Development of artificial photosystems based on designed proteins for mechanistic insights into photosynthesis
Review Editor: Aitziber L. Cortajarena
Abstract
This review aims to provide an overview of the progress in protein-based artificial photosystem design and their potential to uncover the underlying principles governing light-harvesting in photosynthesis. While significant advances have been made in this area, a gap persists in reviewing these advances. This review provides a perspective of the field, pinpointing knowledge gaps and unresolved challenges that warrant further inquiry. In particular, it delves into the key considerations when designing photosystems based on the chromophore and protein scaffold characteristics, presents the established strategies for artificial photosystems engineering with their advantages and disadvantages, and underscores the recent breakthroughs in understanding the molecular mechanisms governing light-harvesting, charge separation, and the role of the protein motions in the chromophore's excited state relaxation. By disseminating this knowledge, this article provides a foundational resource for defining the field of bio-hybrid photosystems and aims to inspire the continued exploration of artificial photosystems using protein design.
1 NATURAL PHOTOSYNTHESIS AND ITS CHALLENGES
Photosynthesis is the process through which green plants, algae, and certain bacteria harness the energy of light to synthesize chemical compounds that are essential to life (Scholes et al., 2011). The light-harvesting in photosynthesis occurs with exceptional efficiency, ensuring the utilization of each photon for light conversion under optimal conditions (Scholes et al., 2011). This efficiency arises from the interconnection between two ultrafast phenomena: photon absorption (Croce & van Amerongen, 2020; Tros et al., 2020) and charge separation (Cardona et al., 2012; Croce & van Amerongen, 2020; Ma et al., 2019; Romero et al., 2017; Tros et al., 2020). Photon absorption occurs in light-harvesting complexes across a range of wavelengths (Croce & van Amerongen, 2014; Lapillo et al., 2020; Loco et al., 2016; Tros et al., 2020; van Grondelle et al., 1994). The charge separation occurs when the energy from the absorbed photons is transferred through these complexes to the reaction centers, where the light energy is converted to redox energy (Romero et al., 2017; Scholes et al., 2011). These intricate processes are orchestrated by photosystems comprised of proteins, chromophores, and redox cofactors such as metals or metal clusters that function in synergy (Sirohiwal et al., 2020). Chromophores such as chlorophyll work as “antenna” of these photosystems, absorbing photons of light and transferring the energy to the reaction center (Balevičius et al., 2017; Simkin et al., 2022). Other chromophores such as carotenoids and phycobilins contribute to broadening the spectrum of the absorbed light and facilitating the dissipation of excess energy (Hashimoto et al., 2015, 2016; Simkin et al., 2022). Proteins provide an environment composed of amino acids for organizing and positioning chromophores within photosystems (Romero et al., 2017) and coordinate the activities in response to changing environmental conditions (Belgio et al., 2014; Manna & Schlau-Cohen, 2022; Takahashi & Badger, 2011; Valkunas et al., 2012). The coupling between protein amino acids and chromophores governs the photosystem assembly (Elias et al., 2023; Mascoli et al., 2021), modulates the electronic properties of individual chromophores (Liguori et al., 2020; Loco et al., 2016), and shapes chromophore interaction networks (Croce & van Amerongen, 2020; Tros et al., 2020) in manners that are still not fully understood.
Investigations have suggested that protein conformational changes regulate the light-harvesting and primary electron transfer reactions of chromophores by orchestrating their extraordinary efficiency (Bhowmick et al., 2023; Cellini et al., 2024; Kulik et al., 2020; Li et al., 2024; Litvín et al., 2005; Russell & Vinyard, 2024; Sauter et al., 2016; Sugo et al., 2022; Terentyev, 2022). Nonphotochemical quenching for photoprotection also unfolds conformational changes across vast interconnected and finely tuned spatiotemporal scales (Accomasso et al., 2024; Lapillo et al., 2020; Nawrocki et al., 2024; Pedraza-González et al., 2023; Ruban et al., 2007). Numerous inquiries have persisted regarding the understanding of the coupling effects between structural changes and chromophore relaxation or heat dissipation processes on photosystems. Addressing these questions is crucial for unraveling the intricacies of photosynthetic systems and controlling the photosynthetic quantum phenomena. This control has the potential to bring advances in the development of bio-inspired energy technologies that are more efficient and sustainable than the existing ones, representing a significant step forward in the global journey toward green energy generation.
For studying the fundamental principles governing natural photosynthesis, different strategies have been developed based on the protein engineering and molecular biology breakthroughs in the 21st century (Chen, 2012). These strategies are based on the engineering of natural photosystems or the design of artificial photosystems.
Natural photosystem engineering is based on modifications of natural photosystems through mutations to understand the role of different amino acids in the light-harvesting process (Bhowmick et al., 2023; Han et al., 2022; Ibrahim et al., 2021). This strategy has been crucial in obtaining key information on photosynthesis functioning, including the activation of energy transfer pathways (Espinoza-Corral et al., 2024; Sil et al., 2022), the role of photoactive proteins in photoprotection (Kuznetsova et al., 2020), or the mechanism of photosynthetic water oxidation (Bhowmick et al., 2023; Guo et al., 2023; Han et al., 2022; Hussein et al., 2023; Ibrahim et al., 2021). An interesting approach that has been reported is replacing the original amino acids with noncanonical ones (Weaver et al., 2021; Weaver & Boxer, 2018). These amino acids have subtly different electronic or steric properties than the original ones, allowing us to study their role in the origin of symmetry breaking (Weaver et al., 2021) or stabilizing charge transfer intermediates (Saggu et al., 2014), among others. The main challenge in performing mechanistic studies based on natural photosystem engineering is their complexity, which makes it difficult to evaluate individual features, limiting the fundamental knowledge that can be achieved.
An alternative approach is designing artificial photosystems by combining recombinant proteins and synthetic chromophores to mimic the key steps in photosynthesis in a simplified manner. These simplified photosystems are called bio-hybrids. The modularity of this approach allows for the creation of custom-made photosystems with the desired properties to answer specific questions on how photosystems work without using the whole photosystem. To develop useful knowledge about photosystem functioning, three key steps need to be taken (Figure 1): (1) design the photosystem's simplification, (2) bio-hybrid synthesis and characterization, and (3) iterations for knowledge generation. In the first step, the process of interest that takes place in the natural photosystem is studied to identify its key properties and design how to simplify them in an engineered bio-hybrid. In the second step, the key features that will generate the bio-hybrid are selected to synthesize it. In the third step, deep characterization of the engineered bio-hybrid is done based on design iterations to generate new knowledge on the photosystem's functioning.
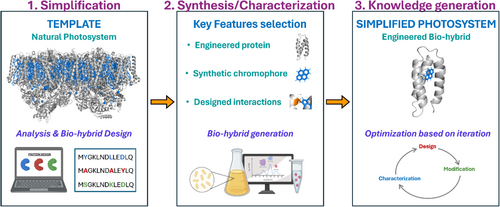
Besides their simplicity, the bio-hybrids have the potential to show mechanisms that are key for the photosystem's functioning but are hindered by the complexity of natural systems. Crucial to develop useful knowledge is to generate the proper design suitable to answer the question of interest. Performing these designs is challenging as there are many parameters to be controlled. This review covers the main advances in the field of bio-hybrid design, with a particular focus on the key considerations for designing bio-hybrids (Section 2), the advances in bio-hybrid engineering (Section 3), and the potential of bio-hybrids to solve open questions in how natural photosystems works (Section 4). Finally, this review shows the remaining challenges in the field (Section 5). By disseminating this knowledge, this article aims to boost the development of new bio-hybrid design strategies that allow to answer open questions on the photosystem's functioning and to optimize the bio-hybrid's for their use in nanotechnological applications.
2 ARTIFICIAL PHOTOSYSTEM'S DESIGN COMPONENTS
Engineered bio-hybrids are composed of synthetic chromophores, protein scaffolds, and the interactions between these two (Figure 2). Over the years, the fundamental properties of these components have been studied in isolated molecular systems. Based on these studies, synthetic chromophores are usually selected based on their photophysical and photochemical properties (Hammarström, 2015; Hou et al., 2019; Pannwitz & Wenger, 2019; Zysman-Colman, 2020). The protein scaffolds are based on re-engineered proteins (Dai et al., 2021; Miller et al., 2007) or designed from scratch (Baker, 2019). In this section, we summarize the basic properties of these components that must be considered when designing the bio-hybrid photosystems.
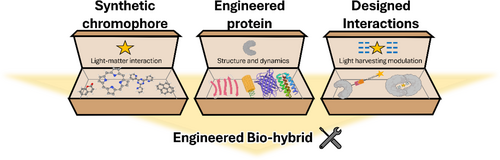
2.1 Synthetic chromophores: Light-harvesting molecules
Chromophores are the molecules that interact with the light in the bio-hybrids (Lasorne et al., 2011). When light of the appropriate wavelength strikes a chromophore, it absorbs the photon's energy, promoting electrons to higher energy levels. How the molecule interacts with the light and its subsequent evolution is determined by its molecular structure, electronic configuration, and its interaction with the surroundings (Gromov et al., 2007). The relaxation process in a chromophore can occur following different pathways. The main decay processes are heat dissipation through chromophore vibrations via internal conversion (IC) or light emission via fluorescence (Mataga et al., 2000). The excitation can also evolve into a triplet state through intersystem crossing (ISC) (Sasikumar et al., 2020; She et al., 2022), into a charge transfer (Lin et al., 2018), or trigger structural rearrangements such as isomerization (Hou et al., 2022; Pfeifer et al., 2024; Pooler et al., 2021; Roke et al., 2018; Stähler et al., 2022).
When two or more chromophores are close to each other, their electronic or vibrational states can interact, resulting in electronic or vibrational coupling, which influences different processes such as charge transfer reactions or exciton migration. Related to the charge transfer, depending on the redox properties of the chromophores, these couplings can trigger photochemical responses, leading to phenomena like charge separation (Hou et al., 2019; Lu et al., 2014; Yanagi et al., 2022), electron transfer (Aramburu-Trošelj et al., 2020; Bayda et al., 2017; Colasson et al., 2020; Hou et al., 2019; Hung et al., 1994; Illescas et al., 2009; Madrid-Úsuga et al., 2019; Sánchez et al., 2003; Stasyuk et al., 2024; Wróbel & Graja, 2011), or proton-coupled electron transfer reactions (PCETs) (Pannwitz & Wenger, 2019; Tyburski et al., 2021). In the context of exciton migration, electronic coupling influences the spread of excited states across multiple chromophores via Förster resonance energy transfer (FRET) (Chen et al., 2024; Haldar et al., 2018; Hsu, 2009; Kashida et al., 2018; Langhals & Walter, 2020), Dexter energy transfer (Bai et al., 2019; Skourtis et al., 2016; Wang et al., 2024), or coherently transferred forming delocalized excitons (Curti et al., 2023; DuBose & Kamat, 2021; Lu et al., 2017; Wang et al., 2024; Weingarten et al., 2017).
In the lab, it is possible to synthesize chromophores with different structures and properties. Understanding the properties of chromophores through molecular studies is key to selecting the appropriate chromophore for developing customized bio-hybrids. Embedded in the protein, the molecular structure of the chromophore and its electronic configuration might change due to protein–chromophore interactions. Studying how the protein environment tunes the chromophore's light-harvesting, redox properties, or the coupling between chromophores allows to draw conclusions regarding the role of the proteins in the photosystem functioning.
2.2 Engineered proteins: A diverse functional scaffold
Proteins stand out as remarkable biological macromolecules, offering a unique combination of chemical diversity, structural versatility, and dynamic flexibility that are unparalleled by any synthetic polymers (Camponeschi et al., 2019; Orozco, 2014; Schiavina et al., 2019; Ye et al., 2018).
2.2.1 The chemical diversity
The chemical diversity of proteins is attributed to the wide variety of amino acids, each with unique chemical traits. The amino acids' properties depend on their size, charge, and polarity encoded in their side chain. Depending on their side-chain, amino acids can be polar or hydrophobic, hydrogen donors, aromatic, redox-active amino acids, and/or contain charged groups that generate intramolecular electric fields. The amino acid composition defines the protein's chemistry and how it interacts with other molecules.
2.2.2 The structural versatility
The structural versatility of proteins comes from the variety of three-dimensional arrangements that showcase different protein folding patterns, ranging from taut and inflexible to pliant and adaptable (Dobson, 2003). Protein folding is mainly defined by three structural elements that form its secondary structure: α-helices, β-sheet, and loops. This secondary structure is comprised of protein backbone interactions and defines how amino acids are organized in the protein. The intricate arrangement of the amino acid side-chain forms the tertiary protein structure. The interaction between the amino acids influences the Gibbs free energy (ΔG) of the protein fold and thus the stability of the protein folding. The protein structure is crucial for the performance of the photosystem, as it defines the chromophore environment and its movement freedom.
2.2.3 The dynamic flexibility
The dynamic flexibility of proteins empowers them to transition between various structural states. These conformational changes take place in a broad range of time and space scales, from bond vibrations and local side-chain rotations, on the femtosecond-to-picosecond time scale, to slow movements that encompass shifts in the secondary structure or protein domains that occur on the millisecond or even slower time scale (Haran & Riven, 2023). These conformational changes are activated by thermal fluctuations or external stimuli such as ligand binding or chromophore excitation, and their amplitude is determined by folding stability. Protein scaffold dynamics are essential in the photosystem function, changing the chromophore properties over time to affect transiently their light-harvesting properties, charge transfer efficiency, or photoprotection (Li et al., 2020).
Nowadays, there are strategies to design proteins with tailored features and methods to synthesize them in the lab in a controlled manner. The interplay between the protein features (chemistry, structure, and dynamic) is key for the final performance of the designed bio-hybrid photosystem and thus must be considered.
2.3 Designed protein–dye interaction: Light-harvesting modulation key
In photosystems, chromophores interact closely with amino acid side-chains. These interactions are deeply rooted in the amino acid composition and chromophore properties, which lead to multifaceted consequences at all levels, including structural alterations and changes in electronic properties (Spezia et al., 2003). This section explains the types of interactions known in photosystems, including local and non-local interactions, and their relevance. Understanding the role of these interactions in photosystem functioning is one of the main challenges in the field of photosynthesis.
2.3.1 Local interactions
Local interactions refer to interactions in which a specific amino acid plays a key role and is divided into non-covalent and covalent interactions. Non-covalent interactions include hydrophobic, van der Waals, hydrogen bonding, electrostatic, and π–π interactions. The strength of these interactions depends on the orientation between the residue and the chromophore itself (Baumgart et al., 2021). Hydrophobic, van der Waals, and hydrogen bonding interactions affect the alignment of chromophores within the protein structure and directly influence their vibrational and rotational freedom (First et al., 2018; Orozco, 2014; Patil et al., 2010; Venugopal et al., 2020). Hydrogen bonds also define the hydration and solvation surfaces of chromophores, which influence their pKa values (Naseem et al., 2013). Electrostatic and π–π interactions directly impact the electron clouds of chromophores, modifying their transition dipole moments (Shao et al., 2022; Sherrill, 2013). Covalent interactions generate covalent bonds between reactive groups in chromophores and amino acids, resulting in a permanent attachment. The interactions can be non-specific or orthogonal—with high specificity—depending on the selected reactive groups. Non-specific interactions have the advantage of broader applicability and adaptability (Roelfes, 2019), while orthogonal interactions provide control of the position of the chromophore in the protein scaffold (López-Andarias et al., 2018; Mejías et al., 2016; Romera et al., 2015; Xu et al., 2021).
2.3.2 Non-local interactions
Non-local interactions occur between chromophores and their environment at a distance. These interactions are responsible for steric and dielectric effects. In relation to the steric effects, protein conformation can affect the chromophore's mobility by changing its shape, which in turn affects its orbital distributions (Houjou et al., 2001; Kabir et al., 2023). Recent studies have suggested that chromophore's electronic states interact with their environment through stochastic energy fluctuations of weak electron-vibration coupling that affect their light-harvesting properties (Houjou et al., 2001; Kabir et al., 2023; Romero et al., 2017; Zhu, Li, et al., 2024). On the other hand, charged amino acids form electrostatic networks that influence the dielectric constant of the protein environment (Allgöwer et al., 2022; Baumgart et al., 2021; Di Luca et al., 2017; Isom et al., 2010; Kaila, 2018; Robinson et al., 2018; Sirohiwal et al., 2020). This effect is screened by water molecules that reduce the strength of the electrostatic interactions between the charged groups within the proteins. The dielectric constant varies within a protein, and chromophores may experience different dielectric environments depending on their location and the nature of the surrounding amino acid residues.
3 STRATEGIES FOR CUSTOMIZED BIO-HYBRID DEVELOPMENT
Bio-hybrid photosystems are modular, which means that the chromophore and protein scaffold can be modified independently. The introduced modifications change the properties of the bio-hybrid in different ways, including its electronic properties, dynamics, and complexity. Thus, it is crucial to have control over how to introduce these modifications in the systems to develop a customized bio-hybrid. Reported strategies for bio-hybrid engineering include site-specific chromophore incorporation (Section 3.1), the design of protein scaffold modifications (Section 3.2), and the generation of hierarchical photosystem by encoded bio-hybrid assembly (Section 3.3). In this section, we summarized the relevance of each strategy for bio-hybrid engineering.
3.1 Site-specific chromophore incorporation
Researchers have developed strategies to combine chromophores and proteins in a controlled manner. The strategies are based on the supramolecular chromophore's binding, its coordination by metals, its linking based on orthogonal interactions, or the use of non-natural amino acids as photoactive units (Figure 3). The strategy used for incorporating the chromophore will influence the final structure of the bio-hybrid, including the chromophore's location and orientation, its stability, and its photophysical and photochemical properties. The advantages and disadvantages of the different strategies reported for chromophore–protein combination are summarized below.
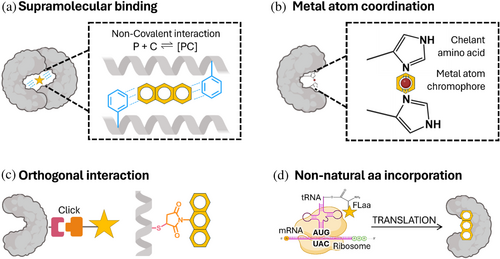
3.1.1 The supramolecular binding approach
The supramolecular binding approach (Figure 3a) relies on non-covalent interactions for incorporating the chromophore into the protein structure, achieving a thermodynamically favorable arrangement at equilibrium. The propensity of the formed complex to separate reversibly into its individual components is measured by the dissociation constant (Kd). By taking advantage of the promiscuity of the lactococcal multidrug resistance regulator (LmrR) protein pocket (PDB ID: 3F8F), it has been shown that aromatic amino acids decrease the Kd for the protein–chromophore complex when compared with hydrophobic amino acids (Ferrara et al., 2022; Mejías et al., 2020), playing its electron density a key role in chromophore affinity (Shao et al., 2022). Charged amino acids have also been used to boost supramolecular interactions, as they are stronger than aromatic or hydrophobic interactions overall (Li et al., 2023). The primary advantage of this approach is its reversibility, which allows for the on-and-off complex formation. Drawbacks include limited control over the arrangement of chromophores in the protein pocket and the equilibrium-based yield of the complex.
3.1.2 The metal coordination approach
The metal coordination approach (Figure 3b) uses chelating amino acids with sulfur, nitrogen, or oxygen, which act as ligands that interact with metal-containing chromophores (Hutchins et al., 2023). The affinity of chromophores to the protein pocket can be tuned by modifying the hydrophobicity of the ligand's environment, as demonstrated using alpha helix bundles modified with axial histidines (Anderson et al., 2014). The binding capacity of proteins increases when using a well-folded scaffold with high conformation flexibility (Curti et al., 2023). The primary advantages of coordinating the chromophores using metals include the stability of the formed complex, the control of the position of the chromophore by the ligands, and the direct effect on the redox properties of the metal by coordination. Their drawbacks include weak control over the orientation of the chromophore, especially if the binding of the chromophores involves protein conformational changes.
3.1.3 The orthogonal interaction approach
The orthogonal interaction approach (Figure 3c) uses covalent interactions to link the chromophore at a specific position of the protein. Chromophores are functionalized with a linker composed of a reactive group that interacts with amino acid side chains in a bio-orthogonal manner, avoiding cross-reactions in the systems (Devaraj & Finn, 2021), and a spacer that provides the movement freedom of the chromophore (Wong & Zimmerman, 2013). The commonly used reactivities are the Michael reaction (Nair et al., 2014), Staundinger ligation (Lin et al., 2005), and click chemistry (Best, 2009; Mehak et al., 2024). Each reactivity has a different labeling efficiency and stability, depending on the chromophore and protein scaffold used (Best, 2009; Scinto et al., 2021). The reactivities can be combined to form multifunctional systems (Xu et al., 2021). The primary advantages of this approach are its selectivity and efficiency in controlling the position and orientation of the chromophore in the photosystem (Best, 2009; Scinto et al., 2021). The drawback is the need for extra purification steps to achieve a clean bio-hybrid.
3.1.4 The non-natural amino acid incorporation approach
The non-natural amino acid incorporation approach (Figure 3d) uses photoactive non-natural amino acids (Phaas) as chromophores. Phaas are modified versions of amino acids that have fluorescent moieties in their side chains (Cheng et al., 2020; Dangerfield & Johnson, 2020; Summerer et al., 2006). Owing to their similarity to natural residues, they can be used to fluorescently label protein backbone directly at the protein expression. The amber codon suppression method is the most used methodology for Phaa incorporation in recombinant proteins. Briefly, the amber codon is assigned to the non-natural amino acid codon by supplying the cells with an exogenous aminoacyl-transfer ribonucleic acid synthetase and its corresponding transfer ribonucleic acid (Ovaa & Kim, 2014; Shandell et al., 2021). This strategy allows the incorporation of the non-natural amino acid at this unique position, minimizing background interference. A family of Phaas has been successfully incorporated using this strategy, including hydroxycoumarin-dimethylglycine (Summerer et al., 2006), acridonylalanine (Jones et al., 2021; Speight et al., 2013), and biphenyl-phenylalanine (Lampkowski et al., 2015). The primary advantages of this approach are the high stability of the generated bio-hybrids due to the lack of labile linkers, the control of the exact location of the chromophore position, and the direct coupling of the chromophore to the protein backbone. The drawbacks are the small variety of Phaas that are still available and the low incorporation yield.
3.2 Design of the protein scaffold modifications
Strategic design of the modifications in the bio-hybrid's proteins is key to drawing conclusions about how photosystems work by tracking the effect of the mutations in the properties of the chromophore. The main strategies used to design these modifications are based on rational design, directed evolution, and computational design (Figure 4). Rational design and directed evolution use existing protein structures as starting points and modify them to enhance their properties or functions. Computational design can use existing proteins or design de novo proteins from scratch based on theoretical calculations. The following sections summarize the key points of each strategy and the challenges behind their use for photosystem engineering.
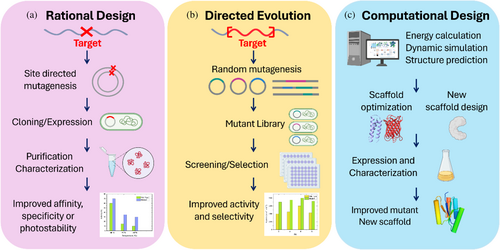
3.2.1 Rational protein design
Rational protein design (Figure 4a) streamlines protein modification by targeting precise functionalities based on existing knowledge from empirical data or theoretical principles. This strategy has been used to design protein modifications that selectively modify the binding affinity of chromophores (Ferrara et al., 2022; Mejías et al., 2020), to combine metal coordination and hydrophobic interactions (Kodali et al., 2017), and to tailor protein pockets selectively for improving the photostability (Mejías et al., 2020) and singlet oxygen photosensitization of the chromophore (Rodríguez-Pulido et al., 2018; Westberg et al., 2015). It has also been used to design orthogonal reactivities for chromophore site-specific incorporation, forming arrays of porphyrins (López-Andarias et al., 2018; Mejías et al., 2016) or to link electron donor and acceptor molecules with specific spacing at engineered reaction centers (Ennist, Stayrook, et al., 2022; Ennist, Zhao, et al., 2022; Keller et al., 2017). Overall, rational design is a time- and cost-efficient method for engineering proteins, offering insights into potential modifications before experimentation. However, some effects of the designed changes can be easily underestimated, resulting in a bio-hybrid structure different from the predicted structure.
3.2.2 Directed evolution
Directed evolution (Figure 4b) creates genetic diversity by introducing mutations into a target gene, followed by a selection process to identify the variants that exhibit the desired traits. Consecutive selection cycles follow a single evolutionary path by accumulating different mutations toward the optimization of the protein function (Arnold, 2018; Wang et al., 2021). This design approach has been extensively used to enhance ligand affinity by a particular scaffold (Zhu, Man, et al., 2024), to increase its thermal stability (Kotzia & Labrou, 2009), or to develop enzymes with enhanced catalytic activity (Xu et al., 2003). However, the complexity of photosystems with multiple interacting components makes it challenging to engineer them using directed evolution, making this strategy poorly used in photosystem engineering.
3.2.3 Computational protein design
Computational protein design (Figure 4c) is based on computer algorithms and machine learning techniques to predict how specific modifications affect the photosystem structure and dynamics. Different strategies are available for predicting the protein structure. For example, AlphaFold uses a multi-sequence alignment strategy to develop a deep learning algorithm based on the protein amino acid sequence (Anon, 2023; Jumper et al., 2021). This tool excels in predicting protein scaffold folding, yet to gain a full understanding of the optimal chromophore binding pocket—including insights into binding forces, chromophore, and protein structure and orientation within the pocket, as well as protein and chromophore dynamics—it is essential to integrate additional computational technique (Curti et al., 2023). Another design tool is Rosetta. Rosetta uses energy minimization of structures based on residue conformation, hydrogen bonds, and electrostatic interactions to make calculations. This approach allows for the direct modeling of the orientation of the chromophore in the binding sites (Baek et al., 2021; Rohl et al., 2004) and protein–chromophore interactions (Baek et al., 2021; Kuhlman, 2019; Wittenberg et al., 2013). To compute pigment-binding protein complex dynamics is molecular dynamics simulations. In molecular dynamics simulations, each molecule is treated as a system of interaction sites for which Newton's equations of motion are solved, providing information on the dynamics of the simulated complex (Liguori et al., 2020). On the other hand, density functional theory uses a quantum mechanical simulation that approximates the Schrodinger equation to calculate the system's energy (Cupellini et al., 1861; Nottoli et al., 2021). Most of the available tools combine approximations to examine orthogonal degrees of freedom (Bondanza et al., 2020), chromophore energetic states (Balevičius et al., 2017), and photo-induced reaction dynamics (Allgöwer et al., 2022; Baumgart et al., 2021; Kaila, 2018; Saura et al., 2022; Wittenberg et al., 2013), among others.
3.3 Generation of hierarchical photosystems
Hierarchical photosystems can be formed through the assembly of multiple bio-hybrids. The assembly starts with monomers, individual bio-hybrids, that interact to build a multifunctional photosystem in a bottom-up fashion (Balevičius et al., 2017; McManus et al., 2016; Solomonov et al., 2024; Sun et al., 2020; Zhu et al., 2021). The structure of the assembly depends on the pattern of designed interactions between the bio-hybrids (McManus et al., 2016; Sun et al., 2020; Zhu et al., 2021). These interactions can be boosted by engineering protein–protein interactions or by designing reactivities with non-protein structures (Figure 5). The assembled photosystems will show properties different from those of single bio-hybrids, coming from the synergy of the different components. The main strategies used to generate photosystem assemblies and their relevance are summarized below.
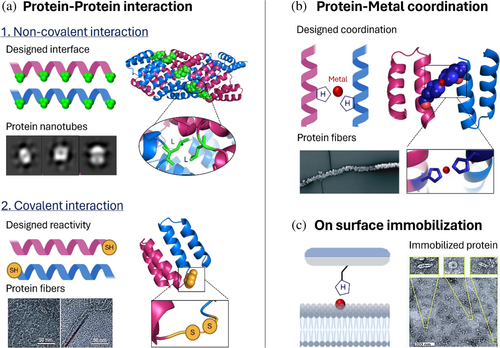
3.3.1 Assemblies based on engineered protein–protein interactions
These assemblies are built by engineering novel interfaces in proteins with amino acids that boost interactions between the protein monomers (Figure 5a). Non-covalent interactions are the most used to trigger protein self-assembly. For example, tetratricopeptide repeat proteins (PDB ID: 2AVP) have been optimized to assemble based on head-to-tail and side-to-side dipole–dipole interactions between alpha helixes (Grove et al., 2013; Mejias, Aires, et al., 2016; Mejias et al., 2021; Mejías et al., 2014; Mejias, Couleaud, et al., 2016). The stability of the assembly depends on the dipole magnitude of the alpha helix, which changes with the aspect ratio of its turn. Assemblies are also generated by designing interfaces in proteins based on hydrophobic, aromatic, or electrostatic interactions to form protein nanotubes (Sanchez-deAlcazar et al., 2018), coiled–coiled heterodimers (Chen et al., 2019), rods (Anaya-Plaza et al., 2023), or ordered crystals (Anaya-Plaza & Kostiainen, 2023; Patrian, Shaukat, et al., 2023). Another strategy is decorating proteins with peptide-binding sites to lead the protein assemblies by peptide–protein interaction (Speltz et al., 2015). Overall, buffer conditions are essential to control the assembly structure, as highlighted in the tobacco mosaic virus (TMV) (PDB ID: 2OM3) assembly that turns from rods to multilayer disks when the pH changes (Miller et al., 2007). Covalently stapled assemblies make it possible to build stable assemblies by shifting the equilibrium toward the oligomerized state through covalent bond formation. The specificity of the covalent interactions used in this strategy provides low cross-reactions and thus full control of the assembly process. For example, click reactions have been extensively used to link helical hairpins that reconfigure the protein active site based on the linkers used (Chino et al., 2017). Another example is the use of non-canonical amino acids such as p-amino phenylalanine and 3-nitrotyrosine to stabilize the interactions between the designed heterodimeric bio-hybrids (De Filippis et al., 2006). Disulfide bonds have also been used to link several alpha helix motifs, forming hydrophobic pockets to allocate heme molecules (Chen et al., 2002) and staple head-to-tail interactions to hold protein units in a linear structure (Mejías et al., 2014).
3.3.2 Assemblies based on coordination chemistry
Coordination chemistry is used to provide a strong and selective chemical connection between proteins using metal ions as bridges (Figure 5b) (Sontz et al., 2014). In particular, proteins are decorated with chelating amino acids such as histidines, forming an intramolecular interface that has been used, for example, to guide the formation of a 16-helix structure with zinc as a bridging metal (Salgado et al., 2007) (PDB ID: 2QLA). Metals have also been used to achieve reversible regulation of the assembly through changes in pH to modify the protonation state of histidines or the addition of EDTA as a chelating agent (Aupič et al., 2024). Rules that control the strength, directionality, and selectivity of the assembly have been presented in an approach called “metal-directed protein self-assembly” (Salgado et al., 2010).
3.3.3 Assemblies generated on lipids, polymers, and surfaces
Assemblies generated on lipids, polymers, and surfaces (Figure 5c) are used to form hybrid assemblies and materials. For example, TMV was decorated with a peptide containing a poly-histidine tag to immobilize it in nickel-chelating lipids (Dai et al., 2023). The immobilization efficiency was more than 97%, and the proteins had the freedom to move through the bilayer. Polymers have also been used to immobilize proteins through imidazolinone formation (Koo et al., 2019). In particular, highly reactive 2-pyridine carboxyl aldehyde polymers were used as a substrate to react with the N-terminal of the proteins to produce linkages that remain stable over the long term but could be reversed through the addition of hydroxylamine. Finally, photosystems have also been integrated into electrodes made of various materials, such as indium tin oxide, which combined with hydrogenase yield H2 and O2 (Mersch et al., 2015). Besides these specific examples, deep research on surface functionalization strategies and changes of the photosystem properties when attached to surfaces are needed to extend the use of these strategies in photosystem development.
4 ADVANCES IN THE PHOTOSYNTHESIS UNDERSTANDING BASED ON ENGINEERED BIO-HYBRIDS
Bio-hybrids have been designed to address questions about how the protein structure affects light-harvesting mechanisms (Section 4.1) and photoinduced redox reaction kinetics (Section 4.2). Crucial to obtaining relevant knowledge on how photosystems work is the design of the bio-hybrid. In this sense, the properties of the engineered bio-hybrid need to be suitable to answer the question of interest. The bio-hybrid design includes the chromophore selection, optimizing its incorporation strategy in the protein scaffold, and selecting the key protein modifications. The effects studied in these artificial photosystems and the main conclusions achieved are described in the following sections.
4.1 Light-harvesting mechanisms in photosystems: Excited state relaxation dynamics
Light-harvesting mechanisms have been studied in bio-hybrids by altering the design of the chromophore composition or its arrangement and tracking the effect of these changes in the excited state relaxation of chromophores (Figure 6). Single chromophore bio-hybrids have been engineered to evaluate environmental effects on the chromophore's IC and ISC, whereas multi-chromophore systems have been used to study chromophore–chromophore coupling mechanism and its role in energy transfer or charge transfer state formation.
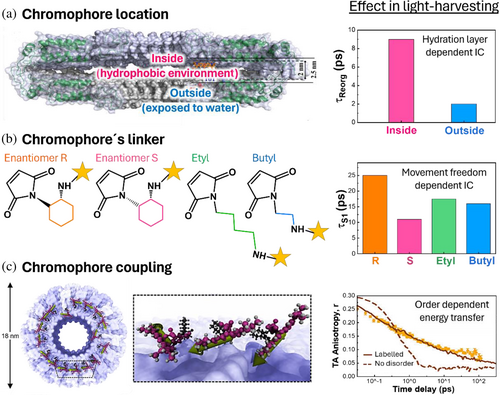
4.1.1 Effects of the protein environment on the chromophore's IC, ISC, and photostability
The role of proteins in tuning the IC rate has been studied taking advantage of the hydrophobic pocket of the TMV protein (Figure 6a,b) (Noriega et al., 2015). By covalently linking a single chromophore at different TMV positions, it has been shown that water molecules trapped in the protein cavity stabilize the excited state of the chromophore by preventing its crossover to energy surface regions where IC is efficient (Figure 6a). Using the same protein scaffold, it has been shown that the linker used to covalently attach the chromophore to the protein plays an active role in its relaxation (Figure 6b) (Delor et al., 2018). In particular, rigid linkers retard chromophore energy losses by decreasing the IC rate. It has also been observed that the protein environment stabilized the chromophore's triplet state (López-Peña et al., 2022). However, there are no mechanistic studies on the effect of amino acids on the ISC kinetics and yield based on engineered bio-hybrids. Based on controlled modifications of the LmrR protein pocket, the crucial role of tryptophan in altering the photostability of chromophores has been demonstrated (Mejías et al., 2020). A custom setup was developed to monitor absorption changes during direct irradiation. By applying arbitrary fitting models to the absorption changes over time, researchers quantified the photodegradation rates of various chromophores in the presence and absence of tryptophan. This approach revealed that tryptophan exhibits distinct photostabilization mechanisms depending on the chemical and redox properties of the chromophores. For instance, with riboflavin, photostabilization is associated with quenching of the sample's emission, indicating rapid electron transfer as the primary stabilization mechanism. Conversely, for rose Bengal, the emission is enhanced in the LmrR pocket, with tryptophan acting as a photoprotector only in the absence of oxygen, suggesting that tryptophan alters the chromophore's electronic states. Photostabilized bio-hybrids have been used to develop fluorescent probes for cell imaging (Iyer et al., 2021) and as emissive components in light emitting devices (LEDs) (Ferrara et al., 2022), showing the relevance of this study.
The structural complexity of the scaffolds used in the studies described above constrains the potential of the engineered bio-hybrids for detailed mechanistic studies. Nevertheless, general conclusions can be drawn: (1) the amino acid composition of the protein pocket modifies the chromophore's photophysical and photochemical properties in a complex manner and (2) the pocket structure plays a vital role in the steric interactions that modify the vibration and conformational freedom of the chromophores, as well as their electronic properties. These factors must be considered when designing bio-hybrids.
4.1.2 Effect of chromophore–chromophore coupling in energy transfer mechanisms and charge transfer state formation
The role of non-adjacent chromophores, static disorder, and chromophore packing in energy transfer have been studied based on multi-chromophore systems (Figure 6c). First, TMV was functionalized with different donor-to-acceptor chromophore ratios (Miller et al., 2007). On the basis of this design, non-adjacent donors actively participate in energy transfer by increasing the FRET efficiency expected for a single event at a certain distance. A similar effect was observed for the intermolecular energy transfer when non-identical light-harvesting complexes were labeled with FRET chromophore pairs (Bischoff et al., 2023) or in designs combing natural and synthetic light-harvesting proteins (Mancini et al., 2017). In this last work, the preconditions for directing the energy transfer have been identified by changing the combinations of used chromophores. To demonstrate the role of the static disorder on long-range energy transfer, transient absorption anisotropy was used (Hamerlynck et al., 2022). Using a multifunctionalized TMV, polarization relaxation was found to follow a stretched behavior due to poor coupling between disordered chromophores. The functional crosstalk of the cofactors was also studied in a large heme porphyrin array developed in TMV, with similar results (Dai et al., 2021). Finally, using a designed de novo protein to control the number of chromophores per protein, the role of the chromophore packing at the chromophore exciton coupling has been highlighted (Kodali et al., 2017). The present work shows that high chromophore density favors low-energy exciton mixing with charge transfer states that could favor charge transfer after light harvesting.
Overall, these studies emphasize the importance of the chromophore's spatial arrangement in bio-hybrids, especially when constructing multichromophore systems. Static disorder due to linkers or protein structure flexibility can lead to inadequate coupling between chromophores, thereby adversely affecting energy transfer efficiency. Thus, using rigid structures is beneficial for developing antenna systems. Moreover, a high density of chromophores is necessary to observe significant effects of exciton mixing and charge transfer states. While the studies provide valuable information on energy transfer mechanisms, practical application in real devices may require additional optimization and context-specific considerations.
4.2 Redox properties, charge separation, and long-range electron transfer mechanisms
Natural reaction centers attain unidirectional, light-activated charge separation in chains of redox cofactors (Allgöwer et al., 2022; Di Luca et al., 2017; Kaila, 2018; Saura et al., 2022). An active effort has been made to mimic photosynthetic reaction centers and study the fundamental principles underlying their efficiency (Figure 7). Below, the designs used to study the protein effects in the redox potential of cofactors, in the charge separation lifetime, and in the intermolecular electron transfer kinetics are reviewed.
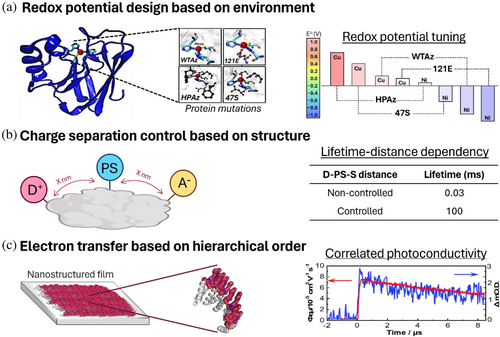
4.2.1 Redox cofactors and photoinduced redox reactions
Proteins with metal binding sites have been designed to study the role of amino acids in metal redox states (Figure 7a) (Hosseinzadeh & Lu, 2016). For example, systematically changing the metal ligands in a rubredoxin scaffold (PBD ID: 4AZU) has shown the relevance of metal coordination geometry and electron density in the metal redox potential (Hosseinzadeh et al., 2016). It was also found that hydrophobicity has a role in the metal redox properties (Hosseinzadeh et al., 2016). Finally, by labeling rubredoxin with a photosensitizer, it has also been shown that the charge transfer rate depends not only on the reaction driving force but also on the distance and relative orientation between the metal and the chromophore (Marguet et al., 2019).
Redox-active amino acids such as tyrosine or tryptophan have also been used to study the role of PCET in photosystems (Tyburski et al., 2021). In this sense, the PCET mechanism has been shown to vary with the pH value, from a stepwise to a concerted PCET reaction when going from a high pH to a low pH, respectively (Glover et al., 2014; Nilsen-Moe et al., 2020) being different for the tyrosine and tryptophan due to their differences in pKa value and oxidizing potential (Glover et al., 2018). It has been hypothesized that protein conformation changes the redox reactivity of the amino acids (Bialas et al., 2019; Nilsen-Moe et al., 2020). In particular, water channels open in the protein due to conformational fluctuations, allowing water to enter the protein pocket to act as the primary proton acceptor in the PCET reaction. In another work, it has been shown that changes in amino acid redox states alter protein conformation by modifying amide hydrogen bonding (Pagba & Barry, 2012). The author shows that PCET reactions in proteins change their IR spectrum, which is attributed to changes in their folding state. Finally, the involvement of tyrosine in the rapid transfer of a hole to a metallic center has been demonstrated by a 20-fold increase in the apparent electron rate when tyrosine is present (Tebo et al., 2021).
Previous works highlight the complexity of designing bio-hybrids for redox reactions, emphasizing the need to consider protein composition and structural dynamics across all redox states. Several key factors must be considered when designing bio-hybrids for redox reactions: (1) hydrophobicity of the protein pocket; (2) electron density of the amino acids; (3) relative distances and orientations between redox cofactors; and (4) flexibility of the protein structure. These factors collectively influence the rate, efficiency, and direction of charge transfer reactions within protein pockets and, thus, must be meticulously designed and optimized. The cooperative effects between these factors are also not fully understood, adding to the complexity of designing bio-hybrids for charge transfer purposes.
4.2.2 Charge separation in multi-chromophore systems
The multi-chromophore systems of electron donor and acceptor molecules combined with engineered proteins have been used to study how inter-factor distances, orientation, redox reaction driving forces, and protein structure affect charge separation rates and efficiency (Figure 7b). One of the first examples that reported a charge separation in such systems was based on streptavidin protein (Keller et al., 2017) (PDB ID: 3P). The main achievement of this work was the protein's multifunctionalization by combining a covalently linked chromophore and donor with a supramolecular assembled acceptor. This multifunctionalization allows the control of the molecule localization in the protein. By linking the molecules at different positions, it was shown that the distances between the elements affected the charge separation lifetime. The best triad showed a 3.1 μs charge separation lifetime. However, the charge separation quantum yield was low due to limited structural control of the bio-hybrid assembly, which does not consider the dynamic flexibility of the protein scaffold.
The lack of controlled photosystem designs for charge separation has been overcome by designing simplified reaction centers based on de novo proteins. A platform based on a de novo helical bundle has been designed with histidines that bind heme molecules with high affinity (Hutchins et al., 2023) (PDB ID: 7AH0). The heme position in the protein pocket was controlled by adjusting the histidine position. On the basis of this design, electronic coupling between hemes has been shown to induce a positive drift in the redox potential of the maquette. To selectively modify the heme redox properties inside the protein, the pocket hydrophobicity was modified (Solomon et al., 2022). In another attempt to design unidirectional reaction centers, de novo proteins have been multifunctionalized with metal cofactor, tetrapyrrole, and heme as electron donors, photosensitizers, and electron acceptors, respectively (Ennist, Stayrook, et al., 2022; Ennist, Zhao, et al., 2022) (PDB ID: 5VJS). In the metal site, tyrosine has been incorporated to mimic the metal-tyrosine oxidation in photosystem II. With this design, a charge separation with a lifetime of 100 ms was achieved. These works show a structural control for the development of biomimetic reaction centers, but strategies to design controlled interactions between the protein and chromophores are still needed for a deeper understanding of the natural charge transfer mechanisms.
4.2.3 Intermolecular electron transfer
A key challenge in designing bio-hybrid photosystems for long-range electron transfer is to obtain a photoactive components organization that maintains molecular coupling (Figure 7c) (Romera et al., 2015). To overcome this challenge, a bottom-up approach has been proposed where, first, the photoactive molecules are organized at the nanoscale in a protein template, and then the nanostructure is scaled up according to the protein self-assembly (Romera et al., 2015). This bottom-up approach has been used to assemble solid films with anisotropic long-range electron transfer (Mejías et al., 2016), electron donor-acceptor systems with optimized electron coupling (López-Andarias et al., 2018), and 3D crystalline structures with enhanced photoconductivity (Liutkus et al., 2020) by combining porphyrins, single-walled carbon nanotubes (SWCNT) and C60 fullerenes, respectively, with designed consensus tetratricopeptide repeat proteins. These studies demonstrate that photoinduced long-range electron transfer relies on three key factors: (1) the redox properties of the cofactors; (2) the distance between them; and (3) the strength of their coupling. Photoactive molecules can serve as bridging agents between protein units, which is relevant for designing functional materials. Further investigation into essential properties, such as long-term stability, is still required to use the bio-hybrids in practical applications.
4.3 Protein dynamics and their role in light-harvesting
The role of protein motions in the efficiency of light energy harvesting in photosystems has been a subject of discussion for the last few years (Allgöwer et al., 2022; Elias et al., 2023; Liguori et al., 2020; Manna & Schlau-Cohen, 2022; Orozco, 2014; Zhang et al., 2014). For example, the coupling of specific protein movements to chromophore electronic states has been suggested to be a general strategy used by nature to selectively drive electronic processes and provide directionality (Romero et al., 2017). On the basis of this model, the energy transfer funnel within the antenna complex could form transiently using thermal fluctuations (~kbT) to overcome energy transfer barriers (Reiter et al., 2023). Photoprotection also relies on protein conformational changes that adjust in response to changing light conditions to reduce their susceptibility to photodamage (Belgio et al., 2014; Malý et al., 2016; Manna & Schlau-Cohen, 2022; Takahashi & Badger, 2011; Valkunas et al., 2012; Zubik et al., 2020). In this case, it has been proposed that the coupling of a fast protein dynamic to a specific slow-motion coordinate could be the basis of regulatory switching (Valkunas et al., 2012). Most of these conclusions are based on calculations performed in computational studies. The lack of strategies to engineer bio-hybrids with dynamic control at different timescales has prevented experimentally studying these effects. Designing bio-hybrids suitable for these studies could allow the understanding of the mechanistic role of protein motions in light-harvesting and heat dissipation dynamics to harness their full power. Owing to the complexity of engineering bio-hybrids for these types of studies, a knowledge gap in this direction persists in this field.
5 IMPLICATIONS, REMAINING CHALLENGES, AND FUTURE PERSPECTIVES
This review presents the potential of engineered bio-hybrids to provide deep insights into the light conversion mechanisms in photosynthesis. The key challenge is to design a simplified photosystem suitable to answer specific questions on the photosystem's functioning. The main focus in this field has been on creating light-harvesting complexes and reaction centers using proteins as structural units to control the distances and orientation between chromophores. On the basis of this simplification, the field has shown that the protein structure plays a fundamental role in processes such as energy and charge transfer and the crucial role of chromophore–chromophore coupling based on a reduced disorder in photosystems. These effects were predicted from the functioning of natural photosystems, but the capacity to directly measure them in a simplified manner has allowed us to obtain key information on efficiency-enhancing factors and rate-limiting steps.
Besides the progress, there are still open questions in photosynthesis functioning, including how the dielectric environment, charged networks, or aromatic interactions of proteins affect the chromophores transitions. In addition, there is an increased interest in understanding the coupling mechanisms of protein dynamics to chromophore relaxation kinetics. Based on simplified photosystems, it is possible to reveal unexpected mechanisms that are hindered by the complexity of natural photosystems. However, a lack of control in predicting the final confirmation of the bio-hybrids limits their use to address these complex questions. New strategies for developing bio-hybrid photosystems with designed conformational changes is an evident research direction that the field must cover. Another limitation in the field is the lack of fundamental theories that gather the main conclusions of existing studies and principles for design strategies. This theory development requires parametrizing protein–chromophore interactions to develop algorithms that describe the action mechanisms of amino acids.
Obtaining general conclusions on designing customized photosystems could substantially enhance both our understanding of photosynthesis and the efficiency of human-made solar-energy conversion systems (optoelectronic or photonic devices). The use of proteins in specific applications promises to replace unsustainable elements in devices, making them more sustainable or generating clean fuels more efficiently. So far, the potential of these advancements has been demonstrated with natural fluorescence proteins and photosystems. For instance, fluorescence proteins have been used to create more sustainable LEDs (Fernández-Luna et al., 2018; Gutiérrez-Armayor et al., 2024; Patrian, Nieddu, et al., 2023) and for lasing applications (Sanchez-deAlcazar et al., 2019) by integrating them with polymers and other matrices. Additionally, natural photosystems (e.g., Photosystems I and II) have been combined with electrodes for solar cell applications, addressing self-quenching challenges typically encountered in solar cells (Cai et al., 2015; Mersch et al., 2015; Wang et al., 2014) or generating photoelectrochemical cells that produce H2 and O2 by encapsulating them in negatively charged phospholipid bilayers (Cai et al., 2015; Niroomand et al., 2018; Wang et al., 2014). These devices leverage the photoluminescence features of proteins, such as high emission efficiency with a narrow spectrum, high photon-flux saturation, and exceptional photostability to enhance device performance. Additionally, proteins offer sustainable production through bacterial synthesis and eco-friendly recycling. However, a significant limitation is the low stability of proteins under device configuration and operational conditions. Several strategies have been proposed to address this issue: (1) genetically modifying proteins to promote protein–protein solid interactions, creating protein macro-oligomers that increase LED stability from a few minutes to up to 100 h (Patrian, Nieddu, et al., 2023), (2) changing the coating strategy to generate mesoporous nanoparticles using silica as a precursor to enhance stability up to 15-fold, (Cai et al., 2015; Niroomand et al., 2018; Wang et al., 2014) or (3) adding additives to materials to enhance electron shuttling (Cai et al., 2015; Niroomand et al., 2018; Wang et al., 2014).
Replacing natural proteins with engineered bio-hybrids, as discussed in this review, has the potential to overcome the challenges described above and significantly improve device performance and stability. By customizing the protein sequence, these bio-hybrids can be engineered to have higher melting temperatures and more stable protein folding, making them suitable for harsh operating conditions. Additionally, the choice of chromophores can be optimized to enhance the absorption cross-section, and the protein environment can be controlled to improve the chromophores photostability and emission yield. The flexibility in selecting chromophores and modifying the protein scaffold opens up possibilities for applications in quantum computing and other photonic technologies. This can be achieved using protein environments to control chromophore quantum states or by employing synthetic chromophores with non-linear properties. Finally, bio-hybrids can serve as ideal models for advancing spectroscopic techniques such as attosecond measurements and time-resolved X-ray spectroscopy and for developing data analysis protocols due to the capability of fine-tuning them in a controlled way. Harnessing the full potential of these innovative materials will necessitate a multidisciplinary collaboration among chemists, biologists, physicists, and engineers, ultimately revolutionizing the paradigm of photoactive devices based on these newly developed bio-hybrids.
AUTHOR CONTRIBUTIONS
Gonzalo Pérez Serrano: Writing – review and editing; investigation. Claudia F. Echavarría: Investigation; writing – review and editing. Sara H. Mejias: Conceptualization; writing – original draft; writing – review and editing; funding acquisition; supervision; investigation; validation.
ACKNOWLEDGMENTS
S.H.M. acknowledges the funding from the Spanish Ministry of Science, Innovation and Universities (project TED2021-131906A-I00) and “La Caixa” Foundation for her fellowship (ID 100010434) that allowed the writing of this review. The writing process was also supported by a 2023 Leonardo Grant for Researchers and Cultural Creators from the BBVA Foundation (LEO23-2-9635). The BBVA Foundation accepts no responsibility for the opinions, statements, and contents included in this article and/or the results thereof, which are entirely the responsibility of the authors. Some figures in the review were Created with BioRender.com.