Prion diseases: Lessons from historical outbreaks and potential emerging ones
Review Editor: Aitziber L. Cortajarena
Abstract
Prion diseases (PrDs) are a unique and fatal class of neurodegenerative disorders caused by misfolded proteinaceous infectious particles, or prions. While the pathogenic form was first documented in humans nearly a century ago, the global monitoring of PrDs only gained momentum after the “Mad Cow” epizootic and its human counterpart of the 1980s and 1990s. Currently, 34 countries track human prion cases annually, with over 27,000 cases. However, true prevalence estimates suggest significantly higher numbers, millions, highlighting the urgency of addressing these enigmatic diseases. Prions are exceptionally resilient, resisting conventional sterilization methods and persisting in environmental reservoirs, such as soil and plants, raising concerns about environmental and cross-species transmission, particularly with the growing prevalence of chronic wasting disease (CWD) in cervids. This review explores the history, pathogenesis, presence, public health implications, and novel innovations in studying and treatment of PrDs. Future priorities should include the development of faster, cost-effective diagnostic tools and systemic therapies to neutralize prions in affected individuals and mitigate environmental risks. Understanding and addressing the challenges posed by prions is critical for global health security in the wake of CWD.
1 INTRODUCTION
It took almost 70 years after the first few documented cases of human prion disease by Drs. Hans Creutzfeldt and Alfon Jakob (independently) for the world to start monitoring the amount of growing cases of human prion disease (Creutzfeldt 1989). Albeit, this switch came at the forced behest of the “Mad Cow” epizootic of the 1980s that affected at least 24 countries at the time (CDC 2024). Currently, 34 countries record and publicly report prion disease (PrD) cases in humans per year (Gao et al. 2024). A study by Gao et al. revealed that in 2024, there were 27,872 cases of PrD among the 34 countries, with the United States in the lead (5156 total cases, France was second with 3276 cases) (Gao et al. 2024). Since 1993, we have begun monitoring seven main human PrDs. They consist of five human-specific diseases—(1) (sporadic) Creutzfeldt-Jakob disease (sCJD), (2) Kuru, (3) fatal familial insomnia (FFI), (4) Gerstmann-Sträussler-Scheinker (GSS) disease, (5) variably protease-sensitive prionopathy (VPSPr)—and two more diseases that affect animals and humans—(6) bovine spongiform encephalopathy (BSE) and (7) variant CJD (vCJD) (caused by the same prion) (Hill et al. 1997). CJD is the most commonly diagnosed PrD, accounting for 24,623 of the 27,872 cases aforementioned. Unfortunately, researchers expect the true numbers of PrD to be somewhere in the millions, after over half a million people were genotyped and compared from 23andMe Inc. genome data in 2016 (Minikel et al. 2016).
PrDs are uniquely terrifying due to their unconventional pathogenesis. Unlike typical pathogens, abnormal prions are not microorganisms but rather misfolded proteinaceous infectious particles, as first identified by Dr. Stanley Prusiner in 1982 (Prusiner et al. 1982a). Since then, we have determined that when the normal cellular prion protein (PrPC) misfolds into the infectious conformation (PrPSc), it can bind and misfold other PrPCs. These rogue proteins are capable of transmitting fatal, chronic diseases between animals and humans. Despite their simplicity, PrPScs exhibit remarkable resilience, remaining active at temperatures exceeding 600°C and resisting conventional sterilization methods such as autoclaving (at standard 120°C), UV radiation, and formaldehyde cleaning (Brown et al. 1990; Brown et al. 2000; Safar et al. 1993). This resilience underscores the critical need to develop effective containment and treatment strategies.
Several outbreaks of PrD over the years have led to extreme decisions, such as when South Korea put a decade-long ban on beef trade from the United States in 2003 after BSE prions were found in a dairy cow in Washington state (Jurenas and Manyin 2010). Although the ban was lifted early in 2008 with the signing of the U.S.-Korean Beef Protocol, fears surrounding prions ignited public riots that year (France24 2008), reflecting the widespread apprehension these diseases incite. A rising PrD, chronic wasting disease in deer and other cervids across the world shows transmissibility to other primates and animals, but no known human cases have surfaced. Nonetheless, historical human outbreaks emphasize the importance of acting quickly to address the rising threat of this cervid outbreak.
The following review will delve into the history, pathogenesis, innovations, and challenges surrounding prion diseases. By examining their prevalence, diagnostic advancements, and ongoing research, this review aims to highlight the urgent necessity of addressing these enigmatic diseases. Understanding their nature and implications is vital for improving public health responses, mitigating risks, and fostering scientific breakthroughs in combating these resilient and deadly proteins.
2 MECHANISMS OF PRION DISEASES
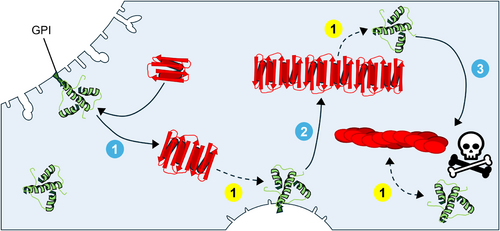
Prions are naturally occurring proteins in humans, expressed predominantly in neuronal cells. The normal cellular form, PrPC, is a membrane glycoprotein post-translationally modified with a glycosylphosphatidylinositol (GPI) anchor that tethers it to the outer leaflet of the plasma membrane, particularly in lipid rafts (Prusiner 1992). The PrPC, encoded by the PRNP gene, typically adopts a structure rich in α-helices. However, mutations or interactions with an abnormal isoform—either exogenous or endogenous—can induce a conformational shift, resulting in a structure predominantly composed of β-sheets. This structural transformation underpins the widely accepted “protein-only hypothesis,” which posits that prions propagate to form associated pathologies without the need of nucleic acids or other cofactors. Abnormal prions belong to a broader class of proteins called amyloids, which are characterized by their ability to misfold other proteins, leading to loss of normal protein function and associated pathologies. The specific mechanism for prion propagation starts with the abnormal β-sheet rich PrPSc monomer or aggregated assemblies and after coming into contact with the normal, cellular counterpart, PrPC, induces the conversion into more of the misfolded, disease-associated form. Figure 1 illustrates the prion propagation cycle using cryo-electron microscopy-derived structures of aRML murine PrPC (Kraus et al. 2021). While the typical PrPSc structure has over four beta-sheets, for simplicity, this structural model of PrPSc will be used in subsequent figures unless otherwise noted, though it is also acknowledged that each prion strain exhibits distinct morphological variations (Alam et al. 2024; Kraus et al. 2021). Furthermore, it is largely understood that the C-terminal domains of both proteins, the β-solenoid C-terminus of the PrPSc and the α-helical C-terminus of the PrPC, are what bind and misfold the PrPC into a larger, pathogenic PrPSc aggregate (Lau et al. 2015). However, the octapeptide-repeat region of the N-terminals in both PrPs is said to play a role in the rate of aggregation and strain specificity (Lau et al. 2015).
Amyloidogenic diseases, including Alzheimer's, Parkinson's, and Huntington's diseases as well as some forms of Type II Diabetes and transthyretin amyloidosis, are all marked by proteins that, when misfolded, propagate this misfolding to normally structured proteins. This feature leads to the development of harmful aggregates, or amyloid fibrils, which impair cellular function. Although PrPScs are categorized as amyloids, they uniquely stand out for their infectious nature, spreading not only between cells but also across organisms.
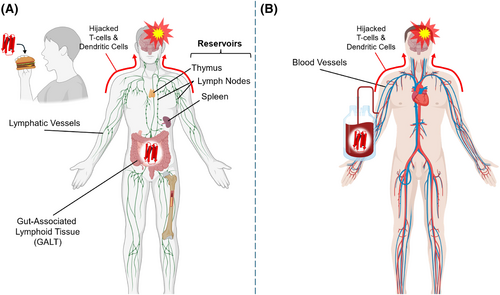
While amyloid diseases like Alzheimer's and Parkinson's involve the misfolding and propagation of specific proteins—such as amyloid-beta in Alzheimer's, or alpha-synuclein in Parkinson's—these misfolded proteins do not (usually) spread beyond the individual (Lücking and Brice 2000; Yankner and Mesulam 1991). Recently, Banerjee et al. demonstrated the successful development of several iatrogenic cases of Alzheimer's disease. They concluded that despite these cases, the transmissibility of the proteins responsible still appears to be significantly rare (Banerjee et al. 2024). Abnormal prions can be transmitted between humans and animals due to their remarkable stability and resistance to degradation by digestive enzymes, allowing them to survive gastrointestinal transit. They can then enter the lymphatic system, specifically the gut-associated lymphoid tissue (GALT) and are transported to immune cells via M-cells within the lining of the gut. PrPSc reaches and travels through neurons using the hijacked immune cells (primarily dendritic cells and T-lymphocytes) and retrograde axon transport (Figure 2a) (Gousset et al. 2009; Moya et al. 2004). While in the lymphatic system, PrPSc can also travel and reside in the many lymph nodes and lymphoid organs, such as the spleen and thymus, propagating their misfolding as their concentration increases, and eventually entering immune cells through circulation (O'Connor et al. 2012; Prinz et al. 2002). Additionally, PrPSc can be introduced into the bloodstream through contaminated blood transfusion and cross the blood–brain barrier through the same mechanisms (Figure 2b) (Hanna Lewicki et al. 2003; Rosicarelli et al. 2005). This distinction makes prions not only amyloidogenic but also transmissible across species (Béringue et al. 2012; Bieschke et al. 2004; Tanaka et al. 2005), adding a public health dimension largely absent in other amyloid-related diseases.
Oddly, not all species of animals are at risk of developing PrDs from certain strains of PrPSc. The reasoning for this is termed nonadaptive prion amplification (NAPA). NAPA is the phenomenon whereby prions can replicate in a host species without necessarily causing disease. This concept helps explain why classical Scrapie, the most common PrD affecting sheep and goats, is considered nontransmissible to humans. Krejciova et al. (2014) showed that differences in protein glycosylation and chaperone interactions influence prion susceptibility, further supporting the species barrier hypothesis. Furthermore, Jones et al. (2023) demonstrated that certain amino acid variations in the PrP gene create structural incompatibilities that prevent efficient conversion of PrPC into PrPSc. Beyond genetic factors, some studies suggest that variations in the gut microbiome may also play a role in determining prion susceptibility. Some microbial communities appear to degrade prions before they can enter systemic circulation, thereby reducing the risk of infection (Dagleish et al. 2010; Jeffrey et al. 2006). Similarly, differences in immune system function, such as variations in microglial response and lysosomal degradation pathways, have been proposed as key factors limiting prion propagation in resistant species (de Melo et al. 2021; Heiseke et al. 2010).
The spread of prions is further complicated by their potential to cross-seed other proteins (Wang and Hall 2018), potentially linking prion diseases to conditions like Alzheimer's or Parkinson's through accelerated protein aggregation (Katorcha et al. 2017). For instance, studies suggest that PrPSc can propagate in the presence of alpha-synuclein, a hallmark of Parkinson's disease, and amyloid-beta, common in Alzheimer's, contributing to prion misfolding (Katorcha et al. 2017; Ren et al. 2019). These interactions broaden the pathological scope of PrDs, presenting unique challenges in distinguishing their effects from those of other neurodegenerative conditions.
In addition to their transmissibility, PrPScs cause damage to the brain by inducing spongiform degeneration. As misfolded PrPSc accumulates, it disrupts neural architecture, forming insoluble aggregates and creating microscopic holes in the brain tissue. These holes give rise to the characteristic “spongy” appearance seen in transmissible spongiform encephalopathies (TSEs); another term commonly used for PrDs. The loss of neurons and supporting glial cells contributes to the rapid cognitive and motor decline associated with prion diseases.
In summary, prion diseases stand out in the broader category of amyloids due to their infectious potential and cross-species transmission. While sharing common misfolding mechanisms with diseases like Alzheimer's and Parkinson's, prions pose a significantly greater propensity to affect and infect more people.
3 HISTORY OF PRION OUTBREAKS
The world often prioritizes attention and resources toward diseases only after significant outbreaks occur, a phenomenon driven by the visible threat to public health, economic stability, and societal norms during such crises. For prion diseases, this reactive approach is evident in the historical handling of outbreaks like Kuru in Papua New Guinea and the BSE epizootic in the United Kingdom. In both cases, widespread concern and research funding surged only after the diseases reached epidemic proportions, revealing their devastating impacts. For instance, the BSE crisis in the 1980s spurred international regulations on cattle feed and the slaughtering process after the link to vCJD became apparent. Similarly, Kuru prompted significant anthropological and biomedical research only after its unique transmission mechanism via ritualistic cannibalism was uncovered. Major studies, such as Gajdusek's Nobel Prize-winning work on Kuru transmission (1976) and Prusiner's discovery of prions as infectious agents (1982), exemplify how outbreaks serve as catalysts for breakthroughs in scientific understanding and policy reform. This pattern highlights a critical gap in proactive disease prevention, with the world often underestimating emerging threats until they manifest as public health crises.
The following discussion on the history of prion outbreaks worldwide will focus on the mechanisms of disease spread, the populations most at risk, and the strategies employed to control and mitigate the outbreaks, where such information is available.
3.1 The Kuru epidemic (1900s–2000s)
The Kuru epidemic, one of the earliest recognized human prion diseases, wiped out about 30% (at least 3000 individuals) of the Fore people of Papua New Guinea in the 20th century (Gajdusek and Zigas 1957). This devastating outbreak is notable for its unique cultural context, its mode of transmission through ritualistic practices, and the eventual measures that led to its decline. Kuru, derived from the Fore word meaning “to shake,” was so named due to the characteristic tremors it caused in its victims.
The Kuru epidemic is believed to have started in the early 20th century, approximately 1901–1902, though it was not widely documented until the 1950s (Goldfarb 2002). It arose in the Eastern Highlands of Papua New Guinea, among the Fore people who practiced ritualistic cannibalism as part of their funerary rites. This tradition involved consuming the bodies of deceased relatives to honor their memory and ensure the safe passage of their spirits. The brains of the deceased, which were considered the seat of wisdom and strength, were often consumed by women and children, while men typically ate the more prestigious parts of the body, including muscle tissue.
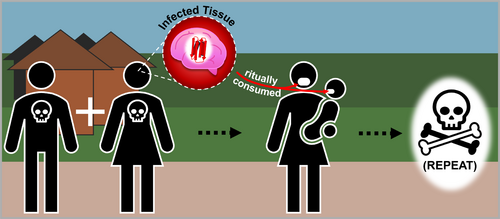
The transmission of Kuru was driven by this ritualistic consumption of brain and central nervous system tissues, which harbor the highest concentrations of PrPScs (Figure 3). Through this practice, infectious prions were introduced into the bodies of participants, facilitating the spread of the disease. Women and children, being the primary consumers of these high-risk tissues, bore the brunt of the epidemic, resulting in a disproportionate impact on these demographic groups. This selective vulnerability not only amplified the toll of the disease but also posed profound challenges to the Fore community's societal continuity, as it undermined the health and survival of key members responsible for caregiving and the perpetuation of cultural traditions.
Additionally, the incubation period for Kuru was unusually long, ranging from 5 to over 40 years (Collinge et al. 2006; Gajdusek 1996), which allowed the disease to persist and spread undetected for decades before symptoms appeared. This allowed for an untold number of individuals to become infected as individuals died before symptoms manifested.
In the 1950s, Australian government officials and anthropologists declared Kuru an epidemic and intervened. It was Dr. Carleton Gajdusek, a researcher, who identified the connection between Kuru and the Fore's cannibalistic practices by successfully transmitting the disease to chimpanzees fed on infected tissue (Steadman and Merbs 1982). Public health efforts focused on educating the Fore people about the risks associated with these rituals and encouraging the abandonment of cannibalism.
Although the practice of ritualistic cannibalism gradually ceased during the 1950s and 1960s, the epidemic persisted for decades due to Kuru's prolonged incubation period. Cases continued to emerge sporadically even into the 21st century, with the last reported case occurring in 2008 (Brandner et al. 2008). This extended timeline highlights the unique challenge posed by prion diseases, which can remain latent for years before symptoms develop.
A year or so after the last reported case of Kuru, scientists discovered a probable resistance factor at codon 129 of PRNP by Mead et al. (2009). The variant, PRNP G127V, was found only in homozygous individuals and was not found in patients infected with Kuru. They concluded that the variant was an acquired prion disease resistance factor, rather than a pathogenic mutation. Knowledge of this novel variant, and those alike, puts us closer to engineering protective proteins against PrPSc.
The Kuru epidemic represents a tragic yet illuminating chapter in the history of human prion diseases. Its spread through ritualistic cannibalism highlights the interplay between cultural practices and disease transmission, while the disproportionate impact on women and children underscores the social dimensions of health risks. The eventual decline of the epidemic demonstrates the importance of culturally sensitive public health interventions. Furthermore, the scientific discoveries stemming from Kuru research have had a lasting impact on the study of prion diseases, contributing to our understanding of diseases like CJD and BSE, and propelling us toward new therapeutic strategies. The story of Kuru remains a vital reminder of how deeply cultural practices can shape the dynamics of disease and the critical role of public health efforts in mitigating their impact.
3.2 The iatrogenic CJD outbreaks (1970s–2010s)
Iatrogenic means having an illness as a consequence of medical examination or treatment. When iatrogenic Creutzfeldt-Jakob Disease (iCJD) was first discovered in the 1970s (Brown et al. 2012), it exposed critical vulnerabilities in medical practices and underscored the importance of implementing strict safety protocols. iCJD demonstrated how medical procedures, although meant to save lives or improve health, could inadvertently become vehicles for the transmission of devastating diseases when proper precautions were neglected.
The origins of iCJD can be traced to several medical practices that involved human-derived biological materials or reused equipment (Figure 4). Among the primary sources of transmission were dura mater grafts harvested from cadavers, cadaveric pituitary glands used in human growth hormone (hGH) therapy, and contaminated neurosurgical instruments (Brown et al. 2012). In each case, prions responsible for the disease persisted due to their resistance to standard sterilization techniques, such as autoclaving, and were inadvertently introduced into patients' bodies. This resilience and resistance against denaturation can be attributed to their propensity to adhere to metallic surfaces via their octapeptide repeat domain (Guadagno and Medina 2023).
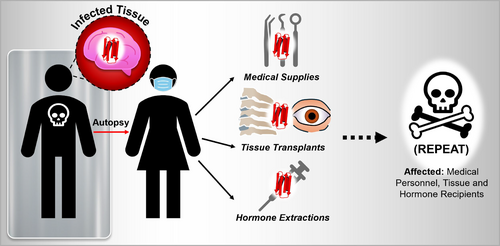
The affected populations were often patients undergoing specialized treatments. For example, children receiving cadaver-derived hGH therapy for growth deficiencies were particularly vulnerable, as the prion-contaminated material was administered during critical periods of growth and development. Similarly, individuals undergoing neurosurgical procedures or receiving dura mater grafts faced heightened risks if the instruments or materials used had been exposed to infected patients. Unlike sporadic or inherited forms of CJD, iCJD was preventable, which made its occurrence all the more tragic.
The clinical progression of iCJD mirrored that of other prion diseases, characterized by rapidly progressive neurological decline, dementia, myoclonus, ataxia, and visual disturbances. However, the disease's long latency period—ranging from a 4.5 to over 40 years—complicated the identification of its origins (Rudge et al. 2015; Will 2003). This prolonged incubation period allowed the disease to spread undetected, remaining dormant until it was eventually linked to specific medical practices 20 years after the illness was discovered (Brown et al. 2012).
Once the connection between iCJD and medical practices was identified, a series of interventions were implemented to mitigate the risk. The use of cadaver-derived hGH was discontinued and replaced with recombinant DNA-derived alternatives, eliminating the potential for prion contamination. Similarly, synthetic materials replaced dura mater grafts harvested from cadavers. More rigorous sterilization protocols for neurosurgical instruments were introduced, including methods specifically designed to target the prions' unique resilience, such as sodium hydroxide treatments, to neutralize the protein.
In addition to these changes, robust monitoring systems were established to identify and track cases of iCJD. These registries provided valuable data that allowed researchers and healthcare providers to better understand the scope of the issue and prevent future outbreaks. Despite these preventive measures, the long incubation period of iCJD meant that new cases continued to emerge for years after the control measures were put in place. By the early 2000s, however, the incidence of iCJD had substantially declined, indicating the effectiveness of these efforts. By 2012, the outbreak had largely subsided, with over 96% of all iCJD cases occurring prior to that year.
The legacy of iCJD extends beyond the containment of the outbreaks themselves. The occurrence of these outbreaks exposed the potential risks associated with medical innovation and emphasized the need for ongoing vigilance in the development and application of new treatments. The outbreaks also spurred significant advances in prion research, enhancing our understanding of how prions resist conventional sterilization techniques and how they might be neutralized. Furthermore, regulatory reforms were enacted to ensure that biological products used in medical treatments met stricter safety standards, and international collaboration became essential in prion disease surveillance and prevention.
The story of iCJD serves as a reminder that even the most well-intentioned medical practices carry inherent risks. Unlike the Kuru epidemic, which was directly linked to cultural practices, iCJD emerged from advancements in healthcare, complicating the ethical and scientific dimensions of the response. The history of iCJD highlights the necessity of balancing innovation with caution and the importance of learning from past mistakes to protect future generations.
3.3 The “Mad Cow” epizootic-epidemic (1980s–2000s)
The “Mad Cow” epizootic-epidemic, one of the most widely recognized outbreaks of prion disease, had a significant impact on public health, animal agriculture, and food safety regulations. Before the epizootic began, it was first seen in the 1980s' United Kingdom, where BSE, commonly known as Mad Cow Disease, was discovered in cattle. BSE is a prion disease that affects cattle's central nervous system, leading to progressive neurological degeneration, including symptoms such as uncoordinated movement, tremors, and changes in behavior. BSE was linked to the consumption of contaminated feed, or meat-and-bone meal, containing infected cow remains, which led to the spread of the disease among cattle populations.
The first cases of BSE were reported in the UK in 1986, and the epizootic quickly escalated as the number of affected cattle grew (Wilesmith et al. 1988). The disease spread within the cattle population through this practice. By 2008, there were more than 184,000 BSE cases in cattle in the UK (Brown et al. 2001).
The connection between BSE and its human counterpart, vCJD, became clear in the 1990s (Bruce et al. 1997; Collinge et al. 1996; Will et al. 1996). The human transmission of the disease is what sparked the common (mis)classification of the “Mad Cow” epizootic as an epidemic. The first human cases of vCJD were reported in the UK in 1996 (Will et al. 1996), raising fears of a new prion disease that could have devastating consequences for public health. vCJD shares many clinical features with classic CJD, including cognitive decline, memory loss, ataxia, and myoclonus. However, vCJD differs in that it primarily affects younger individuals and tends to have a longer disease course before symptoms manifest. The disease's symptoms were initially thought to be consistent with other neurological conditions, which delayed the identification of vCJD as a distinct prion disease related to BSE. For clarity, the abnormal prion responsible for BSE in cattle is the same prion transmitted to humans that develop vCJD.
Scientists immediately evaluated the potential of bovine products to carry transmittable prions. No evidence suggested that bovine milk, colostrum, or tissues of the mammary gland were capable of transmitting prion from BSE-diseased cattle (Vetrugno 2004). It was the consumption of infected beef products that was identified as the key route of transmission for vCJD in humans (Figure 5) (Bruce et al. 1997). As BSE-infected cattle were slaughtered and their meat entered the food supply, people who consumed contaminated beef could have ingested prions, which eventually led to the development of vCJD. It became evident that prions were capable of crossing the species barrier from cattle to other animals and cattle to humans.
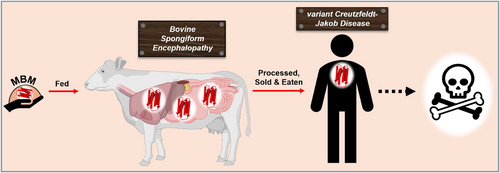
The human toll of the vCJD epidemic was tragic, with 178 confirmed cases of vCJD in the UK and a few additional cases worldwide (Ritchie et al. 2021), though the numbers remained low due to swift interventions in food safety and regulations. The outbreak led to widespread changes in agriculture and food safety practices, including the banning of high-risk materials such as brains and spinal cords from cattle feed, and later a complete ban on feeding animal-derived protein to livestock. These measures were designed to prevent the further spread of BSE in the cattle population and eliminate the risk of human exposure to prions in contaminated beef products. The UK government and international health organizations also implemented stringent testing protocols for cattle to identify and remove infected animals from the food supply.
The impact of the “Mad Cow” epizootic extended far beyond the immediate human health crisis. The outbreak severely damaged public trust in the safety of beef products, leading to significant economic losses in the livestock industry, with countries around the world placing bans on UK beef imports. The fear of vCJD also spread to other countries, including the US, prompting international collaboration to prevent the disease from entering global food chains.
While the “Mad Cow” epizootic and its link to vCJD had a devastating impact, they also spurred significant advances in prion research and prion disease management. The scientific community gained a better understanding of how prions can spread through animal populations and cross species barriers. Enhanced surveillance systems were put in place to monitor and track prion diseases in both animals and humans (discussed further in the following section on Animal and Environmental Monitoring of Prions), and these lessons led to the development of stronger regulatory frameworks to prevent future outbreaks.
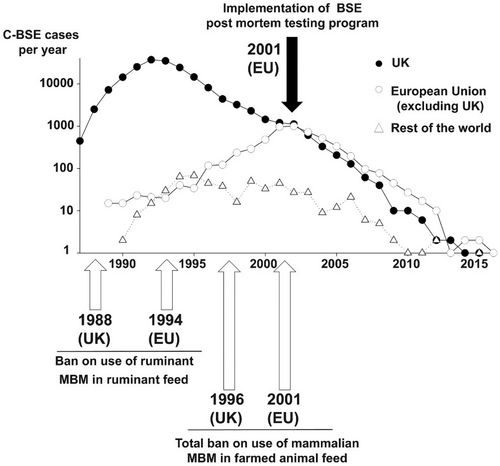
In the years since the human epidemic, the incidence of BSE in cattle has declined dramatically, thanks to the implementation of strict control measures and surveillance (Figure 6) (Houston and Andréoletti 2019). However, the legacy of the “Mad Cow” epizootic persists in the continued efforts to monitor and control prion diseases, ensuring that similar outbreaks do not occur again. This epizootic-epidemic serves as a reminder of the importance of food safety and the potential risks that can arise when new diseases are introduced into the food supply. It also emphasizes the need for global collaboration in managing prion diseases and protecting public health.
3.4 A growing crisis: Chronic wasting disease (1960s to present)
In 1967, researchers from Colorado State University and the University of Wyoming jointly embarked on a long-term project to study the ecology, health, and behavior of wild mule deer over 12 years (Williams and Young 1980). During this period, 53 mule deer displayed unusual behaviors, including extreme weight loss and progressive neurological symptoms, eventually leading to death within 2 weeks to 8 months after symptoms appeared. In 1980, the necropsy of captured deer with similar behavior revealed an abnormal isoform of the prion protein, PrPCWD, confirming the presence of what would come to be known as Chronic Wasting Disease (CWD). This discovery marked the beginning of one of the most perplexing prion disease outbreaks, uniquely affecting deer, elk, and other cervids.
Unlike prion diseases that require direct exposure to infected tissue, such as Kuru or vCJD, CWD spreads efficiently through multiple routes. Infected cervids shed prions in saliva, urine, feces, and even through the natural decomposition of their carcasses (Haley et al. 2011; Henderson et al. 2015). Interestingly, as little as 300 ng of saliva was found to be capable of infecting an exposed white-tailed deer (Denkers et al. 2020). These prions persist in the environment, binding to soil particles and plants, where they remain infectious for years (Gehrenkemper et al. 2021; Georgsson et al. 2006). This environmental resilience has contributed to the relentless spread of CWD across North America and beyond. A typical CWD transmission cycle begins when a cervid, such as a white-tailed deer, consumes vegetation contaminated with prions (Figure 7). As the disease progresses, the infected animal sheds prions through bodily fluids and excreta, contaminating its surroundings. Upon death, additional prions are released into the environment from the carcass, where they persist and initiate the cycle anew.

Recent research has demonstrated that PrPCWD can be transmitted through a behavior known as allogrooming (Inzalaco et al. 2023). This natural grooming practice, where animals remove insects and other arthropods (e.g., ticks) from individuals of the same species, serves as a defense mechanism. Although parasites like ticks have not been shown to transmit prions while feeding (Shikiya et al. 2020), the act of allogrooming itself has been successfully proven to facilitate the transmission of CWD through digesting PrPCWD-positive ticks (Inzalaco et al. 2023). Disturbingly, ticks, specifically hard ticks (Acari: Ixodidae, one of two families of ticks), drop off their host after feeding, up to 3 times during their lifecycle (Apanaskevich et al. 2014), increasing the likelihood of engorging PrPCWD-infected blood and transmitting PrPCWD to other animals. Late last year, Benestad and co-authors showed that black soldier fly larvae (Hermetia illucens) retained infectious prions after being experimentally fed dilutions of (classical) scrapie-infected brain tissue (Benestad et al. 2024). Still, the evidence suggesting the potential for arthropod vectors to transmit the pathogenic isoform is limited.
From its initial discovery through 2012, CWD had expanded far beyond its original endemic zones, with cases reported in deer and elk populations in 22 U.S. states and two Canadian provinces (Haley and Hoover 2015). By 2019, it grew to 26 U.S. states and three Canadian provinces (see Figure 8), Norway, Sweden, and South Korea (Rivera et al. 2019; Vikøren et al. 2019). Since then, CWD has been documented in 36 U.S. states, five Canadian provinces, and Finland (Chiavacci 2022; Hazards et al. 2017; Richards 2025). This geographic spread was facilitated by human activities, including the movement of captive cervids for farming and hunting purposes.
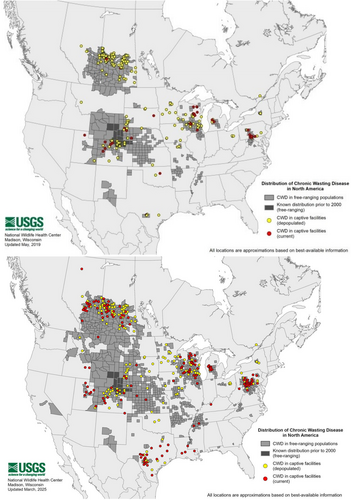
In some regions, CWD prevalence has reached alarming levels, with infection rates exceeding 40% in local deer populations (Carlson et al. 2018). This has raised concerns about potential long-term impacts on cervid ecology and the broader ecosystems they inhabit, as their role as prey species and ecosystem engineers is diminished.
In addition to its ecological implications, CWD has also had profound economic and cultural consequences. Deer and elk hunting generate substantial revenue and are deeply rooted in the traditions of many communities. The spread of CWD has led to increased costs for surveillance, testing, and management, as well as heightened anxiety among hunters about the potential risks of consuming infected meat. On that, no known cases of CWD have appeared in humans. This raises the question of whether humans are secure from the effects of PrPCWD because of phenomena such as NAPA (discussed earlier). Several in vitro studies showed successful conversion of human PrPC via PrPCWD, including one simulated oral exposure (Davenport et al. 2015; Kolodziejczak et al. 2010; Kurt et al. 2015). Additionally, CWD has seen successful transmission to orally exposed nonhuman primates, intracerebrally inoculated cows, and other animals (Hamir et al. 2001; Marsh et al. 2005; Tamgüney et al. 2006). Current evidence suggests that some unknown barrier prevents real oral exposure to humans and cows (Nemani et al. 2020; Williams et al. 2018). But continued monitoring for CWD-positive animals is encouraged until the underlying mechanisms are resolved.
Efforts to control CWD have focused on minimizing its spread and managing infected populations. Strategies include the testing of hunter-harvested animals, culling of infected herds, restricting the transport of live cervids, and banning the use of deer urine as hunting attractants. However, the effectiveness of these measures has been mixed, as the environmental persistence of prions and the wide-ranging nature of cervids make eradication exceedingly difficult, as discussed in the following section.
Some regions have implemented targeted culling and population reduction strategies in areas with high CWD prevalence, including throughout the US, Canada, and several countries in Europe (Carlson et al. 2018; Hazards et al. 2023). In addition, research is ongoing to develop vaccines or treatments for CWD, though none are currently available. Advances in prion detection methods, such as improved diagnostic tests capable of detecting prions in blood or environmental samples, may enhance early detection and containment efforts.
CWD represents a unique challenge in the realm of prion diseases due to its environmental persistence, wildlife hosts, and potential risks to humans. Its spread across continents underscores the difficulties of managing a disease that defies traditional boundaries of transmission and containment. While progress has been made in understanding its biology and dynamics, CWD remains a pressing issue for wildlife management, public health, and ecological sustainability.
4 CURRENT ANIMAL AND ENVIRONMENTAL MONITORING OF PRIONS
4.1 Animal monitoring for prion diseases
Before the “Mad Cow” epizootic, scientists generally believed that a transmission barrier existed across species for TSEs. Now, we know that we do not always know what to expect when it comes to prions. Prion diseases in animals include BSE in cattle, CWD in cervids, scrapie in sheep and goats, and transmissible mink encephalopathy. Emerging prion diseases, such as camel prion disease recently identified in Algeria (Babelhadj et al. 2018), highlight the unpredictable evolution of these pathogens. The zoonotic risk varies among these diseases.
Classical scrapie, which has been known for centuries, primarily affects sheep and goats and has spread globally through trade in livestock. Active surveillance programs in the European Union, the United States, Canada, and other countries have identified cases of classical (acquired) and atypical scrapie (spontaneous), often revealing a higher prevalence than previously estimated. While classical scrapie is associated with multiple prion strains and environmental transmission, atypical scrapie is thought to occur sporadically and is not considered contagious. Despite this, both forms have demonstrated the ability to cross species barriers under experimental conditions (Baker et al. 1993; Cassard et al. 2014; Comoy et al. 2015).
To date, no confirmed human cases of scrapie have been documented, and its impact on public health is considered negligible compared to diseases like BSE. However, the lack of comprehensive long-term studies means that subtle zoonotic risks cannot be ruled out entirely. In animals, thousands of cases of classical scrapie have been reported worldwide, with the European Union accounting for a significant proportion due to extensive surveillance efforts. Atypical scrapie has been detected in countries previously considered free of prion diseases, including Australia and New Zealand (Cook et al. 2016; Kittelberger et al. 2010), indicating a broader distribution than previously recognized.
CWD remains a concern due to its rapid spread and detection in consumable tissues like venison, although no direct human cases have been linked yet. Nevertheless, there have been documented successful transmissions of CWD to non-human primates (Race et al. 2014). CWD prevalence is largest in the U.S. and Canada at the moment (Richards 2025). Additionally, the U.S. Fish and Wildlife Service estimates that one in every 10 people in the U.S. hunts for sport or food, putting a great deal of the population at risk if an outbreak in CWD surfaces (U.S. Fish and Wildlife Service 2022).
BSE has been definitively linked to vCJD in humans, with 232 cases reported worldwide as of now, mostly in the UK (77% of cases) (Gao et al. 2024). Since the “Mad Cow” epizootic, we have identified multiple forms of BSE. Classical BSE (C-BSE), which caused the infamous “Mad Cow” epizootic, is linked to vCJD in humans. Atypical forms of BSE, including L-type (L-BSE) and H-type (H-BSE), were identified through active surveillance programs. The L- and H-types refer to the molecular weights of the protein fragments after proteinase K digestion (lower and higher, respectively) compared to C-BSE. L-BSE, also known as bovine amyloidotic spongiform encephalopathy (BASE), exhibits a distinct biochemical profile, higher virulence, and shorter incubation periods in experimental models, raising concerns about its zoonotic potential (Comoy et al. 2008). Transmission studies have shown that L-BSE may be more transmissible to humans than C-BSE, highlighting the need for ongoing vigilance (Baron et al. 2008; Comoy et al. 2008). In contrast, H-BSE appears to have a lower zoonotic risk (Baron et al. 2011). The origins of these atypical BSE forms remain unclear, with theories ranging from spontaneous prion misfolding to possible low-level environmental transmission.
Global monitoring efforts for animal prion diseases involve a combination of active and passive surveillance, primarily in regions with known prion disease risks. The European Union (EU) has led the way with comprehensive programs initiated during the BSE crisis, which revealed previously unknown atypical BSE and new scrapie strains. In North America, monitoring focuses on CWD, which is endemic in many U.S. states and Canadian provinces, with efforts aimed at curbing its spread in wild and captive cervid populations. Scandinavia has recently intensified surveillance following the discovery of CWD in Norway and Finland (Benestad et al. 2016), while historically unaffected regions like Australia and New Zealand have begun monitoring for atypical prion diseases. However, the challenges are significant. Prion diseases often affect older animals or remain asymptomatic, complicating detection. Surveillance programs require substantial resources and international collaboration, particularly as new forms of prion diseases emerge globally.
4.2 Environmental monitoring for prions
Prions are exceptionally stable in the environment, persisting in soil for years, and environmental monitoring of prions has gained attention due to the role these media play in prion transmission and persistence (Figure 9). Current research has revealed intriguing insights into how prions interact with the environment, highlighting their remarkable stability and ability to influence disease dynamics in wildlife and livestock.

Soil is one of the most studied environmental reservoirs of prions due to its role in the transmission of CWD among cervids and scrapie among small ruminants. Scientists have shown that prions can bind tightly to soil particles, particularly to clay minerals and organic matter, which protects them from degradation (Georgsson et al. 2006; Johnson et al. 2006; Rao et al. 2007; Seidel et al. 2007). This binding enhances their environmental stability, allowing prions to remain infectious for years (Georgsson et al. 2006; Seidel et al. 2007). For example, studies have demonstrated that scrapie prions buried in soil retain their infectivity for more than 16 years (Georgsson et al. 2006).
The interaction between prions and soil minerals is a critical factor in environmental prion monitoring. Clay minerals, such as montmorillonite, have been found to increase prion infectivity and reverse inactivation when bound to them (Booth et al. 2021). This binding likely alters the prion protein structure, enhancing its capacity to cause disease. Conversely, soil organic matter contains billions of microbes per gram which may hold some potential in reducing prion infectivity, though it has only been hypothesized. On the other hand, water appears to reduce the infectivity of prions as opposed to soil (Miles et al. 2011). These findings underscore the complexity of prion interactions with soil and water and the importance of soil composition in determining prion persistence and infectivity.
Recent research has expanded the scope of environmental prion studies to include plant interactions. Experiments have demonstrated that plants can absorb prions from contaminated soil and retain them on their surfaces or within their root systems (Figure 9) (Carlson et al. 2023; Pritzkow et al. 2015). A landmark study by Pritzkow et al. showed that prions could attach to root surfaces and be taken up into the vascular tissues of grass plants (Pritzkow et al. 2015). These plant-associated prions remained infectious, where hamsters fed the prion-contaminated grass leaves developed PrD.
The implications of plant-prion interactions are significant for understanding prion transmission. Plants growing in prion-contaminated environments, such as those frequented by infected wildlife or livestock, could serve as vectors for disease spread. For instance, deer and other animals grazing on vegetation in CWD-endemic areas may ingest prion-contaminated plants, perpetuating the disease cycle. This raises concerns about the potential for prion dissemination through agricultural practices, such as irrigation or the use of manure-based fertilizers (Xu et al. 2013; Xu et al. 2014).
Efforts to monitor environmental prions focus primarily on detecting prions in soil, water, and plant samples from areas of known TSE outbreaks (Saunders et al. 2008). Techniques like protein misfolding cyclic amplification (PMCA) and real-time quaking-induced conversion (RT-QuIC) have been adapted for environmental samples, offering high sensitivity and specificity (discussed more in the following section on Current Diagnostic Techniques for PrPSc). These methods can detect prions at very low concentrations, making them suitable for assessing environmental contamination.
Field studies have concentrated on regions with high CWD prevalence, such as North America, where researchers have detected prions in soil and water near CWD-infected deer populations. Monitoring programs aim to map contamination hotspots, evaluate the risk of disease spread, and inform management strategies, such as restricting animal access to heavily contaminated areas or implementing soil remediation measures.
Despite advances in detection methods, monitoring prions in the environment poses significant challenges. Prions are unevenly distributed in soil and plants, making representative sampling difficult. Their strong adherence to soil particles and plant surfaces also complicates extraction and detection. Additionally, the low concentrations of prions in environmental samples require highly sensitive analytical techniques (PMCA and RT-QuIC), which can be resource-intensive and time-consuming.
The long-term fate of prions in the environment remains poorly understood. While they are known to be highly stable, factors like soil pH, microbial activity, and environmental conditions could influence their persistence and infectivity. Further research is needed to clarify these dynamics and develop effective strategies for mitigating prion contamination.
Research into the environmental monitoring of prions has revealed their remarkable ability to persist in soil and interact with plants, creating potential pathways for transmission. Advances in sensitive detection methods have enhanced our ability to study environmental prions, but challenges remain in understanding their long-term behavior and impacts. As prions continue to pose threats to wildlife and livestock health, environmental monitoring and management strategies will play a critical role in controlling their spread and safeguarding ecosystems and public health.
5 CURRENT DIAGNOSTIC TECHNIQUES FOR PrPSc
The groundbreaking discovery of the infectious agent behind TSEs was made by Dr. Stanley Prusiner in 1982 (Prusiner et al. 1982a), when he identified prions as a novel type of pathogen distinct from bacteria, viruses, or fungi. Prusiner demonstrated that the infectious particle was a misfolded form of the normal cellular prion capable of inducing a conformational change in PrPC to propagate itself. To support this, he used enzymes such as proteases and nucleases to treat the infectious material and tested its infectivity, discovering that it remained infectious after nucleic acid digestion but was slowed by protease treatment (Prusiner et al. 1982a, 1982b). This pivotal work earned him the Nobel Prize in Physiology or Medicine in 1997 and transformed the understanding of protein misfolding diseases, laying the foundation for diagnostic methods to differentiate between normal and pathological prion proteins.
Current techniques for prion detection include Western blotting, enzyme-linked immunosorbent assay (ELISA), PMCA, RT-QuIC, Fourier-transform infrared spectroscopy (FT-IR), circular dichroism (CD), and cryogenic (transmission) electron microscopy (Cryo-EM) (Table 1). Each of these methods has its own advantages and limitations, and they are often used in combination to reach robust conclusions.
| Technique | Ultrasensitive | High-throughput | Minimal resources | For (semi-) quantitation | For structural characterization |
|---|---|---|---|---|---|
| RT-QuIC | 1 | 1 | 0 | 1 | 0 |
| PMCA | 1 | 0 | 0 | 1 | 0 |
| ELISA | 0 | 1 | 0 | 1 | 0 |
| FT-IR | 0 | 1 | 1 | 0 | 1 |
| CD | 0 | 0 | 1 | 0 | 1 |
| Cryo-EM | 1 | 0 | 0 | 0 | 1 |
| Western Blotting | 0 | 0 | 0 | 1 | 0 |
Western blotting has been a cornerstone technique for prion protein detection and was used by Prusiner to identify prion as the main culprit in TSEs (Prusiner et al. 1982b). This method separates proteins by size through gel electrophoresis after proteinase K digestion to eliminate normal PrPC, leaving only the resistant PrPSc for identification via antibodies. Western blotting, first described by Towbin et al. (1979) and later adapted for prion studies (Fido et al. 1995), is highly specific and widely validated. However, its sensitivity is moderate, requiring substantial amounts of tissue, often from postmortem brain samples. Additionally, it is labor-intensive and time-consuming, limiting its use for rapid or early-stage diagnosis.
The enzyme-linked immunosorbent assay (ELISA) has been adapted to detect prion proteins using specific antibodies. ELISA uses antibodies to bind PrP in a sample, with a secondary enzyme-linked antibody generating a detectable signal proportional to the amount of protein present. Commercial kits, such as those developed by Bio-Rad, make this technique accessible and suitable for high-throughput screening. ELISA is faster and simpler than Western blotting, and it can detect prion proteins in bodily fluids such as cerebrospinal fluid (CSF) and blood. However, its sensitivity is lower (10−12 to 10−5 g/mL) (Biocompare 2024; Moynagh and Schimmel 1999), especially for detecting small amounts of PrPSc, and it requires highly specific antibodies to minimize cross-reactivity with PrPC. Current antibodies used in both western blotting and ELISA either target specific regions of either protein (typically the core of the protein) (e.g., ICSM18, 6H4, R017, and R073) (Bian et al. 2017; Reilly et al. 2022; Taraboulos et al. 1990) or target the beta-sheet structure for amyloid analysis (e.g., 15B3 and PRC1) (Bian et al. 2017; Korth et al. 1997; Stravalaci et al. 2016). It should be noted that PRC1 has been shown to bind to PrPCWD but not all other prions (Bian et al. 2017).
Protein misfolding cyclic amplification (PMCA) represents a significant advancement in prion detection. Developed by Saborio et al. (2001), PMCA mimics the pathological process of prion propagation by converting PrPC to PrPSc in vitro. The method involves cyclic incubation (analogous to DNA amplification by polymerase chain reaction) of PrPSc with PrPC, followed by ultrasonic treatment. During incubation, PrPSc aggregates incorporate PrPC, increasing the size of the oligomeric structures. Sonication fragments these aggregates, generating more seeding surfaces for PrPSc formation in subsequent cycles. Finally, gel separation of the incubation-solution cycles is imaged using immunoblotting to confirm PrPSc presence. This technique is extremely sensitive, capable of detecting (10−18 to 10−12 g/mL) levels of PrPSc in peripheral samples such as blood, urine, or CSF (Bougard et al. 2016; Gonzalez-Montalban et al. 2011; Moda et al. 2014). However, PMCA is time-intensive, requiring multiple cycles to amplify the signal, and it is prone to contamination, which can lead to false positives. Similar false-positive results could be expected in the case of non-prion amyloid aggregate presence in the tissue samples. For instance, amyloid β (Aβ) oligomers are commonly detected in cerebrospinal fluid that can potentially template PrPC aggregation. Additionally, its implementation requires specialized expertise and equipment.
Real-time quaking-induced conversion (RT-QuIC), introduced by Atarashi et al. in 2011 (Atarashi et al. 2011a, 2011b), is another highly sensitive (down to 10−18 to 10−16 g/mL) and specific assay for PrPSc (Green 2015; Orrú et al. 2011). This technique detects prion proteins by monitoring the conversion of recombinant PrPC (rPrPC) into the misfolded form in the presence of PrPSc. The availability of recombinant PrP has been instrumental in enabling this method, providing a consistent and controlled substrate for conversion assays while eliminating the need for tissue-derived PrP sources (further discussed in the following section). Similar to PMCA, samples are mixed with rPrPC and incubated in a plate reader in the presence of a fluorescent dye. PrPSc templates the aggregation of rPrP,C which causes an increase in dye fluorescence. RT-QuIC allows for real-time monitoring of prion aggregation, making it faster than PMCA. It has shown high diagnostic accuracy even in non-invasive samples like cerebral spinal fluid and olfactory mucosa, making it increasingly valuable for pre-mortem diagnosis of prion diseases. Nevertheless, it requires precise optimization of reaction conditions and access to specialized reagents and instruments. Finally, a better understanding of the potential cross-seeding of PrPC by non-prion aggregates is required to exclude the possibility of false-positive sensing in individuals diagnosed with amyloid diseases such as type II diabetes, Alzheimer's, and Parkinsons' disease.
Still, PMCA and RT-QuIC are the techniques of choice when it comes to prion disease diagnosis (quantitation) (Dong and Satoh 2021). In the U.S., however, RT-QuIC is the sole focus of ante- or peri-mortem CWD (USDA 2024). Antemortem CWD describes CWD-infected animals that are either asymptomatic or showing symptoms before death. Most monitoring is done on already dead animals, and without high error as it is well known that the concentration of PrPSc/PrPCWD is highest in the brain and nervous tissues, with very low abundance in the peripheral tissues and bodily fluids. For example, documented concentrations of PrPCWD in white-tailed deer range from ~10−15 to 10−18 M in urine and feces based on subsequent inoculations of mice (calculated using 1 femtogram prion = LD50) (Haley et al. 2009). This makes ante- or peri-mortem detection using RT-QuIC questionable as it reaches its well-established limits (Table 2). Not to mention the expected time to reach these detection limits is days for both PMCA and RT-QuIC (Table 2). Recently, Bravo-Risi et al. demonstrated that PMCA was more accurate at identifying prion-infected cervids peri-mortem than RT-QuIC; however, the true positive rate was slightly above 50% (Bravo-Risi et al. 2023). One may wonder if a more sensitive, high-throughput technique may surface to enhance the peri-mortem study of PrDs, including CWD.
Beyond that, Fourier-transform infrared spectroscopy (FT-IR) and circular dichroism (CD) are spectroscopic methods used to identify structural differences between PrPC and PrPSc. FT-IR examines the secondary structure of proteins based on their infrared absorption, while CD analyzes differences in the way circularly polarized light interacts with PrPC and PrPSc. Both techniques are non-invasive and label-free, eliminating the need for antibodies or protease digestion. However, their sensitivity is much lower than that of other methods, and they require high concentrations of prion proteins, making them impractical for detecting small amounts of PrPSc or for use in early diagnostics. Instead, these methods are primarily employed in research to study prion protein structures (Rao et al. 2007).
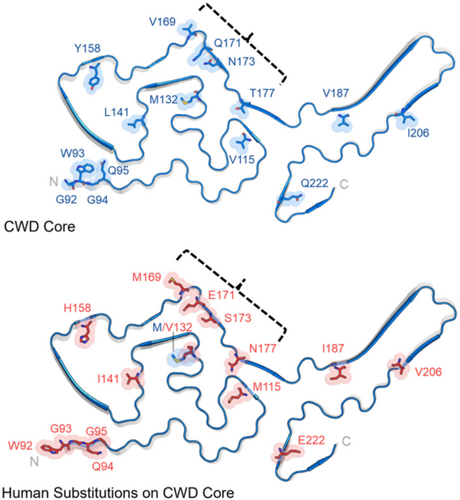
More recently, A major breakthrough in determining the structural conformations of PrPC and PrPSc came with the utilization of cryogenic electron microscopy, or Cryo-EM. Unlike traditional methods that rely on indirect structural inferences (e.g., CD and FTIR), Cryo-EM directly captures fibril morphology, offering a clearer understanding of how misfolded PrPSc templates its own propagation. Advances in Cryo-EM, particularly the development of direct electron detectors (following the release of the DE-12 detector in 2008 and the famed Falcon direct electron detectors since 2011) and improved image-processing algorithms, have allowed researchers to resolve prion fibril architectures at near-atomic resolution (van Duinen et al. 2017). In 2016, a team led by Drs. Howard Young, Jesús Requena, and Holger Wille discovered that the β-solenoid architecture of PrP is the transmissible form (Vázquez-Fernández et al. 2016). Thereafter, strain-specific variations in conformation were revealed in many prions (Alam et al. 2024; Hoyt et al. 2022a, 2022b). In 2024, Alam et al. revealed the 3D structure of the CWD prions and determined the presence of unique bending of the loops within the infectious filaments compared to extractions from both cervids and rodents, including models for GSS (Figure 10) (Alam et al. 2024). Still, Cryo-EM requires highly purified prion preparations and sophisticated instrumentation, making its widespread application challenging. Nonetheless, as imaging techniques continue to advance, Cryo-EM is expected to further refine our understanding of prion biology, hopefully contributing to improved diagnostics and therapeutic strategies.
Each of these techniques offers unique contributions to the field of prion diagnostics, with distinct strengths and limitations. Western blotting is a foundational method that provides high specificity for PrPSc, making it reliable for confirming diagnoses, but it is labor-intensive and requires substantial tissue, often postmortem. ELISA is well-suited for routine screening due to its scalability and speed, but its sensitivity for detecting low levels of PrPSc is limited. Advanced molecular techniques such as PMCA and RT-QuIC offer unparalleled sensitivity, enabling pre-mortem diagnosis and detection of minute amounts of PrPSc, but these methods require significant expertise, costly reagents, and precise optimization of reaction conditions. Spectroscopic techniques like FT-IR and CD provide valuable structural insights into prion proteins, which are essential for research, but their low sensitivity and reliance on high protein concentrations limit their clinical utility. Ultimately, the choice of diagnostic method depends on the clinical or research context, often necessitating a combination of complementary techniques for accurate and comprehensive prion detection.
6 PRION DECONTAMINATION
Prions are notoriously difficult to deactivate due to their unique protein-only structure and remarkable resistance to conventional sterilization methods. Many traditional approaches, including standard autoclaving, chemical disinfectants, and radiation, have proven ineffective. Standard autoclaving at 121°C for 30 minutes, sufficient to sterilize most pathogens, fails to neutralize prions, which retain infectivity under these conditions (Spiropoulos et al. 2019). Studies focused on determining the most effective temperature have determined that PrPSc continues to remain infectious at 600°C or lower (dry heat) (Brown et al. 2000). Common chemical disinfectants like alcohols, aldehydes (formaldehyde and glutaraldehyde), chlorhexidine, and quaternary ammonium compounds are similarly ineffective, with some even stabilizing the prion protein structure (McDonnell and Burke 2003). Radiation techniques such as gamma and UV irradiation also fail because prions lack nucleic acids, the primary target of radiation damage in most microorganisms. Even robust proteases commonly employed for lysis are incapable of degrading PrPSc. Other simple measures like boiling water are equally ineffective, leaving prions highly resilient in many environments.
Despite these challenges, several methods have proven effective for prion decontamination (Table 3). Sodium hypochlorite (bleach) solutions with 20,000 ppm (2%) available chlorine are highly effective, requiring a contact time of at least 1 h to neutralize prions (Fichet et al. 2004). Sodium hydroxide (1M NaOH) is another reliable option (Flechsig et al. 2001), effectively hydrolyzing prions and making it suitable for glassware and non-corrosive materials, though its corrosiveness limits applications on sensitive equipment (Fichet et al. 2004). Combining chemical treatments with autoclaving enhances efficacy; for instance, pre-soaking instruments in bleach or NaOH followed by autoclaving ensures complete prion inactivation (Fichet et al. 2004). Alkaline detergents, especially when combined with heat, are another effective strategy, while plasma-based sterilization technologies are gaining attention for their potential to safely treat heat-sensitive materials (Fichet et al. 2004).
| Chemical(s) | Minimum time | Noncorrosive (Y/N)? | Variants of PrPSc tested in reference | Reduced infectivity (active/inactive) | Reference |
|---|---|---|---|---|---|
| NaOCl (20,000 ppm or 2%) | 1 h | N | Hamster 263 K | <1 out of 1,000,000 | Fichet et al. (2004) |
| NaOH (1N, 20°C) + autoclave in water (134°C) | 1 h, 18 min | N | Hamster 263 K | <1 out of 1,000,000 | Fichet et al. (2004) |
| Alkaline detergent (1.6%, 43°C) | 15 min | Y | Hamster 263 K | <1 out of 1,000,000 | Fichet et al. (2004) |
| Enzymatic detergent (0.8%, 43°C) + hydrogen peroxide gas plasma sterilization (1.5 mg/L, 25°C) | 5 min, 3 h | Y | Hamster 263 K | <1 out of 1,000,000 | Fichet et al. (2004) |
| Vaporized hydrogen peroxide (2 mg/L, 30°C) | 3 cycles | Y | Hamster 263 K | 31 out of 1,000,000 | Fichet et al. (2004) |
| 1% SDS + 0.5% acetic acid (25°C, pH 3.6) + autoclave in solution (134°C) | 15 min | Y | Human sCJD (normal) and Hamster 139H | <1 out of 1,000,000 | Peretz et al. (2006)) |
- Note: Reduced infectivity was calculated from provided (referenced) log reductions for each treatment and represents the number of active infectious particles out of the number of inactivated infectious particles.
The application of these methods depends on the context and specific challenges. Metals like stainless steel, that are often used to work in sterile fields, bind tightly to PrPSc, resisting typical formaldehyde treatment (Flechsig et al. 2001; Zobeley et al. 1999). In biosafety cabinets, where bleach's corrosive nature damages metal components, alternatives like hydrogen peroxide vapor, enzymatic cleaners, or (acidic) sodium dodecyl sulfate (SDS) are better suited (Fichet et al. 2004; Peretz et al. 2006). Healthcare settings, which require prion decontamination of surgical instruments and endoscopes without damage, benefit from pre-cleaning with alkaline detergents or enzymatic treatments followed by validated autoclaving protocols. Environmental decontamination, particularly for prions bound to soil, remains complex; while localized contamination can be treated with bleach or NaOH, large-scale decontamination is impractical. Laboratory settings often rely on a combination of chemical pre-treatment and autoclaving to decontaminate glassware and instruments, though enzymatic solutions are increasingly used for sensitive equipment.
Additionally, variations of PrPSc should be carefully considered, as Peretz et al. observed a 105-fold increase in resistance to inactivation in human-derived sCJD PrP compared to the commonly tested hamster PrPSc (Peretz et al. 2006). Therefore, caution is advised when implementing the discussed decontamination methods in laboratory or workplace settings. In other words, if the specific PrPSc variant has not been tested, experiments should be conducted to ensure effective decontamination before proceeding with further work.
Prion decontamination is complicated; their unique resistance to typical sterilization settings for heat, ultraviolet light, ethanol, proteases, and peroxides makes them incredibly challenging to eliminate from biological material and contaminated equipment. Effective decontamination is critical to safeguarding healthcare professionals and research scientists working to combat this formidable threat. Continued research is essential to develop simpler, highly effective prion decontamination methods that minimize equipment corrosion while maintaining robust inactivation.
7 ADVANCES IN TREATMENT OF PRION INFECTION
Historically, prion infections were considered untreatable. However, advances in our understanding of prion biology have led to the development of innovative approaches aimed at early detection, slowing disease progression, and reducing prion load. While these treatments have not yet transformed outcomes, they represent significant steps forward in the fight against PrDs.
For decades, the treatment of prion diseases was primarily supportive, focused on alleviating symptoms such as dementia, ataxia, and myoclonus. The neurodegenerative process caused by PrPSc aggregation in the brain progressed unchecked, leading to rapid cognitive decline and death within months to years of symptom onset. Early attempts to treat prion diseases were largely unsuccessful, as therapies targeting conventional pathogens like viruses or bacteria were ineffective against these proteinaceous infectious agents. The extraordinary stability of prions, coupled with their resistance to proteolytic degradation and lack of nucleic acids, made traditional therapeutic strategies unviable.
Early detection of prion diseases is essential for initiating therapeutic interventions before significant neurodegeneration occurs. Diagnostic tools like RT-QuIC and PMCA have greatly enhanced sensitivity and specificity in identifying misfolded prion proteins. These methods have been modified to detect PrPSc in cerebrospinal fluid, blood, or nasal brushings, providing reliable biomarkers of prion disease even during asymptomatic stages. RT-QuIC, for example, has been reported to have sensitivity and specificity exceeding 90% in detecting sCJD in large numbers of samples (Rhoads et al. 2020). By enabling earlier diagnosis, these tools create opportunities for therapeutic interventions aimed at halting disease progression.
The inhibition of prion aggregation is a central focus in prion therapy development. Compounds such as quinacrine and anle138b have shown potential in experimental models. Quinacrine, a repurposed antimalarial drug, demonstrated the ability to reduce prion propagation in vitro (Barret et al. 2003), although clinical trials yielded mixed results in prion-infected patients (Collinge et al. 2009). More promising is anle138b, a small molecule that has been shown to prolong survival in animal models by preventing the aggregation of PrPSc. Though these molecules are not yet approved for human use, they highlight the therapeutic potential of targeting prion aggregation pathways.
Current research on antibodies targeting PrPC or PrPSc as a strategy to reduce prion load and prevent disease progression has shown promise in animal models and even some (small) human trials. There are antibodies that bind to misfolded prion proteins, marking them for clearance by the immune system. However, challenges include delivering antibodies across the blood–brain barrier and avoiding interference with normal cellular prion proteins. Despite these obstacles, preclinical models have demonstrated reduced prion propagation and prolonged survival with antibody treatments, highlighting their potential as a therapeutic tool. For example, PRN100 (ICSM18) and ICSM35, humanized anti-PrPC monoclonal antibodies, have shown efficacy in extending survival of prion-infected mice (White et al. 2003). The PRN100 antibody has also reached a small human trial of 6 participants (two men and four women) diagnosed with iCJD and sCJD as described in Mead et al. (2022). The study demonstrated that at a 48-h titration of 10 mg/kg followed by 120 mg/kg PRN100 every 2 weeks until death resulted in weaker symptom effects and reduced prion load in postmortem brain histology.
Reducing the expression of PrPC is another promising strategy, as PrPC serves as a substrate for conversion into the infectious PrPSc. Techniques like RNA interference (RNAi) and antisense oligonucleotides (ASOs) have been used to silence the PRNP gene, thereby reducing PrPC levels in the brain (Golding et al. 2006; Raymond et al. 2019; Reidenbach et al. 2019). Preclinical studies in mice have shown a reduction in prion propagation and extended survival (Raymond et al. 2019). Early-phase clinical trials in humans are ongoing, and initial results suggest these methods could be an effective way to slow disease progression (Reidenbach et al. 2019).
While curative treatments are not yet available, we are knowledgeable enough to, at present, manage the symptoms of PrDs. Medications such as benzodiazepines and antiepileptic drugs help control myoclonus, while other pharmacological and non-pharmacological interventions address sleep disturbances, anxiety, and depression. Supportive care, including physical therapy and counseling, improves the quality of life for patients and their families (Zerr and Schmitz 2021). These efforts, though not curative, represent an essential component of prion disease management.
Innovative approaches, such as immunotherapy, repurposing FDA-approved drugs, and cell-based therapies, are actively being investigated for prion diseases. Advances in nanotechnology are enabling the development of novel drug delivery systems that can cross the blood–brain barrier, enhancing the efficacy of therapeutic agents. Additionally, studies are exploring the use of CRISPR-Cas9 for precise genetic editing to knock out the PNRP gene or correct mutations associated with inherited prion diseases (Kaczmarczyk et al. 2016; Mehrabian et al. 2014).
Despite these advances, prion diseases remain fatal once symptomatic. However, with improved early detection methods, a better understanding of prion biology, and innovative therapeutics in development, the hope is to transform prion diseases from inevitable death sentences into manageable conditions. Continued research and collaboration among scientists, clinicians, and public health officials are essential to achieving this goal.
8 SUMMARY
If nothing else, the following points should serve as the key takeaways: Prions are infectious proteins that misfold other prion proteins, driving the formation of infectious aggregates. Prion misfolding can be accelerated in the presence of other amyloids, thereby accelerating disease progression. This is because misfolded prions (PrPSc) can template the misfolding of other aggregation-prone proteins, like alpha-synuclein and amyloid-beta, creating additional toxic aggregates that amplify cellular stress, disrupt proteostasis, and promote widespread neurodegeneration. Prion diseases vary in their clinical presentation and disease duration, but all are characterized by spongiform degeneration, which results in brain damage and neurological symptoms. Currently, we are facing a global prion crisis, as wild cervids (deer, elk, and moose) are testing positive for the chronic wasting disease prion at a growing rate, raising concerns about environmental and cross-species transmission. Prions are exceptionally stable in the environment, persisting in soil for years, and studies have shown that they can be taken up by plants, which can transmit the prions to animals upon ingestion. Their unique resistance to typical sterilization settings for heat, ultraviolet light, ethanol, proteases, and formaldehyde makes them incredibly challenging to eliminate from biological material and contaminated equipment. There is no cure for prion diseases, but current treatments focus on identifying the presence of misfolded prion proteins early on and slowing disease progression with symptomatic management and experimental therapeutics aimed at reducing prion load or halting aggregation. Future research should prioritize the development of faster, more affordable techniques for prion detection and the creation of safer, more systemic therapies to remove or neutralize prions in affected individuals and the environment.
AUTHOR CONTRIBUTIONS
Aidan P. Holman: Conceptualization; writing – original draft; visualization. Dmitry Kurouski: Supervision; writing – review and editing; project administration; resources.
ACKNOWLEDGMENTS
Some figures were partly made using images from Biorender.com and Microsoft PowerPoint.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




