DrugDomain: The evolutionary context of drugs and small molecules bound to domains
Review Editor: Nir Ben-Tal
Abstract
Interactions between proteins and small organic compounds play a crucial role in regulating protein functions. These interactions can modulate various aspects of protein behavior, including enzymatic activity, signaling cascades, and structural stability. By binding to specific sites on proteins, small organic compounds can induce conformational changes, alter protein–protein interactions, or directly affect catalytic activity. Therefore, many drugs available on the market today are small molecules (72% of all approved drugs in the last 5 years). Proteins are composed of one or more domains: evolutionary units that convey function or fitness either singly or in concert with others. Understanding which domain(s) of the target protein binds to a drug can lead to additional opportunities for discovering novel targets. The evolutionary classification of protein domains (ECOD) classifies domains into an evolutionary hierarchy that focuses on distant homology. Previously, no structure-based protein domain classification existed that included information about both the interaction between small molecules or drugs and the structural domains of a target protein. This data is especially important for multidomain proteins and large complexes. Here, we present the DrugDomain database that reports the interaction between ECOD of human target proteins and DrugBank molecules and drugs. The pilot version of DrugDomain describes the interaction of 5160 DrugBank molecules associated with 2573 human proteins. It describes domains for all experimentally determined structures of these proteins and incorporates AlphaFold models when such structures are unavailable. The DrugDomain database is available online: http://prodata.swmed.edu/DrugDomain/.
1 INTRODUCTION
Proteins are vital components of cells, playing essential roles in regulating a myriad of cellular processes. The three-dimensional (3D) structure of a protein provides valuable information about protein interactions and functions. Protein domains are functionally, evolutionarily, and structurally distinct units that ensure evolutionary viability by fulfilling specific functions (Buljan & Bateman, 2009; Grishin, 2001). The identification and classification of protein domains into a hierarchy of evolutionary relations can lead to a better understanding of protein function by analyzing the known functions of their homologous relatives. Until recently, major structure-based classifications of protein domains have principally focused on the classification of experimentally determined protein structures: SCOP (Andreeva et al., 2020), and CATH (Sillitoe et al., 2021). Our team has developed and maintains the Evolutionary Classification Of protein Domains (ECOD), which primarily groups domains based on homology rather than topology (Cheng et al., 2014; Schaeffer et al., 2019). This feature aids in detecting instances of homology between domains with differing topologies. Another significant aspect of ECOD is its focus on distant homology, resulting in a comprehensive repository of evolutionary connections among categorized domains.
Artificial intelligence provides potent tools for scientific research across various fields, and structural computational biology is no exception. AlphaFold (AF), a recently developed deep learning method, demonstrated the capability to predict protein structure with atomic-level accuracy and has thus become an indispensable tool in structural biology (Jumper et al., 2021). Utilizing AF models, ECOD became one of the first databases to incorporate domain classification both for the entire human proteome (Schaeffer et al., 2023) and the whole proteomes of 48 model organisms (Schaeffer et al., 2024). AF has significantly expanded the available tools for computational structural biology related to drug discovery, target prediction, protein–protein, and protein–ligand interaction, and prediction of complex structures (Akdel et al., 2022; Medvedev, Schaeffer, Chen, et al., 2023; Yang et al., 2023).
Many proteins convey their functions through interaction with small organic molecules. These interactions play crucial roles in various biological processes, including enzymatic reactions, signal transduction, and regulation of gene expression. Today the majority of FDA-approved drugs in the market and their generics are small molecules (Makurvet, 2021). In the last 5 years, small molecules accounted for 72% of all FDA-approved drugs (178 out of a total of 247 drugs) (de la Torre & Albericio, 2024). Each drug has one or more protein targets with an affinity defined through experimental methods. However, many proteins are multidomain and it is commonly unknown to which domain a drug binds in most cases. Especially in the cases where the target is a large receptor. Some multidomain proteins exhibit multidomain binding sites (Kruger et al., 2012). For example, human prostaglandin D-synthase (PDB: 3EE2) binds Nocodazole (DB08313) through both domains: Thioredoxin-like (ECOD: e3ee2A2) and Repetitive alpha hairpins (ECOD: e3ee2A1) (Weber et al., 2010). Human topoisomerase II beta (PDB: 3QX3) binds anticancer drug Etoposide (DB00773) using two out of four classified domains: helix-turn-helix (ECOD: 3qx3A1) and HAD domain-like (ECOD: e3qx3A11) (Wu et al., 2011). Homologous proteins exhibit highly similar active sites, capable of accommodating similar chemical compounds (Medvedev et al., 2019, 2021). Understanding the locations and principles that govern multi-domain drug binding sites may help us to better identify them in homologous proteins and complexes made up of homologous proteins. Chemical compound repositories such as PubChem (Kim et al., 2023) and DrugBank (Wishart et al., 2018) contain information about domains of the target protein derived from Pfam (Mistry et al., 2021), which is a sequence-based classification. However, no indications are provided about to which domain(s) the compound interacts. Previously several resources were created that described ligand domain mapping using CATH and SCOP (structure-based) domain classification (Bashton et al., 2008; Bashton & Thornton, 2010; Chalk et al., 2004) and Pfam database (sequence-based) (Mistry et al., 2021) classification (Kruger et al., 2012). However, these resources are out-of-date and presently unavailable for usage by the scientific community. Currently, no structure-based protein domain classification includes this type of data. Databases that offer extensive protein–ligand interaction data, including BindingDB (Gilson et al., 2016) and BioLiP2 (Zhang et al., 2024), still lack domain-level annotations for target proteins.
Here, we have developed the DrugDomain database, which reports those ECOD domains of proteins targeted by small molecules and drugs from DrugBank. The current version not only encompasses experimentally defined protein structures but also incorporates AF models in cases where such structures are unavailable. DrugDomain is available online at: http://prodata.swmed.edu/DrugDomain/.
2 DrugDomain DATABASE FEATURES AND STATISTICS
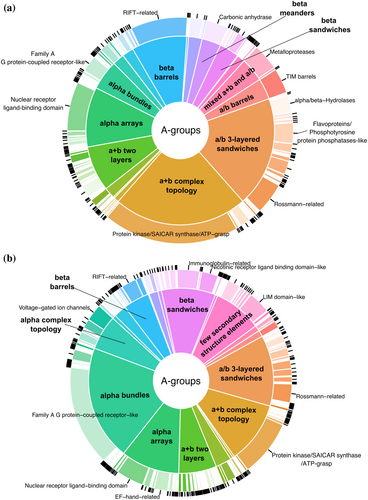
We developed the DrugDomain database (http://prodata.swmed.edu/DrugDomain/) with web interfaces that display two types of database hierarchy: protein and molecule-centric. DrugDomain catalogs ECOD domains whose residues are located within 5 Å of the DrugBank molecule's atoms. The distribution of DrugBank molecules interacting with ECOD homologous groups and architectures is shown in Figure 1. For the target proteins whose 3D structure was experimentally determined with any DrugBank molecule, the top three ECOD A-groups of the interacting domains include a + b complex topology, a/b three-layered sandwiches, and alpha arrays (Figure 1a). The majority of small molecules interacting with a + b complex topology domains are associated with protein kinases, which are one of the most druggable protein domains and is the domain most commonly encoded among genes associated with cancer (Anderson et al., 2023; Medvedev, Schaeffer, Pei, et al., 2023; Wang et al., 2020). The a/b three-layered sandwiches are mostly represented by Rossmann-like proteins, which were shown to bind the majority of organic molecules superclasses (Medvedev et al., 2019, 2021). For the proteins lacking their interacting DrugBank molecule in any experimental structure classified by ECOD, we predicted interacting ECOD domains using the AlphaFill algorithm (Hekkelman et al., 2023) (see below for details). In this case, the three most populated ECOD A-groups containing modeled interacting DrugBank molecules include (1) the alpha bundles with G protein-coupled receptors (GPCRs), one of the most druggable protein domains together with kinases (Wang et al., 2020), followed by (2) the a/b three-layered sandwiches and (3) a + b complex topology (Figure 1b).
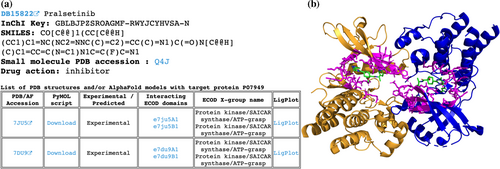
The protein-centric hierarchy begins with the alphabetically sorted list of UniProt accessions (http://prodata.swmed.edu/DrugDomain/proteins/). Each accession leads to the dedicated webpage, which lists the UniProt accession, UniProt entry name, gene name, and protein name followed by the list of DrugBank molecules and drugs that target this protein. The list of molecules includes links to DrugDomain data webpages, DrugBank accession, molecule name, drug action data from DrugBank, affinity data from BindingDB, InChI Key, and SMILES formula. The molecule-centric hierarchy starts with the list of DrugBank accessions (http://prodata.swmed.edu/DrugDomain/molecules/). Each accession leads to the dedicated webpage, which lists the DrugBank accession, molecule name, InChI Key, SMILES formula, and small molecule PDB accession followed by the list of human proteins that are targets for this molecule. The list of proteins includes links to DrugDomain data webpages, UniProt accession, drug action data from DrugBank, protein names, and affinity data from BindingDB. The last level of our database hierarchy (DrugDomain data webpages) contains DrugBank accession, molecule name, InChI Key, SMILES formula, small molecule PDB accession, and drug action data from DrugBank followed by the table of PDB structures and/or AF models with target particular protein. The table contains different information depending on the availability of experimentally determined 3D protein structures together with a particular DrugBank molecule. Such structures are available at RCSB Protein Data Bank for 2149 molecules interacting with 783 target proteins, encompassing 5702 PDB structures. For these cases, we identify residues whose atoms are located within 5 Å of the DrugBank molecule's atoms and map these residues to existing ECOD domains. In such instances DrugDomain data webpage's table includes PDB accession linked to RCSB, downloadable PyMOL script, an indication that this interaction between molecule and protein target was confirmed experimentally, a list of ECOD domains interacting with the molecule with links to the ECOD database, names of corresponded ECOD X-groups (possible homology level) and 2D diagrams of ligand–protein interactions (LigPlots) generated using LigPlot+ (Laskowski & Swindells, 2011) (Figure 2a). The PyMOL script downloads the PDB structure from RCSB, colors ECOD domains by different colors, colors residues interacting with the molecule in magenta and sets their representation as sticks (Figure 2b). We specified links to DrugDomain data webpages for corresponding domains in the ECOD database (e.g., http://prodata.swmed.edu/ecod/complete/domain/e7du9A1). If the small molecule does not interact with a particular protein, which is listed as its target, within a PDB structure (e.g., when the PDB structure includes more than one protein/UniProt ID), the AF model is shown instead of the PDB structure.
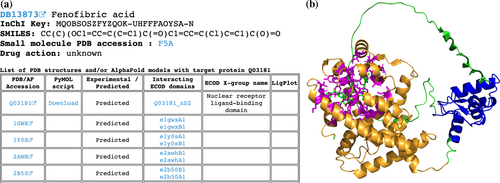
The subset of DrugBank molecules that are not present in experimentally defined PDB structures includes 3480 molecules targeting 2361 human proteins. Among those proteins, experimentally defined PDB structures exist for 1776 (75%) but without the DrugBank molecules of interest; only AF models exist for 573 (24%) proteins and there are no structural data for 12 (<1%) proteins (e.g., such huge proteins as E3 ubiquitin-protein ligase UBR4 (Q5T4S7), which contains 5183 amino acids). In these cases, we applied the AlphaFill algorithm (Hekkelman et al., 2023) that uses sequence and structure similarity to retrieve small molecules and ions from experimentally determined structures to predicted protein models by AF. Using AlphaFill models we identify residues whose atoms are located within 5 Å of the DrugBank molecule's atoms of interest (if present) and map these residues to ECOD domains identified for the whole human proteome using AF models (Schaeffer et al., 2023, 2024). If the molecule of interest is not present in the AF model, all other present ligands are considered as such. The DrugDomain data webpage's table in these cases includes AF accession, downloadable PyMOL script, an indication that this interaction between molecule and protein target was not confirmed experimentally (and was predicted), a list of ECOD domains interacting with the molecule with links to the AF-based ECOD database and names of corresponded ECOD X-groups. If experimentally defined PDB structures exist for a particular protein, the table also lists them with domains from the main ECOD database (with experimental structures), which correspond to domains from the AF model. In these instances, PyMOL script is not provided due to the absence of the molecule of interest in the PDB structure (Figure 3a). The PyMOL script includes the AF model, colors it by ECOD domains, colors residues interacting with the molecule in magenta and sets their representation as sticks (Figure 3b). Full information about ECOD domains and residues interacting with DrugBank molecules are also available for download in a plain text format.
3 COLLECTION OF DrugBank MOLECULES DATASET
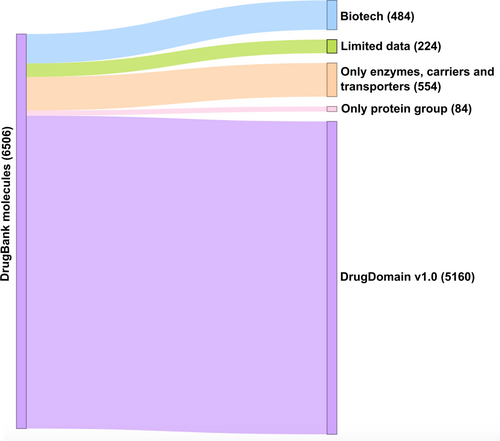
To obtain all drugs and small molecules that target human proteins we retrieved all DrugBank accessions (Wishart et al., 2018) related to proteins from the reference human proteome (UP000005640) using UniProt KB (UniProt, 2019). Overall, using this approach we obtained 6506 DrugBank molecules (approximately 30% of DrugBank) that are associated with 3193 human proteins. The DrugDomain database focuses on small molecules, so we have excluded 484 DrugBank entities representing biologics (“biotech” type of molecules in DrugBank). Additionally, 224 molecules were removed from the dataset due to incomplete DrugBank records, for example, birch bark extract (DrugBank accession: DB16536) and KW-6356 (DB17080). Each molecule can have multiple targets, enzymes, transporters, and carriers associated with it in DrugBank. The purpose of this study is to catalog the interaction between DrugBank small molecules and the structural domain(s) of their target protein. Moreover, as we focus on targets that are human proteins, some molecules do not have targets in our dataset. For example, antibiotics that target bacterial proteins are excluded from the DrugDomain database (Figure 4). Some molecules include protein groups as targets, which are often represented by various receptors. For example, Amoxapine (DB00543) targets, among others, the GABA receptor, which consists of 16 subunits, each of which is a different protein. In the majority of cases, drug targets include a particular receptor subunit known to bind the drug. However, in some cases, only protein groups are present as drug targets (e.g., for Mephentermine (DB01365)) and we removed such molecules from our current dataset. Thus, the remaining set, which was used for the development of the DrugDomain database version 1.0, includes 5160 DrugBank molecules associated with 2573 human proteins (Figure 4).
4 FUTURE PERSPECTIVES
The DrugDomain database version 1.0 represents the initial step of its development. We plan to significantly expand the range of represented molecules and include all DrugBank entities in our database. Additionally, we are going to incorporate not only the protein targets of molecules but also enzymes, transporters, and carriers associated with these molecules. Considering the conservation rate of residue positions will enhance the accuracy of predicting amino acids that interact with small molecules. Finally, based on ECOD evolutionary classification and homologous evidence between proteins we anticipate to suggest new potential targets for known drugs. By focusing on evolutionarily conserved domains, we can prioritize targets that are likely to be functionally essential. Homologous proteins exhibit highly similar active sites, capable of accommodating similar chemical compounds. Better understanding along these lines opens up opportunities for discovering novel targets.
AUTHOR CONTRIBUTIONS
Kirill E. Medvedev: Conceptualization; methodology; software; investigation; validation; formal analysis; data curation; visualization; writing – original draft; project administration. R. Dustin Schaeffer: Software; writing – review and editing; funding acquisition. Nick V. Grishin: Conceptualization; resources; funding acquisition; supervision; writing – review and editing.
FUNDING INFORMATION
The study is supported by grants from the National Institute of General Medical Sciences GM127390 (to N.V.G.), GM147367 (to R.D.S.), the Welch Foundation I-1505 (to N.V.G.), the National Science Foundation DBI 2224128 (to N.V.G.).
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no competing interests associated with the manuscript.