Domain swapping in the kinase superfamily: OSR1 joins the mix†
As explained in the accompanying Editorial, this article is not only a scientific commentary, but also serves to introduce new interactive molecular graphics features for Protein Science. In the electronic version of the article, each Protein Data Bank (PDB) identification code is a hypertext link to an interactive image of the protein in question. Similarly, the words “interactive figure” in figure captions are hypertext links to interactive versions of the figures.
Protein kinases constitute a large family of proteins that participate in the majority of cell signaling pathways.1 Kinases act as mediators of a variety of biological processes in both normal physiology and pathogenesis, through specific interactions with upstream and downstream signaling molecules. Most kinases are multidomain proteins consisting of a catalytic kinase domain and several regulatory domains. The structures of kinase domains are conserved and have a two-lobed architecture consisting of a predominantly β-sheet N-terminal lobe and a predominantly α-helical C-terminal lobe.2 In contrast, the regulatory domains of different kinases often have distinct overall folds and local structural motifs required for maintaining pathway specificity. Structure determinations of protein kinases have provided more detailed descriptions of the regulation of kinase activity at the molecular level; however, the mechanisms of critical events, such as autophosphorylation, are still not fully understood.
Some recently determined structures of kinase domains, including Ste20-like kinase (SLK; PDB 2JFL, 2J51) and lymphocyte-originated kinase (LOK; PDB 2J7T) of the Ste20 family and death-associated kinase 3 (DAPK3, PDB 2J90) and checkpoint kinase 2 (CHK2, PDB 2CN5) of the CaMK family, suggest that domain swapping serves as a possible mechanism for trans autophosphorylation between two identical protein kinases.3, 4 In a domain-swapped dimer, one structural element of a molecule is replaced by the identical element from the partner molecule. Domain swapping is a general mechanism for forming protein oligomers and an efficient one from the evolutionary point of view, because the interactions between monomers in the domain-swapped interface are native-like and new recognition sites need not be evolved. In the February, 2009 issue of Protein Science, Lee et al.5 reported the X-ray crystal structure of the kinase domain of oxidative stress responsive 1 (OSR1), which represents another example of a domain-swapped protein kinase. A similar report by Villa et al.6 appeared in the December 2008 issue of Proteins: Structure, Function and Bioinformatics. The structural coordinates have been deposited in the Protein Data Bank (PDB) as 3DAK and 2VWI, respectively. OSR1 is a Ser/Thr protein kinase belonging to the Ste20 family. It is one of the two human homologues of the putative Drosophila mitogen-activated protein kinase kinase kinase kinase (MAP4K) Fray,7 and a component of the recently identified WNK-OSR1/SPAK pathway, which is responsible for cell volume control and ion homeostasis in mammals and is activated by osmotic stress.8 In the pathway, with-no-lysine kinases (WNKs) activate OSR1, which in turn activates the Na+/K+/2Cl− cotransporters through direct phosphorylation.9, 10 Mutations in WNKs lead to Gordon's syndrome, an autosomal dominant form of hypertension.11 Full-length OSR1 contains an C-terminal extension, residues 291–527; within this sequence, the unique PF1 region, residues 291–344, is required for kinase activity, although the basis for this requirement is unknown.7 The structures of the OSR1 kinase domains reported by Lee et al.5 and Villa et al.6 used constructs that encompassed residues 1–295 and 1–303, respectively, and neither contains a complete PF1 region.
Both structural studies show that the OSR1 kinase domain forms a domain-swapped dimer (Fig. 1). The dimer interface is formed by exchange of the P+1 loop, located in the C-terminus of the activation segment, and the following helix αEF (Fig. 2). The interface is stabilized by a salt bridge between Glu196 in helix αEF of one monomer and Arg279, located between helices αI and αJ, in the other monomer, as well as van der Waals contacts between several hydrophobic residues from helix αEF of one monomer and the hydrophobic pocket located between helices αG and αF of the partner monomer. The activation segment, which commonly is 20–30 residues in length and contains one or several phosphorylation sites, is a critical structural element for regulation of kinase activity.12 Electron density is not clearly defined in either study for the phosphorylation site, Thr185, or the C-terminal part of the activation loop. The partially disordered activation segment suggests that the OSR1 kinase domain structures represent an inactive conformation, because a correctly positioned activation segment is required for ATP binding.
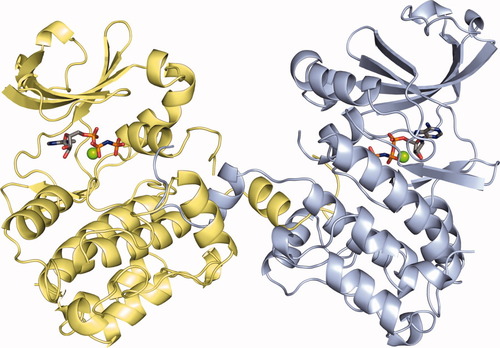
The kinase domain of oxidative stress responsive 1 (OSR1) forms a domain-swapped dimer. Each monomer binds one molecule of Mg-AMP-PNP. The two monomers from the coordinate file PDB 3DAK are depicted in blue and yellow, respectively; the AMP-PNP molecules are shown as CPK-colored bonds; and the Mg2+ ions are shown as green spheres. An interactive view is available in the electronic version of the article. Interactive View
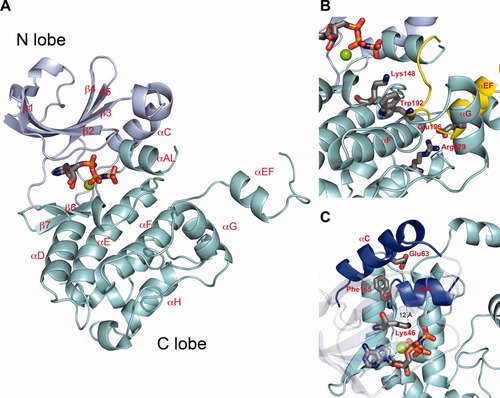
Structure of the OSR1 kinase domain monomer. (A) The first monomer from the coordinate file PDB 3DAK is shown. OSR1 has the typical bilobal kinase architecture consisting of a largely β-sheet N-terminal lobe (blue) and a helical C-terminal domain (aquamarine). (B) The domain-swapped interface between monomers contains a salt bridge between Arg279 of one monomer and Glu196 of the partner molecule, a cation-π interaction between Lys148 of one monomer and Trp192 of the partner molecule, and hydrophobic interactions between residues in αF and αG of one monomer with residues in αEF of the partner. (C) The structure shows characteristics of an inactive kinase, including absence of an ion pair between Lys46 and Glu63. An interactive view is available in the electronic version of the article, which also depicts superpositions with other kinase structures.. Interactive View
As a consequence of domain swapping, the activation segment of the OSR1 kinase domain is displaced from the core of the molecule. This outward movement has been observed in the structure of catalytically active cyclin-dependent kinase 2 (Cdk2) in complex with a phosphatase.13 Solution NMR and kinetic data on dimeric p21-activated kinase 2 (PAK2) combined with the crystal structure of the highly homologous p21-activated kinase 1 (PAK1, PDB 1YHV) in its active state suggest that dimerization, which allows positioning of the activation loop of one monomer in the active site of the partner molecule, is essential for the trans autophosphorylation reaction.14, 15 The structural similarity of OSR1 dimer to these previously characterized active complexes supports a role of domain swapping in the trans autophosphorylation reaction. However, the OSR1 dimer also displays features similar to several kinases for which the crystal structures of inactive dimers have been reported,2, 16-18 in particular the lack of a properly positioned Asp-Lys-Thr catalytic triad. These observations imply that if domain swapping is associated with trans autophosphorylation, then an additional conformational transition is required to activate the kinase.
Lee et al. did not identify any key differences in the amino acid sequences between homologous domain-swapped and non-swapped kinases. Swapped domains often do not share similarity in sizes and sequences, based on the currently available high-resolution structures.19 Owing to the relatively small differences in free energy of monomers and oligomers, many domain-swapped oligomers show low-affinity and are only observed in crystals.20 Indeed, OSR1 kinase domain is monomeric in solution.5, 6 In contrast, the previously identified domain-swapped kinase domains of SLK, LOK, and DAPK3 form stable dimers in solution.4 Biochemical data for these kinases strongly support the involvement of dimers in the autophosphrylation reaction. However, the functional relevance of dimerization cannot be judged solely from the affinity in aqueous solution and coimmunoprecipitation experiments indicate that both the kinase domain alone and the full-length OSR1 are capable of forming oligomers in cells.10
Intrinsic conformational plasticity is critical for structural transitions between active and inactive states of protein kinases, and biophysical evidence suggests that conformational dynamics may be coupled to catalysis.21-24 Results from NMR spectroscopy and molecular dynamics simulations have linked conformational flexibility to domain swapping in other protein molecules.25, 26 Therefore, domain swapping may be a consequence of the intrinsic conformational flexibility of kinase domains. The impact of domain swapping on the autophosphorylation reaction, if indeed they are coupled, remains to be fully elucidated, and additional structural and biochemical data are required to clarify the functional role of domain swapping for OSR1. Nonetheless, the observation of domain swapping in OSR1 serves as indirect evidence for the expanding role of conformational dynamics in protein kinase catalysis and regulation.




