Cupryphans, metal-binding, redox-active, redesigned conopeptides
Abstract
Contryphans are bioactive peptides, isolated from the venom of marine snails of the genus Conus, which are characterized by the short length of the polypeptide chain and the high degree of unusual post-translational modifications. The cyclization of the polypeptide chain through a single disulphide bond, the presence of two conserved Pro residues, and the epimerization of a Trp/Leu residue confer to Contryphans a stable and well-defined structure in solution, conserved in all members of the family, and tolerant to multiple substitutions. The potential of Contryphans as scaffolds for the design of redox-active (macro)molecules was tested by engineering a copper-binding site on two different variants of the natural peptide Contryphan-Vn. The binding site was designed by computational modeling, and the redesigned peptides were synthesized and characterized by optical, fluorescence, electron spin resonance, and nuclear magnetic resonance spectroscopy. The novel peptides, named Cupryphan and Arg–Cupryphan, bind Cu2+ ions with a 1:1 stoichiometry and a Kd in the 100 nM range. Other divalent metals (e.g., Zn2+ and Mg2+) are bound with much lower affinity. In addition, Cupryphans catalyze the dismutation of superoxide anions with an activity comparable to other nonpeptidic superoxide dismutase mimics. We conclude that the Contryphan motif represents a natural robust scaffold which can be engineered to perform different functions, providing additional means for the design of catalytically active mini metalloproteins.
Introduction
The design of proteins with novel chemical structures and biological functions is a powerful tool to study the principles and mechanism of protein folding and bioactivity, and to develop original catalysts and materials with unique chemical properties. From this viewpoint, natural scaffolds, which display high stability and tolerance to multiple amino acid substitutions, represent ideal candidates to test our knowledge of the principles underlying protein structure and function as well as to develop new bioactive (macro)molecules through rational redesign strategies.
A class of (macro)molecules so far poorly exploited in protein design studies is represented by conopeptides, disulphide-constrained bioactive peptides which constitute the main component of the venom of marine snails of the genus Conus.1, 2 Conopeptides have been evolved by Conus snails through a sort of natural combinatorial chemistry to yield powerful and highly specific toxins targeted to ion channels and receptors of Conus natural prey to achieve their neuromuscular block and capture.1, 3, 4 Disulphide-constrained conopeptides display the interesting property of highly hypervariable intercysteine loops, while preserving a stable and compact fold ensured by disulphide bridges and a high proportion of proline residues.5-7 Among disulphide-constrained conopeptides, the simplest prototype is represented by Contryphans, characterized by a eight to nine amino acids sequence length, a single disulphide bond, and a D amino acid residue (D-Trp or D-Leu) in position 4.8-14 Recently, a member of this family, Contryphan-Vn, has been purified in our lab and characterized from a functional and a structural viewpoint.13, 15, 16 Contryphan-Vn modulates the conduction of calcium- and voltage-gated potassium channels both in vertebrate and invertebrate model systems.15 Despite Contryphan-Vn displays several amino acid substitutions with respect to other members of the family, its three-dimensional structure is nearly superimposable to that of other contryphans.16 This observation, together with independent data obtained by engineering experiments carried out on Contryphan-R,17 leads to conclude that Contryphans represent a structural scaffold, which tolerates multiple amino acid substitutions preserving a stable and well-defined three-dimensional structure.
In this work, we exploited the potential of the Contryphans scaffold to design two copper-binding peptides with redox activity as an initial step toward the use of disulphide-constrained conopeptides in the engineering of highly stable, easy to synthesize mini metalloenzymes. The affinity and specificity for copper of the two peptides, named Cupryphan and Arg–Cupryphan, have been investigated using optical, fluorescence, electron spin resonance (EPR), and nuclear magnetic resonance (NMR) spectroscopy. Cupryphan and Arg–Cupryphan bind copper with fairly good affinity (Kd values in the 100 nM range) and with a well-defined coordination environment. Both peptides are redox-active and display a superoxide dismutase activity, which, in the case of Cupryphan (k = 105M−1 s−1), is comparable to that of other nonpeptidic superoxide dismutase mimics.
Abbreviations: Boc, butyloxycarbonyl group; CM, carboxymethylated; DIPEA, N,N-diisopropylethylamine; ES-IT, electrospray ion trap; Fmoc, N-9(fluorenyl) methoxy carboxycarbonyl group; HATU, O-(7-azabenzotrazol-1-yl)-N,N,N′,N′-tetramethyluronium; Ot-Bu, tert-butyl ester; TFA, trifluoroacetic acid; Trt, S-trityl group.
Results and Discussion
Design of the metal-binding site
Contryphan-Vn was used as a template for the design of a metal-binding, disulphide-constrained peptide. Analysis of the amino acid sequence of Contryphans reveals that amino acids at positions 2, 6, and 8 in the Contryphan-Vn sequence are not conserved in other Contryphans, despite a fairly good conservation of the overall molecular structure.16 Thus, residues Asp2, Lys6, and Trp8 were selected to be substituted by three histidine residues. Further, an additional His residue was added at the C-terminal end of the peptide, outside the disulphide ring, generating the new amino acid sequence shown in Figure 1.
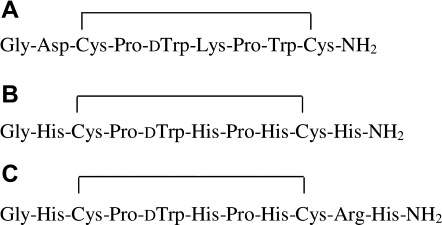
Amino acid sequence of Contryphan-Vn (A) and of the redesigned peptides Cupryphan (B) and Arg–Cupryphan (C).
The feasibility of the formation of a copper-binding site in the novel peptide was confirmed by modeling its three-dimensional structure based on Contryphan-Vn structure (PDB code 1NXN16). The side-chain conformation of the four histidine residues was adjusted to form a metal-binding site with tetragonal geometry, taking as a reference the copper-binding site of bovine superoxide dismutase (PDB code 2SOD18). Stereochemical compatibility of the metal-binding site formation was confirmed by energy minimization in explicit solvent using CHARMM.19 The final model is shown in Figure 2.
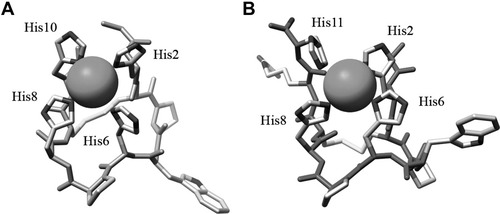
Energy-minimized three-dimensional model of Cupryphan (A) and Arg–Cupryphan (B). The copper ion is represented by a sphere. The figure was prepared using UCSF Chimera.20
Cupryphan metal-binding ability probed by optical and fluorescence spectroscopy
Evidence of Cu2+ binding to the peptide was first obtained by fluorescence quenching experiments.21 Addition of copper quenches the fluorescence emitted by the unique Trp residue of the peptide (DTrp5), as shown in Figure 3.21 Fluorescence quenching is dependent on metal concentration and reaches a maximum at a [Cu2+]/[peptide] ratio higher than 1:1. All of these characteristics are consistent with an energy-transfer mechanism from the tryptophan residue to the copper bound at the binding site. Thus, the engineered peptide was named Cupryphan from the contraction of the words cupreous and Contryphan.
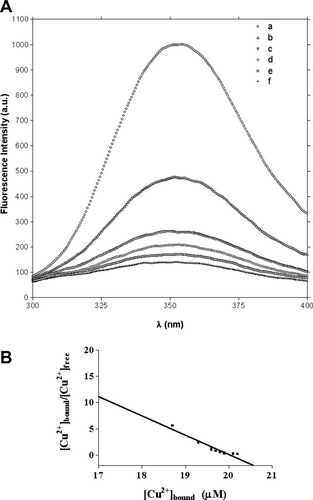
A: Emission fluorescence spectra of Cupryphan in the presence of increasing amounts of Cu2+ ions. (a) apo Cupryphan (2.1 × 10−5M); (b) a + CuCl2 5.5 × 10−6M; (c) a + CuCl2 1.0 × 10−5M; (d) a + CuCl2 1.5 × 10−5M; (e) a + CuCl2 2.0 × 10−5M; and (f) a + CuCl2 2.5 × 10−5M. B: Determination of the dissociation constant of Cu2+ to Cupryphan by fluorescence quenching experiments. Shown is the Scatchard transformation of fluorescence intensities at 352 nm in the presence of increasing amounts of Cu2+ ions.
Fluorescence quenching because of copper binding to Cupryphan was used to determine the binding affinity of Cupryphan for Cu2+. The maxima of fluorescence intensity curves at 352 nm as a function of copper concentration were fitted with Scatchard transformation (see Fig. 3), obtaining a copper dissociation constant of 1.3 (± 0.2) × 10−7M. The good fit of the data with a linear function indicate that only one class of copper-binding site(s) is present in Cupryphan. In addition, fluorescence data indicated the presence of only one binding site per molecule. This is evident from the X-axis intercept value (2.06 × 10−5M) in the Scatchard transformation plot, which represents the total Cu2+ bound at infinite Cu2+ concentration (i.e., when [Cu2+]bound/[Cu2+]free = 0). In fact, this value correlates well with the total peptide concentration value (i.e., 2.1 × 10−5M).
Further evidence of copper–peptide binding was obtained by optical spectroscopy analysis. Addition of a stoichiometric amount of CuCl2 to the peptide (1.0 mM final concentration) induces the appearance of absorption bands with maxima at 312 and 580 nm (see Fig. 4), the first indicative of a Cu2+-histidine charge-transfer,22 the second due to electronic transitions of copper d–d orbitals and typical of copper complexes with nitrogen ligands.22, 23 The maximum and the shape of the absorption band centered at 580 nm did not change with pH in the 6.0–9.5 range (data not shown), indicating a high pH stability of holo Cupryphan. The calculated molar extinction coefficient of Cupryphan at 580 nm is 50 M−1 cm−1. No absorption bands appear in the optical spectrum of Contryphan-Vn after addition of copper ions (data not shown), indicating that the natural peptide does not bind copper ions.
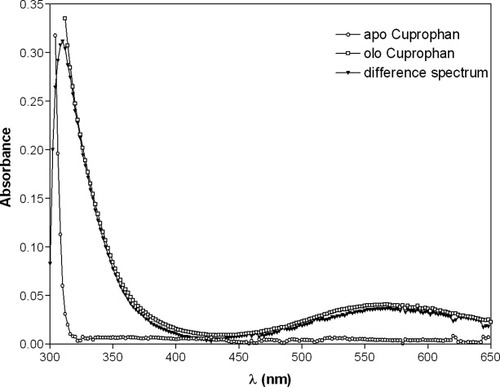
Optical spectra of apo (○) and holo (□) Cupryphan (1 in 50 mM sodium acetate buffer, pH 6.5). The difference spectrum is also shown (▾) to evidentiate the appearance of a band at 312 nm, indicative of a Cu2+–histidine charge-transfer.22
To investigate the possible competition of other metal ions in the binding to the peptide, tryptophan fluorescence quenching experiments by copper ions were carried out in the presence of approximately a fivefold excess of Zn2+ or Mg2+ (ion and peptide concentrations were 100 and 21 μM, respectively). The calculated copper dissociation constant in both cases is 1.4 (±0.1) × 10−7M, a value essentially identical to the one determined in the absence of Zn2+ or Mg2+. This result indicates that Zn2+ and Mg2+ do not appreciably outcompete copper for binding to Cupryphan. In this regard, it must be noted that addition of Zn2+ and Mg2+ does not lead to quenching of tryptophan fluorescence, thus a direct determination of the dissociation constant of these two ions for Cupryphan was not possible using fluorescence spectroscopy.
EPR spectroscopy characterization of Cupryphan
The EPR spectrum of Cupryphan, recorded at liquid nitrogen temperature (see Fig. 5), revealed a homogeneous signal, indicating the presence of only one copper species bound to the peptide. Coordination geometry appears to be axial, with values of the spectroscopic parameters (g|| and A||) typical of hexacoordinate complexes with an axial symmetry. Moreover, in the g⟂ region (Fig. 5, panel B), five to seven superhyperfine lines are observed with position and coupling constant (≈12 G), typical of copper(II) complexes with at least two magnetically equivalent histidine residues on the coordination plane.
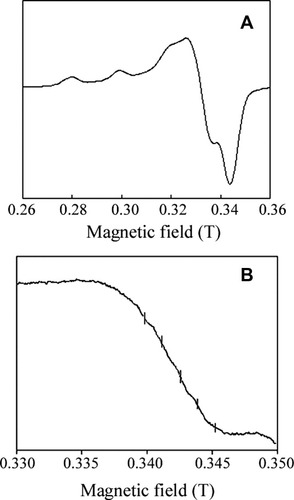
EPR spectra of Cupryphan. (A) ν = 9.556 GHz, Power = 20 mW, modulation = 10 G, T = 115 K; (B) ν = 9.556 GHz, Power = 20 mW, modulation = 4 G, T = 115 K. The vertical lines indicate the minimum number of superhyperfine lines observable.
The presence of a single class of binding sites per molecule was further corroborated by saturation experiments in the 20–200 mW power range (not shown). In fact, the signal lineshape did not change upon increasing the power, suggesting that it likely arises from a single copper coordination environment.
NMR spectroscopy characterization of Cupryphan
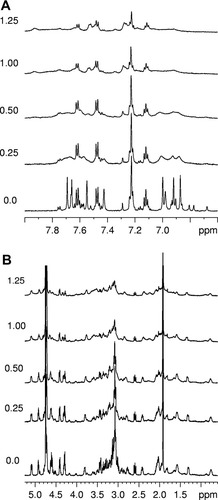
Copper interaction with the native Contryphan-Vn peptide was first investigated. The assignment of the 1H spectrum of Contryphan-Vn is reported elsewhere.16 The addition of CuCl2 to a 3.1 × 10−4M solution of Contryphan-Vn (peptide/Cu ratio 1:0.27) caused a decrease of the intensity of all 1H amino acid signals, whereas the corresponding linewidths were not significantly changed (see Fig. 6). Only the α-CH2 signal of Gly at 3.78 ppm disappeared because of the large line broadening. The increase of the Cu2+ concentration resulted in a further decrease of amino acid signal intensities along with a partial signal broadening (see Fig. 6). Moreover, new signals, well evident at the 1:1.08 peptide/Cu2+ ratio, appeared together with the α-CH2 signal of Gly at 3.94 ppm. The new signals probably belong to a Cu2+–peptide complex. Substantial signal broadening because of fast paramagnetic relaxation impeded the application of common 2D NMR experiments for spectral assignment and structure determination of this Cu2+/peptide complex. NMR data suggest that the interaction between native Contryphan-Vn and Cu2+ is characterized by low affinity as only a partial decrease of the native peptide signals was observed even at 1:1 peptide/Cu2+ ratio.

Titration of Contryphan-Vn with CuCl2 monitored by 1H NMR. The molar ratio CuCl2/Contryphan-Vn is reported on the left side of each spectrum. The arrows indicate the new signals probably belonging to a Cu2+/peptide complex. (A) 7.7–7.0 ppm region of 1H spectrum; (B) 5.2–2.9 ppm region of 1H spectrum.
The copper interaction with Cupryphan was studied by the addition of different aliquots of CuCl2 to a 2.7 × 10−4M solution of Cupryphan. To study this interaction, a complete NMR assignment of Cupryphan was performed. The 1H and 13C NMR spectral assignment of Cupryphan, obtained by means of 2D experiments, is reported in Table I. As in the case of Contryphan-Vn,16 Pro4 residue in Cupryphan is in the cis conformation, whereas Pro7 is found in the trans conformation.
| Amino acid | Atom | Chemical shift (ppm) | ||||
|---|---|---|---|---|---|---|
| Cα | Cβ,β′ | Cγ,γ′ | Cδ | Others | ||
| Gly1 | 1H | 3.090 | ||||
| 13C | 44.9 | |||||
| His2 | 1H | 4.646 | 3.190; 3.095 | 7.004 | 7.713 (Hε) | |
| 13C | 54.7 | 29.7 | 118.8 | 137.5 (Cε) | ||
| Cys3 | 1H | 4.806 | 3.089; 2.623 | |||
| 13C | 55.0 | 38.9 | ||||
| Pro4 | 1H | 4.429 | 2.091; 1.324 | 1.600; 0.782 | 3.240; 3.383 | |
| 13C | 61.3 | 33.3 | 22.3 | 48.3 | ||
| D-Trp5 | 1H | 5.117 | 3.459; 3.097 | 7.253 | 7.642 (Hε); 7.253 (Hζ3); 7.147 (Hη); 7.506 (Hζ2) | |
| 13C | 54.2 | 28.9 | 126.2 | 119.9 (Cε); 123.0 (Cζ3); 120.2 (Cη); 113.1 (Cζ2) | ||
| His6 | 1H | 4.947 | 3.147; 2.793 | 7.018 | 7.434 (Hε) | |
| 13C | 49.2 | 34.3 | 118.6 | 137.3 (Cε) | ||
| Pro7 | 1H | 4.308 | 2.445; 1.965 | 2.043 | 3.826; 3.275 | |
| 13C | 63.8 | 30.5 | 25.9 | 49.5 | ||
| His8 | 1H | 4.615 | 3.094; 3.030 | 6.944 | 7.683 (Hε) | |
| 13C | 54.4 | 29.7 | 118.6 | 137.3 (Cε) | ||
| Cys9 | 1H | 4.321 | 3.104 | |||
| 13C | 55.2 | 39.0 | ||||
| His10 | 1H | 4.730 | 3.325; 3.155 | 6.891 | 7.571 (Hε) | |
| 13C | 28.3 | 117.0 | 137.3 (Cε) | |||
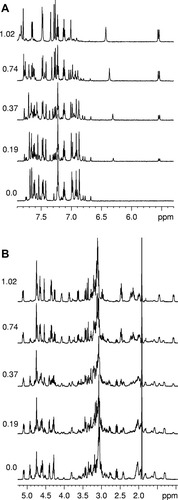
The first CuCl2 addition (peptide/Cu2+ ratio 1:0.25) caused a drastic broadening of Hδ and Hε proton signals of the imidazole rings of all four histidine residues: the signal halfwidth increased by a factor of four or six depending on the signal (see Fig. 7). On the other hand, the signals of Trp and of other amino acid residues remained unchanged or only slightly broadened. Stepwise addition of CuCl2 led to a further broadening of His signals and to a decrease of the intensity of other 1H amino acid signals without any appreciable broadening, similarly to what observed for Contryphan-Vn titration (see Fig. 7).
Titration of Cupryphan with CuCl2 monitored by 1H NMR. The molar ratio CuCl2/Cupryphan is reported on the left side of each spectrum. (A) 8.0–6.6 ppm region of 1H spectrum; (B) 5.2–0.5 ppm region of 1H spectrum.
These results suggest that at low Cu2+/Cupryphan ratios (<1), a peptide–Cu2+ complex is formed with a specific interaction between the metal and all the four His residues of the peptide, as indicated by the broadening of histidine signals.24 At high Cu2+/Cupryphan ratios (>1), when the specific binding is already saturated, a nonspecific interaction between Cu2+ and other amino acid residues is manifested by the stepwise intensity decrease of all the amino acid signals upon further addition of CuCl2 to the peptide solution, as in the case of Contryphan-Vn.
The selectivity of Cupryphan for copper was further assessed by NMR titration of the apo form of Cupryphan with Zn2+. Stepwise additions of ZnCl2 to a solution of Cupryphan (0.94 mM) give rise to a decrease of 1H NMR signals of Cupryphan and a simultaneous increase of a new set of signals that can be ascribed to a Cupryphan–Zn2+ complex (see Fig. 8). Assignment of the 1H and 13C resonances of the Cupryphan–Zn2+ complex was obtained by means of 2D NMR experiments (see Electronic Supp. Info.).
Titration of Cupryphan with ZnCl2 monitored by 1H NMR. The molar ratio ZnCl2/Cupryphan is reported on the left side of each spectrum. (A) 8.0–5.3 ppm region of 1H spectrum; (B) 5.2–1.0 ppm region of 1H spectrum.
The 1H NMR titration allowed an estimation of the Kd, obtained by a nonlinear fitting of the experimental data relative to Cupryphan/Zn–Cupryphan ratios (see Materials and Methods section) at different total Zn2+ concentrations. A Kd value of 9 (±4) × 10−6M was obtained, almost two orders of magnitude higher than that determined for the binding of copper to Cupryphan (see fluorescence quenching experiments), confirming the selectivity of Cupryphan for copper with respect to other divalent cations.
To obtain some structural information on the Cupryphan–Zn2+ complex, a ROESY experiment was performed. However, the NH protons signals of all amino acid residues are rather broad because of the relatively high exchange rate with H2O/HDO at the experimental pH value and peptide concentration. Consequently, the ROESY correlations between NH and side chain protons of amino acids were not observed, making impossible a valid structural analysis of the peptide conformation based solely on NMR data.
Design and characterization of the Arg–Cupryphan variant
In the attempt to improve the metal-binding affinity of Cupryphan, a second variant was designed in which an Arg residue was inserted between Cys9 and His10 residues of Cupryphan (see Fig. 1). The rationale behind this choice was to confer higher flexibility to the C-terminal portion of the peptide allowing an easier access of His10 to the copper site while lowering at the same time the pKa values of the His residues by a general electrostatic effect of the Arg guanidinium group. The three-dimensional structure of this second peptide was also modeled and the stability of the copper-binding site confirmed by energy minimization (see Fig. 2).
The novel peptide, named Arg–Cupryphan, was characterized by fluorescence and NMR spectroscopy. In detail, fluorescence quenching experiments confirmed binding of copper with a 1:1 stoichiometry and a Kd = 1.0 (±0.4) × 10−7M, a value slightly lower than that determined for Cupryphan. A slightly different copper coordination environment of Arg–Cupryphan with respect to Cupryphan was also confirmed by a 20 nm blue shift of the optical absorption peak in the visible region (centered at 560 nm when compared with 580 nm for Cupryphan) and by a twofold increase of the molar extinction coefficient (ε560 = 100 M−1 cm−1 when compared with ε580 = 50 M−1 cm−1 for Cupryphan).
The 1H NMR experiments performed on Arg–Cupryphan (6.8 × 10−4M) upon addition of different aliquots of CuCl2 showed the same changes observed for Cupryphan, that is, an initial significant broadening of Hδ and Hε signals of all four histidine residues and a progressive decrease of the intensity of the other 1H amino acid signals at high (>1) Cu2+/peptide ratios (data not shown).
Determination of superoxide dismutase activity of Cupryphans
The possibility that the copper ion bound to Cupryphans could be reversibly reduced, a prerequisite for any copper-mediated catalytic activity, was tested by assaying the ability of Cupryphan to dismutate superoxide anions. Superoxide dismutase activity of Cupryphans was determined using the pyrogallol enzymatic assay.25 A solution of superoxide dismutase 0.1 μM in 20 mM Tris HCl buffer pH 8.2 was used as a standard, and a solution of Cupryphan or Arg–Cupryphan 6.8 × 10−4M in 50 mM sodium acetate buffer pH 6.5 was used to determine Cupryphans superoxide dismutase activity.
The rate of autoxidation of pyrogallol was recorded in the presence of superoxide dismutase, reaching a 50% inhibition of the reaction rate after addition of superoxide dismutase at a concentration of 2.15 × 10−9M.
The same experiment was performed in the presence of Cupryphan, reaching 50% inhibition at a peptide concentration of 8.5 × 10−5M. Taking as a reference the enzymatic activity of superoxide dismutase (3.92 × 109M−1 s−1),26 and from the concentration of Cupryphan required to attain 50% inhibition of pyrogallol autoxidation, a superoxide dismutase activity rate for Cupryphan of ∼1 × 105M−1 s−1 was calculated (Table II). Surprisingly, Arg–Cupryphan superoxide dismutase activity was significantly lower than that of Cupryphan (approximately one order of magnitude, Table II). In this regard, it must be noted that an analysis of the copper-binding proteins present in the Protein Data Bank reveals that no copper site is present with the sequence pattern His-X-Arg-His, which suggests that the poor superoxide dismutase activity of Arg–cupryphan could be due to a peculiar geometry of the metal site. An alternative explanation is that Arg10 exerts a “disturbing” effect on the electrostatic field generated by the copper ion, which leads to a lower productivity of the superoxide–copper encounter rate, a position-specific effect well known to occur in superoxide dismutase charge mutants.27, 28 Nonetheless, both peptides were found to be redox-active, highlighting the ability of the engineered copper sites to reversibly cycle between oxidized and reduced redox states.
| [SOD] | [Cupryphan] | [Arg-Cupryphan] | |
|---|---|---|---|
| 50% inhibition | 2.15 × 10−9M | 8.5 × 10−5M | 8.0 × 10−4M |
| K1, K2 | 3.92 × 109M−1 s−1 | 1.0 × 105M−1 s−1 | 9.4 × 103M−1 s−1 |
Materials and Methods
Peptide synthesis
Contryphan-Vn, Cupryphan, and Arg–Cupryphan were synthesized by standard Fmoc chemistry on an automated Peptide Synthesizer (Pioneer, Applied Biosystems). The protected peptides were grown on a PAL-resin with a high level of modification, using the HATU/DIPEA amino acid activation, according to manufacturer's instructions. Side chain protection scheme was the following: Asp(Ot-Bu), Cys(Trt), Trp(Boc), and Lys(t-Boc). After synthesis, both peptides were released and deprotected by treatment with TFA/H2O/triisopropylsilane (90:5:5 by volume) for 1 h at room temperature. The cleavage mixture was filtered under vacuum into t-butyl-methyl ether at −20°C. Peptides were collected by centrifugation at 2500 rpm for 20 min at 4°C and washed with t-butyl-methyl ether. The pellet was dissolved in 50% acetonitrile and lyophilized. Peptides were loaded on a Vydac C18 semipreparative column (10 mm × 250 mm, 5 μm particle size, 300 Å pore size) and isolated from by-products with an HPLC apparatus (model K1001, Knauer GmbH, Berlin, Germany), using a linear gradient of 5–80% acetonitrile, containing 0.2% TFA, in 60 min. Elution was monitored by absorbance at 230 nm with an on-line UV detector (model K2501, Knauer GmbH, Berlin, Germany) and peptide fractions were manually collected. The major peptide-containing fractions were air oxidized at 0.01% concentration (w/w) by magnetic stirring in 0.1M NH4HCO3 for 24 h at room temperature. The cyclic peptides were finally purified from dimers/multimers by HPLC, as described for purification of synthetic products (see earlier), and characterized by infusion in an electrospray mass spectrometry (ES-IT, mod. LCQ, ThermoElectron, San Jose, CA).
Optical spectroscopy
UV–vis electronic absorption spectra were recorded on an Agilent spectrophotometer HP8452A. Protein concentration was determined by UV absorption at 280 nm, by using a molar extinction coefficient (ε280) of 5699 M−1 cm−1.29
Fluorescence spectra of Cupryphan and Arg–Cupryphan were recorded at 25°C with a Perkin-Elmer LS50B spectrofluorimeter equipped with a thermostated cell holder. The excitation wavelength was 280 nm and the excitation and emission slits were set at 10 and 5 nm, respectively. Emission spectra were recorded in the 300–400 nm range. Peptides were dissolved at a concentration of 2.1 × 10−5M in 50 mM sodium acetate buffer (pH 6.5). For copper-binding studies, CuCl2 (5.0 × 10−4M) was added in aliquots and fluorescence was measured after 5 min after addition. The dissociation constant values given in the text are the average of at least five independent experiments.
EPR spectroscopy
Low-temperature X-band EPR spectra were recorded on a Bruker ECS 106 spectrometer equipped with an ER4111VT temperature controller. In detail, spectra were recorded at 9.556 GHz and 115 K. Cupryphan concentration was 1.0 in 50 mM sodium acetate buffer, pH 6.5.
NMR spectroscopy
NMR spectra were recorded at 300K on a Bruker AVANCE AQS 600 spectrometer operating at 600.13 MHz and equipped with a Bruker multinuclear z-gradient probehead. Peptide solutions were prepared in D2O or in H2O with 10% of D2O. pH values of Cupryphan and Arg–Cupryphan solutions were adjusted to 7.5–8.0 by the addition of 0.1M NaOH in D2O to deprotonate the NH/ND group of imidazole ring of histidine residues. When only the deprotonated form is present, the chemical shifts of Hδ and Hε signals of the imidazole ring in the 1H NMR spectrum are not changed by further addition of NaOH.
 (1)
(1)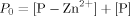 (2)
(2)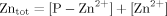 (3)
(3)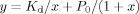 (4)
(4)SigmaPlot 8.0 for Windows program was used for nonlinear regression and fitting of the experimental data to obtain Kd value.
In all of the 1H spectra, a soft presaturation of the HOD residual signal was applied.30 1H and 13C assignments were obtained using total correlated spectroscopy (1H-1H TOCSY)31 and heteronuclear single quantum correlation (1H-13C HSQC) experiments.31 All 2D experiments were carried out using 1024 data points in the f2 dimension and 512 data points in the f1 dimension; the recycle delay was 2 s. The TOCSY experiment was performed with a spin-lock duration of 80 ms. The HSQC experiment was performed using a coupling constant of 150 Hz. The 1H-1H TOCSY and the 1H-13C HSQC experiments were processed in the phase-sensitive mode with (2048 × 2048) and (1024 × 1024) data points, respectively. Rotating frame nuclear Overhauser effect spectroscopy (1H-1H ROESY)32 with a spin-lock time of 150 ms was used to assign the sequence of amino acid residues.
1H and 13C chemical shifts are reported in ppm with respect to sodium 3-(trimethylsilyl-2,2,3,3-tetradeutero)propionate, which gives a singlet signal at 0.0 ppm and was used as an internal standard.
Molecular modeling
Initial structural models of Cupryphan and Arg–Cupryphan were built using the average solution structure of Contryphan-Vn as a template (PDB code 1NXN16). Inserted His residues conformation was chosen using the standard Deep View rotamer library33 so as to form a tetragonal metal-binding site with geometry and ligand distances comparable to the copper-binding site of bovine Cu,Zn superoxide dismutase (PDB code 2SOD18). The holo forms of Cupryphan and Arg–Cupryphan were then equilibrated in water by energy minimization in explicit solvent using the CHARMM macromolecular mechanics package19 and the CHARMM27 parameters and force field.34 The three-site TIP3p model35 was used for water molecules. In detail, hydrogen atoms were added to the modeled peptides using the HBUILD routine of the CHARMM package. The minimized peptides were placed in a truncated octahedron, constructed from a cubic volume of water molecules of dimension 56.57 Å × 56.67 Å × 56.57 Å, and water molecules overlapping with protein atoms (cutoff = 2.8 Å) were removed. Solvated structures, containing ∼2900 solvent molecules, were further energy minimized to an energy gradient of 0.05 kcal/mol.
Superoxide dismutase activity assay
Superoxide dismutase activity of Cupryphan and Arg–Cupryphan was determined using the pyrogallol enzymatic assay.25 The assay was carried out at 30°C, in 20 mM Tris-HCl buffer pH 8.2, containing 1 mM EDTA and 200 μM pyrogallol, recording the absorption increase at 420 nm for 3 min. Bovine erythrocytes Cu,Zn superoxide dismutase (0.1 μM in 20 mM Tris HCl buffer pH 8.2) was used as a standard.
Conclusions
The increasing knowledge of the principles underlying protein structure and function and the need of novel environment friendly biocatalysts for biotechnological purposes has fuelled in the last few years the search for stable scaffolds that can be engineered to obtain (macro)molecules with given structural and/or functional properties. In this regard, conopeptides represent robust scaffolds highly tolerant to sequence mutation, as they have been developed by Conus species through a sort of combinatorial chemistry to target in highly specific and efficient manner the ion channels and receptors of their natural prey. In fact, conopeptide scaffolds are rigid and stable, in order to bind with high affinity complementary molecular surfaces on their targets, and are tolerant to multiple sequence mutations, as they have been adapted during cone snails evolution to tolerate hypervariability of the intercysteine loops to bind with high specificity a huge variety of different functional targets.1, 3, 4
We tested the potential of conopeptides as scaffolds for the engineering of novel, metal-based, biocatalysts starting from the simplest prototype of disulphide-constrained conopeptides: the Contryphans. The results of this work indicate that indeed this class of peptides can be successfully exploited to engineer novel, stable and redox-active macromolecules. In fact, Cupryphans bind copper in a stable manner and with a fairly low dissociation constant. In addition, the engineered metal center is able to catalyze redox reactions, as demonstrated by Cupryphans superoxide dismutase activity. A point that deserves further investigation is represented by the determination of the precise copper coordination environment at an atomic level. NMR data indicate that all the four His residues of the peptides coordinate the copper ion. However, the paramagnetic nature of copper does not allow the determination of the structure of Cupryphans in solution, which would greatly help in designing derivatives with increased metal affinity. Further, use of a zinc derivative of Cupryphan in NMR ROESY experiments did not yield the cross-peaks required for solution structure determination, making X-ray analysis the only choice for the atomic structure determination of Cupryphan.
As a final remark, the peptidic nature of Cupryphan, together with the ease of chemical synthesis makes it a promising starting point for the development of more efficient superoxide dismutase mimics and antioxidant molecules as a natural follow up of this work.
Acknowledgements
The authors thank Dr. Maurizio Minetti for help in performing the EPR studies and for helpful discussion.




