In This Issue
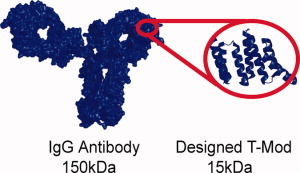
762 Redesign of a protein–peptide interaction: Characterization and applications
Meredith E. Jackrel, Roberto Valverde, and Lynne Regan
The specific identification of tagged proteins is essential for a wide range of biological applications. Most of these techniques currently employ antibodies, but antibodies are by no means ideal—they are large, unstable, and time-consuming to produce, and their affinity cannot be rationally tuned. In this study, the authors present the design of a new class of detection agents based on the tetratricopeptide repeat protein scaffold and demonstrate the facile replacement of these proteins, termed T-Mods, for antibodies in both detection and purification applications.
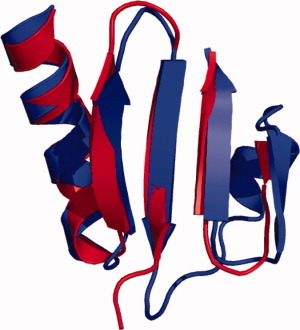
850 A protein encoded by a new family of mobile elements from Euryarchaea exhibits three domains with novel folds
J. Keller, N. Leulliot, N. Soller, R. Vincentelli, P. Forterre, and H. van Tilbeurgh
Sequencing of viral genomes and plasmids sequences has revealed that they encode for a higher proportion of orphan proteins than cellular genomes. This could be due to rapid divergence of their protein sequence compared with their cellular homologues. Alternatively, viruses and plasmids might represent an immense reservoir of new protein folds, outnumbering those already identified. Keller et al. investigated these questions by studying protein structures from archaeal plasmids and viruses. In this study, the authors demonstrate that the structure of a large protein (pT26-6p) encoded by a Thermococcus plasmid consists of three domains each with a unique fold.
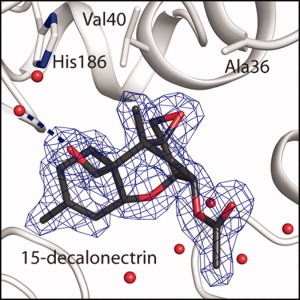
747 Structural and functional characterization of TRI3 trichothecene 15-O-acetyltransferase from Fusarium sporotrichioides
Graeme S. Garvey, Susan P. McCormick, Nancy J. Alexander, and Ivan Rayment
Fungal head blight is a devastating disease of cereal crops caused by a fungal species that belong to the genus Fusarium and produce a wide variety of mycotoxins. This study focuses on the structure and function of TRI3, an acetyltransferase that modifies the C15 position of the 12,13-epoxytrichothecene skeleton. Shown here is the structure of 15-decalonectrin bound to the active site, which is highly conserved across all Fusarium species. Together with a genetic deletion analysis this study shows that Tri3 is an obligatory enzyme in the trichothecene biosynthetic pathway and hence is a potential target for controlling fungal head blight.