 The majority of radium-223 studies were conducted at tertiary or academic sites, and the currently published real-world analyses do not differentiate treatment by site of care, which leaves a paucity of data characterizing any potential treatment differences for mCRPC patients receiving this therapy in academic center (AC) and community practice (CP) settings.13, 16-24 Because of the morbidity and survival benefits offered by radium-223, it is important to understand its current use in both AC and CP settings to ensure access regardless of site of care. In this retrospective analysis, we aimed to address this data gap by assessing the use and survival outcomes of radium-223 in patients treated in AC versus CP sites.
2 MATERIALS AND METHODS
2.1 Data source
This was a retrospective non-interventional chart review of patients from four AC and six CP institutions located throughout the United States with a high volume of prostate cancer patients that integrate radium-223 into the treatment of mCRPC. Specific institutions involved in this study included Tulane Cancer Center, Tisch Cancer Institute, 21st Century Oncology, University of Pittsburgh Medical Center Hillman Cancer Center, Wichita Urology Group, Chesapeake Urology Research Associates, Advanced Radiation Centers, Associated Medical Professionals, Karmanos Cancer Institute, and Prostate Oncology Specialists. Participating providers reported their primary specialty as oncology (50%), urology (30%), or nuclear medicine (20%). “High volume” was defined as treating at least 2000 prostate cancer patients per year. Patient-level deidentified data were extracted using an electronic case report form that was completed by the participating investigator and managed by a designated contract research organization. To reduce selection bias, investigators were provided a list with instructions on how to select patients alphabetically based on randomly assigned letters. Appropriate institutional review board approval was obtained from each participating center.
2.2 Study design and patient population
Eligible patients were adults with mCRPC and a confirmed diagnosis of bone metastases who received one or more doses of radium-223 outside of a clinical trial between January 2014 and June 2017 and who had at least 4 months of follow-up data or a date of death or hospice referral recorded within the 4-month minimum follow-up time. Because diagnosis of mCRPC was by investigator determination within the medical chart, the following consistent definitions were employed: castration resistance was defined as progression in patients with castrate-level testosterone after continuous hormonal therapy, confirmed by two rises in prostate-specific antigen (PSA) levels at least 1 week apart (clinical and radiographic progression were excluded from this definition), and metastatic disease was defined as the presence of metastases confirmed by imaging tests including bone, computed tomography, or magnetic resonance imaging scans. Alternatively, use of agents approved in the metastatic setting in patients who are castrate resistant could be used to confirm metastatic disease.
2.3 Baseline variables
Baseline variables were collected at the date of radium-223 initiation. Data collected included patient demographics, such as age, site of treatment, and type of insurance; disease characteristics, such as time from diagnosis of mCRPC to initiation of radium-223 and number of bone metastases; skeletal symptoms, including incidence of SSEs; medical history (a Klabunde comorbidity score was calculated for each patient); treatment history; laboratory parameters; and radium-223-related variables, including date and number of injections and reasons for initiation and discontinuation of radium-223.
2.4 Outcomes
The primary outcomes included OS and evaluation of differences in radium-223 outcomes between sites of care. Secondary outcomes included a comparison of baseline clinical and demographic characteristics, reasons (based on a predefined list of nonmutually exclusive answers) for initiation and discontinuation of radium-223, and treatment sequencing after radium-223 between sites of care. OS (in months) was defined from the date of initiation of radium-223 to death from any cause. Patients alive at last follow-up/end of study period (November 3, 2017) were censored.
2.5 Statistical analysis
Summary statistics, including means and proportions, were used to describe baseline demographic and clinical characteristics such as treatment sequences, reasons for initiation and discontinuation of radium-223, laboratory values, and physician and patient characteristics. Differences between groups were assessed using Wilcoxon-rank sum test for continuous variables and Fisher's exact test for categorical variables and compared between site of care (AC vs. CP). OS was estimated from the date of radium-223 initiation using the Kaplan–Meier method and compared between site of care (AC vs. CP) using a log-rank test. The log-rank method was used to compare survival distributions between patients who received 1–4 versus 5–6 doses of radium-223. All analyses were conducted using SAS, version 9.4 (Cary).
3 RESULTS
3.1 Baseline demographic and clinical characteristics
A total of 200 patients were included in this analysis. Of the 200 patients, 43% received treatment at an AC and 57% at a CP. The mean age of patients at radium-223 initiation was 73.6 years overall; however, patients treated in CPs were significantly older than those treated in an AC (74.9 vs. 71.9 years; p = .031). Key median baseline laboratory values in patients treated at AC and CP settings included PSA (58.7 and 33.0 ng/dl, respectively), hemoglobin (12.0 and 12.5 g/dl, respectively), alkaline phosphatase (123 and 89.0 U/L, respectively), lactate dehydrogenase (225.0 and 208.0 U/L, respectively), and platelet count (218.5 109/L and 210.0 109/L, respectively). In addition, patients treated in CPs were treated with radium-223 within a shorter period of time from diagnosis of mCRPC (1.3 vs. 1.9 years; p < .001), had more comorbid conditions (Klabunde score at radium-223 initiation 1.1 vs. 0.7; p = .020), and received a greater mean number of radium-223 doses (5.4 vs. 4.8; p = .001). Tables 1 and 2 show the baseline demographic and clinical characteristics of the patients.
| Variable | Total N = 200 | AC n = 86 | CP n = 114 | p Value* |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, mean (SD) | 73.6 (9.1) | 71.9 (8.6) | 74.9 (8.5) | .031 |
| Years from mCRPC diagnosis, mean (SD) | 1.6 (1.6) | 1.9 (1.7) | 1.3 (1.5) | <.001 |
| Primary insurance plan, n (%) | ||||
| Commercial | 50 (25.0) | 19 (22.1) | 31 (27.2) | .128 |
| Medicare | 88 (44.0) | 39 (45.3) | 49 (43.0) | |
| Medicaid | 9 (4.5) | 1 (1.2) | 8 (7.0) | |
| Othera | 53 (26.5) | 27 (31.5) | 26 (22.8) | |
| Patient distance from radium-223 treatment facility (miles), mean (SD) | 28.5 (51.7) | 35.8 (68.7) | 21.2 (22.7) | .226 |
| Refined comorbidity score (Klabunde), mean (SD) | 1.0 (1.2) | 0.7 (0.9) | 1.1 (1.4) | .020 |
| Laboratory values, medianb (IQR) | ||||
| PSA (ng/dl) | 41.2 (14.0, 231.3) | 58.7 (16.8, 244.4) | 33.0 (6.9, 159.7) | NR |
| Hemoglobin (g/dl) | 12.2 (11.1, 13.0) | 12.0 (10.7, 13.0) | 12.5 (11.5, 13.0) | |
| ALP (U/L) | 103 (66.0, 179.0) | 123.0 (70.5, 204.0) | 89.0 (59.8, 172.0) | |
| LDH (U/L) | 223.5 (191.0, 281.5) | 225.0 (192.8, 281.3) | 208.0 (178.3, 289.5) | |
| Platelet count (109/L) | 215 (170.0, 258.0) | 218.5 (175.0, 267.8) | 210.0 (155.0, 246.0) | |
| Bone metastasesc, n (%) | ||||
| 0–6 | 78 (39.0) | 28 (32.6) | 50 (43.9) | NR |
| 7–20 | 38 (19.0) | 14 (16.3) | 24 (21.1) | |
| >20 | 16 (8.0) | 4 (4.7) | 12 (10.5) | |
| Previous SSEd, n (%) | ||||
| Yes | 64 (32.0) | 28 (33.0) | 36 (32.0) | .210 |
| No | 124 (62.0) | 50 (58.0) | 74 (65.0) | |
| Treatment characteristics | ||||
| Bone-targeting agents started before radium-223 initiation | ||||
| Bisphosphonate | 25 (12.5) | 13 (15.1) | 12 (10.5) | NR |
| Denosumab | 122 (61.0) | 56 (65.1) | 66 (57.9) | |
| Previous mCRPC therapies completed before radium-223 initiation | ||||
| 0 | 77 (38.5) | 25 (29.1) | 52 (45.6) | .004 |
| 1 | 63 (31.5) | 24 (27.9) | 39 (34.2) | |
| 2 | 37 (18.5) | 21 (24.4) | 16 (14.0) | |
| ≥3 | 23 (11.5) | 16 (18.6) | 7 (6.1) | |
| Previous therapies completed before radium-223 initiatione, f | ||||
| Abiraterone | 62 (31.0) | 39 (45.3) | 23 (20.2) | <.001 |
| Enzalutamide | 38 (19.0) | 25 (29.1) | 13 (11.4) | .002 |
| Sipuleucel-T | 64 (32.0) | 18 (20.9) | 46 (40.4) | .004 |
| Docetaxel | 39 (19.5) | 29 (34.9) | 10 (8.8) | <.001 |
| Cabazitaxel | 6 (3.0) | 5 (5.8) | 1 (0.9) | .086 |
- Note: p Values represent a comparison between site of care (AC vs. CP).
- Abbreviations: AC, academic center; ALP, alkaline phosphatase; CP, community practice; IQR, interquartile range; LDH, lactate dehydrogenase; mCRPC, metastatic castration-resistant prostate cancer; NR, not reported; PSA, prostate-specific antigen; SSE, symptomatic skeletal event.
- a Other includes veteran affairs, no insurance/charity care/self-pay, and unknown.
- b Median was calculated from available information as there was considerable information missing.
- c Unknown in 68 patients.
- d Unknown in 12 patients.
- e Previous therapy: started/completed before radium-223 initiation.
- f Previous approved therapies: abiraterone, enzalutamide, docetaxel, cabazitaxel, and sipuleucel-T.
| Variable | Total N = 200 | AC n = 86 | CP n = 114 | p Value* |
|---|---|---|---|---|
| Number of radium-223 doses received, mean (SD) | 5.2 (1.5) | 4.8 (1.6) | 5.4 (1.3) | .001 |
| Number of radium-223 doses administered, n (%) | ||||
| 1–4 | 44 (22.0) | 27 (31.4) | 17 (14.9) | .022 |
| 5–6 | 156 (78.0) | 59 (68.6) | 97 (85.1) | |
| Treatment strategy with radium-223, n (%) | ||||
| Monotherapy | 86 (43.0) | 35 (40.7) | 51 (44.7) | .665 |
| Concurrent + layered | 114 (57.0) | 51 (59.3) | 63 (55.3) | |
| Therapy used concurrently/layered with radium-223, n (%)a | ||||
| Abiraterone | 49 (24.5) | 22 (25.6) | 27 (23.7) | .869 |
| Enzalutamide | 73 (36.5) | 32 (37.2) | 41 (36.0) | .883 |
| Sipuleucel-T | 1 (0.5) | 0 | 1 (0.9) | 1.000 |
| Docetaxel | 4 (2.0) | 4 (4.7) | 0 | .033 |
| Cabazitaxel | 0 | 0 | 0 | .000 |
| First therapy used following completion of radium-223, n (%)b | ||||
| Abiraterone | 23 (11.5) | 10 (11.6) | 13 (11.4) | 1.000 |
| Enzalutamide | 17 (8.5) | 5 (5.8) | 12 (11.5) | .309 |
| Sipuleucel-T | 7 (3.5) | 2 (2.3) | 5 (4.4) | .701 |
| Docetaxel or cabazitaxel | 38 (19.0) | 27 (31.4) | 11 (9.6) | <.001 |
| None | 115 (57.5) | 42 (48.8) | 73 (64.0) | .043 |
| Bone-targeting therapies in combination with radium-223, n (%) | ||||
| Bisphosphonate | 21 (10.5) | 10 (11.6) | 11 (9.6) | NR |
| Denosumab | 118 (59.0) | 53 (61.6) | 65 (57.0) | |
- Note: p Values represent a comparison between site of care (AC vs. CP).
- Abbreviations: AC, academic center; CP, community practice; mCRPC, metastatic castration-resistant prostate cancer; NA, not applicable; NR, not reported.
- a Concurrent + layered therapy: overlapping with radium-223 use.
- b Previous approved therapies: abiraterone, enzalutamide, docetaxel, cabazitaxel, and sipuleucel-T.
Most patients (61.5%) received at least 1 therapy before radium-223; the most common therapy for those treated at CPs was sipuleucel-T (n = 46, 40.4%), while the most common therapy in ACs was abiraterone acetate (n = 39, 45.3%).
3.2 Treatment patterns
The mean number of doses of radium-223 received was 5.2 (SD: 1.5), and this differed between site of care as noted above. Patients who completed 5–6 doses of radium-223 were more likely to have radium-223 initiated early in their treatment sequence for mCRPC than those who only completed 1–4 doses (p = .005). In the overall population, bisphosphonates or denosumab were commonly started before radium-223 (12.5% and 61%, respectively; 15.1% and 65.1%, respectively, at ACs; 10.5% and 57.9%, respectively, at CPs). Bisphosphonates and denosumab were also frequently continued in combination with radium-223 (10.5% and 59%, respectively; 11.6% and 61.6%, respectively, at ACs; 9.6% and 57.0%, respectively, at CPs). Also, 43% of patients received radium-223 monotherapy, and 57% received it concurrently with other agents (mainly abiraterone acetate and enzalutamide). After discontinuation of radium-223, 42.5% of patients went on to receive an additional line of mCRPC therapy. The most common therapy after radium-223 in all patients was docetaxel or cabazitaxel (n = 38, 19%), followed by abiraterone acetate (n = 23, 12%), enzalutamide (n = 17, 9%), and sipuleucel-T (n = 7, 4%) (Table 2).
In the subset of patients (n = 78) who received chemotherapy (specifically docetaxel or cabazitaxel), 56% (n = 44) received it before or during radium-223 administration and 44% (n = 34) received it after radium-223. Notably, patients who were treated at an AC were more likely to receive either docetaxel or cabazitaxel at some point in their treatment sequence (66% overall; 34.9% before radium-223 initiation, 31.4% after) compared to patients who were treated in a CP (18% overall; 8.8% before radium-223 initiation, 9.6% after).
3.3 Reasons for radium-223 initiation and discontinuation
Participating investigators could report multiple reasons for initiation or discontinuation of radium-223. In addition to the presence of bone metastases, the most common reason selected for radium-223 initiation in the total population was disease progression (43%) (Figure 1A). Other reasons selected for initiation included to prolong survival (32.5%), treatment guidelines (30.5%), patient symptoms (25.5%), impact on quality of life (23.5%), and unknown (7.5%). Patients treated at an AC compared to a CP were more likely to be initiated on radium-223 due to treatment guidelines (39.5% vs. 23.7%; p = .0199) and less likely due to patient symptoms (12.8% vs. 35.1%; p = .0003) or impact on quality of life (8.1% vs. 35.1%; p < .0001). There were no differences in other reasons for initiation.
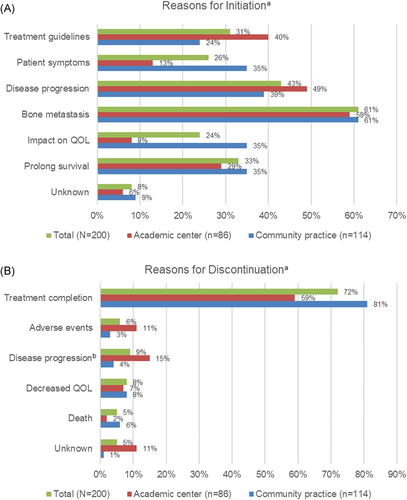
In total, 72% of patients completed treatment, defined as receiving 5–6 doses, with radium-223 (n = 143); significantly fewer patients treated at an AC completed therapy compared to those in a CP (59.3% vs. 80.7%; p = .0014). Overall, for patients who did not complete radium-223 treatment, the most frequent reason for discontinuation was progression (28%). Other reasons included decreased quality of life (23%), adverse events (19%), death (14%), and unknown reasons (16%). By site of care, patients treated at an AC were more likely to discontinue therapy due to adverse events (10.5% vs. 2.6%; p = .0323) or experience disease progression (15.1% vs. 4.4%; p = .0117) than those treated in a CP (Figure 1B).
3.4 Overall survival
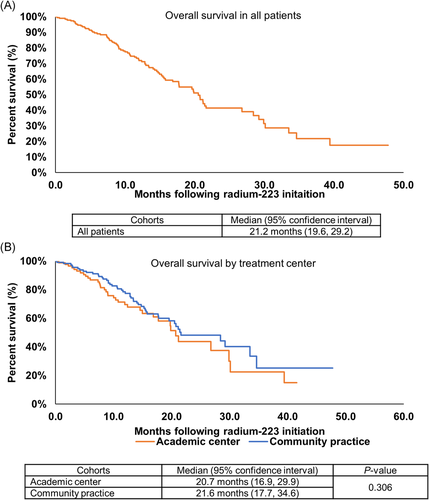
At a median follow-up for all patients from the last dose of radium-223 of 8.8 months (interquartile range [IQR]: 4.5, 15.1), 42% of patients had died. The estimated median OS from radium-223 initiation was 21.2 months (95% confidence interval: 19.6, 29.2) (Figure 2A). The median OS was not significantly different based on whether patients received radium-223 at a CP versus AC (21.6 vs. 20.7 months; p = .306) (Figure 2B). Notably, OS was significantly longer for patients who received 5–6 doses of radium-223 versus those who received 1–4 doses (23.3 vs. 6.4 months, respectively; p < .001).
3.5 Laboratory values
Laboratory values were assessed at initiation of radium-223, discontinuation, and 3 months postdiscontinuation to identify any signs of prolonged bone marrow suppression. Due to the retrospective nature of the data collection, limited values were available for inclusion at each of these time points. Despite the majority of radium-223 being administered in combination with other agents, there were no laboratory measurements indicating prolonged bone marrow suppression from initiation to discontinuation to 3 months postdiscontinuation, including hemoglobin (median: 12.2 g/dl [IQR: 11.1, 13.0; n = 141]; 11.5 g/dl [IQR: 9.9, 12.4; n = 137]; 10.4 g/dl [IQR: 8.8, 12.2; n = 77]) and platelet counts (median: 215 109/L [IQR: 170, 258; n = 138]; 192 109/L [IQR: 151, 230; n = 132]; 160 109/L [IQR: 107, 235; n = 75]).
4 DISCUSSION
This retrospective chart review evaluated the use of radium-223 in the real-world setting in a geographically diverse population. Previously, the majority of radium-223 research had been conducted at tertiary or academic sites, but recent publications have begun to illustrate real-world outcomes and treatment patterns with radium-223 in a variety of practice sites.18, 20-22, 24 However, this is the first real-world study to our knowledge to characterize differences in patterns and outcomes in CP and AC.13, 16, 17, 19, 23 The median OS of patients receiving radium-223 in our study was 21.2 months, a result that numerically exceeds the survival seen in the pivotal ALSYMPCA trial (14.0 months); contributing factors may include additional available treatments, treatment patterns, or early onset of therapy, among others.3
Given the need for multidisciplinary care and coordination to provide radium-223 treatment, understanding differences in treatment patterns and outcomes in different practice settings is imperative to identify potential barriers and further understand how this treatment can be more effectively used. In the United States, ACs only account for approximately 20% of cancer treatment sites, while health system or hospital-owned sites account for 37% and physician-owned sites account for 42%.25 In our study, despite patient characteristics and utilization differences, we did not note any significant difference in outcomes between CP and AC settings. A specific safety concern, prolonged myelosuppression, was not frequently observed 3 months posttherapy. These data support the general effectiveness and safety demonstrated in ALSYMPCA to real-world practice.
Next, patients who were able to receive 5–6 doses of radium-223 had a significantly longer median survival than those who only received 1–4 doses. This confirms findings reflected in multiple other studies13, 19, 26, 27 and is notable given that a higher proportion of patients in our study who received radium-223 in CPs were able to complete 5–6 doses (85.1% vs. 68.6%). Patients who received 1–4 doses compared to 5–6 were more likely to discontinue treatment due to adverse events and decreased quality of life (18.2% vs. 2.6% and 25.0% vs. 2.6%, respectively; p < .01), both of which may be modifiable with close patient management to help increase doses received and improve outcomes. However, approximately 50% of patients who were unable to complete therapy had progressive disease or died, highlighting the need to better understand the underlying tumor genomic alteration for those with primary refractory disease to radium-223 in order to better plan treatment sequences for this patient population.
Finally, notable in our study population was the use of other agents in combination with radium-223. When the pivotal ALSYMPCA trial was accruing, abiraterone acetate and enzalutamide were not available. In this real-world analysis, radium-223 was given in combination with these agents in 61% of patients. This is an important reflection of real-world practice and point to examine, as conflicting outcomes have been reported in studies thus far evaluating radium-223 in combination with abiraterone acetate or enzalutamide. In a Phase 2, open-label study including 25 patients who received abiraterone acetate and 15 patients who received enzalutamide, both concurrently with radium-223, the combinations were well tolerated, with no excess of SSEs observed.26 In contrast, the results of the Phase 3 ERA 223 trial noted an increased frequency of bone fractures with radium-223 and abiraterone combination therapy.28 Four patients in our study, all at AC sites, received radium-223 in combination with docetaxel. The efficacy and safety of this combination was evaluated in a Phase 1/2 trial; results included a PSA decline of more than 50% in 61% of patients receiving the combination and a progression-free survival of 12 months, as compared to 54% and 9.3 months in the docetaxel alone arm, respectively, and a greater suppression of osteoblastic activity.29 Febrile neutropenia was a dose limiting side effect. The promising results of this trial are being further evaluated in a Phase 3 trial, DORA.30 The majority of patients in our analysis received bone antiresorptive agents, including bisphosphonates or denosumab, which have been associated with reduced skeletal fractures and other SSEs when given concurrently with radium-223, a potentially critical component to future evaluation of combination therapies.21 A recent analysis confirmed these findings in the real world and showed that patients who received combination therapy and bone antiresorptive therapies had lower rates of SSEs than those who did not receive bone antiresorptive therapies and highlighted that these agents are underutilized.21 Ongoing prospective trials are evaluating varying combinations of these therapies.
4.1 Limitations
As with any retrospective study, there are inherent limitations. This study was descriptive and not powered for comparisons between subgroups. Also, comparisons may be biased by unobserved differences between groups or immortal time bias.31 For example, it appears patients treated earlier with radium-223 before chemotherapy had improved survival, but because the subgroups were defined by post-baseline criteria, the criteria may not be independent of the outcome and additional variables may be driving the perceived difference, such as disease burden, stage at treatment initiation, or other unaccounted for variables such as access to care. An attempt to mitigate selection bias was made by incorporating a randomization method for eligible patient selection. Patients in the AC cohort had numerically higher baseline PSA and alkaline phosphatase laboratory values than those in the CP cohort, but we did not compare these values statistically. These differences may reflect a higher tumor burden in patients seeking care at AC, a selection bias in which patients chose to receive treatment at an AC as a second opinion or pursuing a clinical trial, or others. While these factors may have influenced the makeup of our cohorts, it also reflects real-world practice differences and therefore, our results showing no difference in outcomes between practice sites remain relevant and reassuring. While this analysis focused on patients treated at ACs vs CPs, there are other factors that may affect treatment patterns within each type of location, including primary specialty of provider (urology, oncology, nuclear medicine), geographic location (urban, suburban, rural), number of years of experience of treating provider, and others that were not included in the analyses in this study. Recent studies have shown an OS benefit in patients with mCRPC that have genetic aberrations associated with homologous recombination deficiency (HRD) receiving radium-223. As we did not have this data, we were unable to control for the presence or absence of HRD in our cohorts or its potential impact on our results.32, 33 Finally, data collection was dependent upon the completeness of the information contained in each chart and reported on the electronic case report form.
5 CONCLUSIONS
This study represents a real-world evidence study of patients on radium-223, with patients included from a broad geographic scope and from both AC and CP settings. Differences in patient characteristics and practice patterns between AC and CP settings did not result in a difference in OS, an important point demonstrating that patients in CPs can potentially benefit similarly compared to those at ACs. While there was no difference in OS, patients treated in ACs received chemotherapy more often as part of their treatment at any point compared to those at CPs. In this real-world cohort, a large proportion of patients received radium-223 concurrently with abiraterone acetate or enzalutamide (24.5% and 36.5%, respectively), and the majority of patients were able to complete 5–6 doses of radium-223. Outcomes of patients who received only 1–4 treatments, primarily due to disease progression, were extremely poor. Future research should continue to examine optimal management strategies and treatment sequencing in patients with mCRPC.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the contributions of Stacey Simmons and Adrianna Valderrama to the conceptualization, design, and analysis of the data supporting this manuscript. Bayer HealthCare Pharmaceuticals, Inc. was involved in the study design, analysis, and publication. All authors vouch for the accurate representation of the data within this manuscript.
CONFLICT OF INTERESTS
Sreevalsa Appukkuttan and Jeffrey Weiss* are employees of Bayer Healthcare Pharmaceuticals Inc., the funding body for this study. Che-Kai Tsao has no relevant disclosures. Oliver Sartor is a consultant for Advanced Accelerator Applications (AAA), Astellas, AstraZeneca, Bayer, Blue Earth Diagnostics, Inc., Bavarian Nordic, Bristol Myers Squibb, Clarity Pharmaceuticals, Clovis, Constellation, Dendreon, EMD Serono, Fusion, Isotopen Technologien Meunchen, Janssen, Myovant, Myriad, Noria Therapeutics, Inc., Novartis, Noxopharm, Progenics, POINT Biopharma, Pfizer, Sanofi, Tenebio, Telix, and Theragnostics; he receives grant or research support from Advanced Accelerator Applications, AstraZeneca, Bayer, Constellation, Dendreon, Endocyte, Invitae, Janssen, Merck, Progenics, Sanofi, and SOTIO.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- * Affiliation at the time study was conducted.




