Miniprotein engineering for inhibition of PD-1/PD-L1 interaction
Review Editor: Aitziber L. Cortajarena
Abstract
Miniproteins constitute an excellent basis for the development of structurally demanding functional molecules. The engrailed homeodomain, a three-helix-containing miniprotein, was applied as a scaffold for constructing programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) interaction inhibitors. PD-L1 binders were initially designed using the computer-aided approach and subsequently optimized iteratively. The conformational stability was assessed for each obtained miniprotein using circular dichroism spectroscopy, indicating that numerous mutations could be introduced. The formation of a sizable hydrophobic surface at the inhibitor that fits the molecular target imposed the necessity for the incorporation of additional charged amino acid residues to retain its appropriate solubility. Finally, the miniprotein effectively binding to PD-L1 (KD = 51.4 nM) that inhibits PD-1/PD-L1 interaction in cell-based studies with EC50 = 3.9 μM, was discovered.
1 INTRODUCTION
Miniproteins, which combine features of peptides and proteins, have recently emerged as molecules of high interest (Baker et al., 2017; Ożga and Berlicki, 2022a). Due to the feasibility of solid-phase synthesis, complete control over the amino acid sequence and incorporation of any building block is possible (Drewniak-Świtalska et al., 2021; Imperiali and Ottesen, 1998, 1999). Moreover, the relatively extensive structure of miniproteins allows the creation of molecules with advanced functions. Miniprotein folding can be driven by the formation of a hydrophobic core, metal binding, or cysteine disulfides (Huang et al., 2016; Porter Goff et al., 2019; Zhu et al., 2003). Miniproteins occur in nature, can be engineered domains of native proteins, or are available as created artificially using an in silico approach (Gates et al., 2018; Korendovych and DeGrado, 2020; Rocklin et al., 2017). Although the high number of nature-derived miniproteins is described, their structural diversity was significantly enlarged by de novo designed molecules, including those containing non-canonical secondary structures (Bejger et al., 2021).
Miniproteins were proved to be excellent scaffolds for developing functional structures exhibiting biological and catalytic activities (Camarero, 2019; Ożga and Berlicki, 2022b). Biological activity can be achieved by modulation of the native activity of miniproteins, grafting epitopes on their surfaces, or de novo design (Ciesiołkiewicz et al., 2022; Crook et al., 2020). A wide range of activities has been reported using miniproteins, including anticancer (Bryan et al., 2021), immunosuppressive (Gründemann et al., 2019; Wang et al., 2014), analgesic (Romero et al., 2017; Wallace, 2006), and antiviral (Cao et al., 2020). In most cases, its molecular mode of action is based on binding to specific receptors or inhibition of protein–protein interactions, and usually, a large area of interaction with the target can be reached (Crook et al., 2020; Valeur et al., 2017).
Programmed cell death protein 1 (PD-1) is a key molecule in regulating the immune system (Cheng et al., 2013; Ishida et al., 1992). PD-1 occurs on the surface of various immune cells, including T cells, and prevents excessive immune responses (Francisco et al., 2010). PD-1 interacts with its ligands, either PD-L1 or PD-L2, which may be present on the surface of cancer cells and antigen-presenting cells, leading to the silencing of the immune response (Akiyama et al., 2020; Andorsky et al., 2011). Therefore, programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) interaction is an immune checkpoint considered an excellent target for cancer immunotherapy (Iwai et al., 2017; Yang and Hu, 2019). Until now, all marketed drugs binding to PD-1 or PD-L1 are antibodies (Davis and Lewis, 2022; Gunturi and McDermott, 2015; Robert et al., 2014). However, inhibitors of this protein–protein interaction (PPI) of smaller size are also known (Zarganes-Tzitzikas et al., 2016). In particular, macrocyclic peptides (Guardiola et al., 2021; Magiera-Mularz et al., 2017; Patil et al., 2018; Rodriguez et al., 2023) and branched aromatic compounds (Guzik et al., 2017; Kamal et al., 2023; Lu et al., 2022; Skalniak et al., 2017; Wang et al., 2021) have been shown to exhibit such activity. Moreover, Craik, Wang, and co-workers described cysteine-rich miniprotein, MOPD-1, incorporating three disulfide bonds, which binds to PD-L1 (Yin et al., 2021).
Here, we evaluate the possibility of using the native miniprotein engrailed homeodomain (ENH) as a scaffold for constructing PD-L1 binders. ENH is a miniprotein built from three α-helices and shows high conformational stability (Clarke et al., 1994). The inhibitor structures were obtained by computationally designed modification of one side of EHN and validated experimentally. Moreover, further optimization of binding activity and physicochemical properties was performed.
2 RESULTS AND DISCUSSION
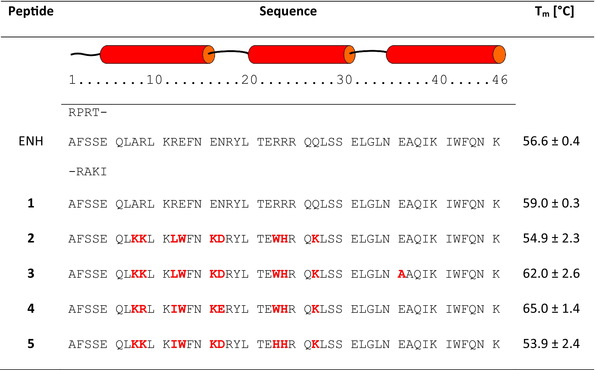
PD-1 and PD-L1 proteins interact via large, nearly flat surfaces of two four-stranded β-sheet structures of interacting partners (Figure S1 in Data S1) (Zak et al., 2015, 2017). A miniprotein scaffold suitable for the development of an effective binder to PD-L1 protein which will bock its interaction with PD-1, should combine the following features: (a) known three-dimensional structure, (b) high conformational stability, (c) sequence length not exceeding 55 amino acid residues to allow efficient solid phase synthesis, and (d) at least one flat surface. The ENH, composed of three α-helices, was chosen (Figure S2 in Data S1) (Clarke et al., 1994). Although this miniprotein does not contain β-sheet and thus could not directly mimic PD-L1 binding partner, namely, PD-1, it could be noticed that one of its sides, formed by two parallel helices, is nearly flat. Moreover, ENH has a well-packed hydrophobic core, which causes high conformational stability of this miniprotein with a Tm value of 56.6°C (Table 1).
|
- Note: Modified amino acid residues are shown in bold and colored red.
- Abbreviations: ENH, engrailed homeodomain; PD-1/PD-L1, programmed cell death protein 1/programmed death-ligand 1.
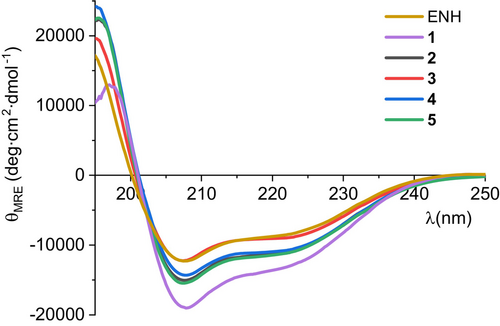
The analysis of the crystal structure of ENH miniprotein (PDB id 1ENH) (Clarke et al., 1994) revealed that neither C- nor N-terminal fragments contribute to forming a hydrophobic core. Thus, the sequence was shortened by four residues at each terminus. The miniprotein with a shorter sequence length, 1 (46 vs. 54 amino acid residues), was not only more feasibly obtained by solid phase peptide synthesis (SPPS) but also showed higher content of helical structures (47.8% vs. 37.3% as calculated by the DichroWeb server) (Miles et al., 2022) with the SELCON3 algorithm (Sreerama and Woody, 1993) on the basis of circular dichroism spectra (Figure 1), and higher melting temperature (59°C vs. 56.6°C, respectively, Table 1).
Initially, the ENH miniprotein was docked to the PD-L1 protein, assuming possible interactions by the flat side of ENH created by two antiparallel α-helices and PD-L1 surface natively interacting with PD-1. Subsequently, 10 positions of ENH were identified to be mutated to make the appropriate interface for PD-L1 binding.
The design of surfaces intended for interaction with PD-L1 was based on the construction of two fragments: hydrophobic and polar, similar to previously published macrocyclic inhibitors such as BMS-57, BMS-71 (Magiera-Mularz et al., 2017), and pAC65 (Rodriguez et al., 2023). Therefore, amino acid residues placed in the center of this surface should be lipophilic (positions 12, 13, 23, 24, and 31 of miniprotein 1), while those on borders should be hydrophilic (positions 8, 9, 16, 17, and 27 of 1). After the initial docking of miniprotein 1 to PD-L1, it was concluded that the amino acid residue side chain at position 23 of 1 is close to Tyr123 and Met115 of PD-L1, which beneficially bound the Trp side chain of macrocyclic peptides studied previously (Magiera-Mularz et al., 2017). The second hydrophobic region of PD-L1 consisted of Ile54, Tyr56, Gln66, Val68, and Met115 and was found to be close side chains of residues at positions 12, 13, and 31. The combination of Trp residue with lipophilic Val, Leu, Ile, or Phe was assumed at that region. The results of the above-discussed analysis were used as input for the FastDesign protocol from the Rosetta software suite.
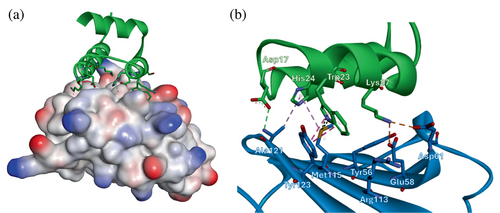
Among the results of these calculations, four sequences with top scores were chosen for experimental validation (miniproteins 2–5, Tables 1 and S1 in Data S1). The analysis of the model of the miniprotein 2/PD-L1 complex (Figure 2) revealed several intermolecular interactions that stabilize it. The side chain of Trp23 of miniprotein 2 creates π-π stacking with an aromatic fragment of Tyr123 of PD-L1, while the side chain of Trp13 is docked to the hydrophobic cavity formed by Ile54, Tyr56, and Met115. Lys8 and Lys9 of miniprotein 2 created charge-assisted hydrogen bonds with Glu71 and Asp73 of PD-L1, while Lys27 interacted with Glu58 and Asp61.
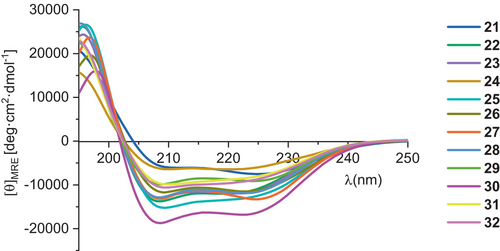
All designed peptides were synthesized using microwave-assisted automated solid phase synthesis with Fmoc chemistry and N,N′-diisopropylcarbodiimide (DIC)/Oxyma coupling reagents. Purified peptides were evaluated using circular dichroism (CD) spectroscopy for conformational stability. All miniproteins showed CD spectra similar to starting structure 1, indicating a high α-helices content (Figure 1).
Moreover, CD-monitored temperature dependences confirmed cooperative folding with estimated Tm values (53.9–65.0°C) similar to the parent compound 1 (Table 1 and Figure S3 in Data S1). Subsequently, the binding affinity measurements of studied miniproteins toward PD-L1 protein were performed using biolayer interferometry (BLI, Figures 3 and S9 in Data S1). Binding constants were obtained by evaluating the kinetics of miniprotein binding to the sensor loaded with PD-L1 (Figure 3b, time 0–300 s) and its dissociation (for sensor immersed in buffer solution, Figure 3b, time 300–750 s). The miniprotein/PD-L1 interaction can also be evaluated in an equilibrium state by dependence of equilibrated response (Req) on ligand concentration (example in Figure 3c). As expected, the starting scaffold showed no activity. The PD-L1 affinities of four designed inhibitors were significant and similar (KD in the range of 1.48–2.43 μM). These results confirmed the correctness of our design strategy as conformationally stable and active miniproteins were obtained. However, there was a space for optimizing these structures in two aspects, namely, activity and solubility. In the case of miniproteins 2–5, a significant loss of solubility in comparison to peptide 1 was observed. In particular, compounds 3 and 4 partially precipitated during thermal denaturation studies. At this stage of analysis, miniprotein 2 was chosen for further optimization.
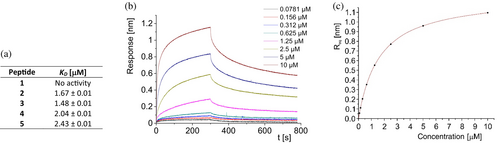
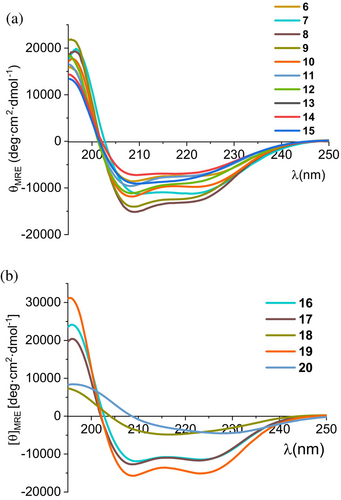
In the second round of optimization, 10 miniproteins with single mutations compared to sequence 2 were investigated (Table 2). In general, these changes were intended to increase specific interactions with the target and were proposed after careful manual inspection of miniprotein 2/PD-L1 complex. Mutations Lys9Trp, Lys16Leu, Asp17Leu, His24Trp, and Glu31Ile in peptides 6–10 aimed to increase hydrophobic interactions with the target residues Val68, Ile54, Ala121, Met115, and Tyr56, respectively. The incorporation of ε-carboxymethyl-tryptophan (sequence 11) was based on the structure of macrocyclic peptide published previously (Rodriguez et al., 2023). Subsequently, we designed miniproteins with nor-leucine residue incorporated at positions 12, 27, and 30 (peptides 15, 12, and 13), also by similarity to results published for macrocyclic peptides. The experimental evaluation of miniproteins 6–15 revealed that only four compounds fold. We added to the set of studied mutants miniproteins 16–20, which had two modifications.

|
- Note: Amino acid residues mutated in the first step are marked bold red, while those changed in this optimization are marked bold green; X and Y denote nor-leucine and ε-carboxymethyl-tryptophan, respectively. Data is unavailable due to the lack of a sigmoidal shape of the CD-monitored temperature denaturation curve.
- Abbreviations: CD, circular dichroism; PD-1/PD-L1, programmed cell death protein 1/programmed death-ligand 1.
Peptides 16 and 17 exhibited the highest Tm values for compounds studied in the second round of optimization. Except for peptides 18 and 20, all others showed CD spectra with minima characteristic of the α-helix (Figure 4), and similar to the spectrum of the starting oligomer—miniprotein 1.
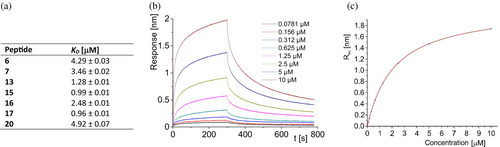
Using BLI, seven miniproteins that showed cooperative folding were evaluated for binding toward PD-L1 (Figures 5, S10, and S11 in Data S1). KD values measured for these compounds were in the low micromolar range. The combination of high conformational stability and high PD-L1 affinity was optimal for inhibitors 16 and 17. However, the measurements of their PD-1/PD-L1 interaction inhibition using the homogeneous time resolved fluorescence (HTRF) method showed 61% and 10% inhibition for 16 and 17, respectively, at an inhibitor concentration of 5 μM. Due to the higher inhibition of the PD-1/PD-L1 interaction, miniprotein 16 was chosen for the next optimization step.
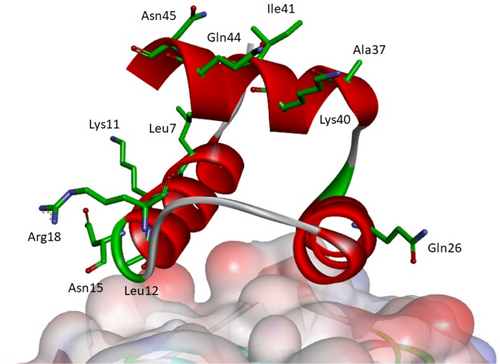
The subsequent optimization step aimed to increase the solubility of studied miniproteins by mutations of the side chains of surface amino acid residues that do not interact with PD-L1. Several positions of possible mutations were identified (Figure 6). The isoelectric point (pI) and the content of charged residues were considered (Tables 3 and S2 in Data S1). The first attempts were based on the assumption that the pI of the inhibitor should be similar to that of the PD-1 or the other published miniprotein inhibitor (MOPD-1). Thus, miniproteins 21, 22, and 24 were constructed with decreased pI. Secondly, we used CamSol software (Sormanni et al., 2015) to indicate mutations that should increase solubility, and sequence 23 was proposed with mutations of surface residues Leu12His and Ile41Glu (Sormanni et al., 2015). Peptides 25, 26, and 27 have adjusted the number of charged residues to miniproteins 1 or 2. Finally, sequences 28 and 29 have an increased number of charged residues (related to peptide 16) with high pI and sequence 30 with low pI.
|
- Note: Mutations of the first and second optimization steps are shown in bold and red or green, respectively. Mutations introduced for solubility enhancement are shown in bold colored blue. Data is unavailable due to the lack of a sigmoidal shape of the CD-monitored temperature denaturation curve.
- Abbreviations: CD, circular dichroism; PD-1/PD-L1, programmed cell death protein 1/programmed death-ligand 1.
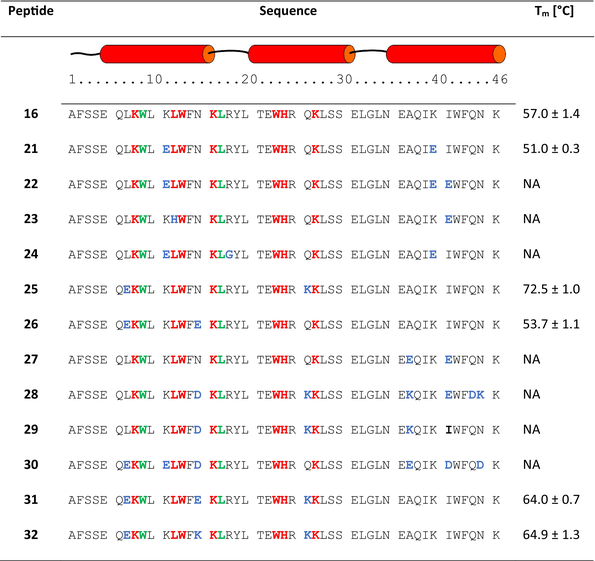
Although all peptides synthesized in this optimization step (21–30) showed CD spectra characteristic for α-helices (Figure 7), only three miniproteins (21, 25, and 26) were folding cooperatively according to CD-monitored thermal denaturation studies (Table 3 and Figures S6 and S7 in Data S1). Interestingly, all conformationally unstable miniproteins have modifications in the C-terminal helix. However, among stable miniproteins, an improved PD-L1 affinity was found (peptide 25, KD = 1.31 μM, Figure 8). Peptide 25 is also characterized by a high Tm value of 72.5°C and good solubility. Therefore, we decided to explore mutants of 25 further, creating sequences 31 and 32 (Table 3). Sequence 31 was obtained by keeping the pI value above 10, adding a Glu residue at position 15, and shifting Glu residues from position 6 to 7 as in sequence 26, which also retained a high Tm value. Sequence 32 includes the Glu15Lys mutation, which increased the pI value. Both newly designed peptides 31 and 32 showed CD spectra indicating high α-helix content (Figure 7) and cooperative folding characterized by Tm values of 64.0 and 64.9°C, respectively (Table 3 and Figure S8 in Data S1).
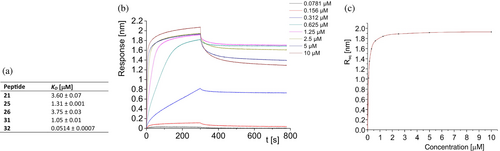
BLI measurements indicated that miniproteins 32 exhibit high affinity to PD-L1 with KD of 51.4 nM, respectively (Figures 8 and S11 in Data S1). Although the side chain of amino acid residue at position 15 cannot directly interact with PD-L1 according to our model, the difference at this position (Glu vs. Lys) causes a difference in the affinity of miniproteins 31 and 32 toward PD-L1 over one order of magnitude. It could be concluded that this change can affect the conformation of neighboring Lys14 or the overall charge of the miniprotein.
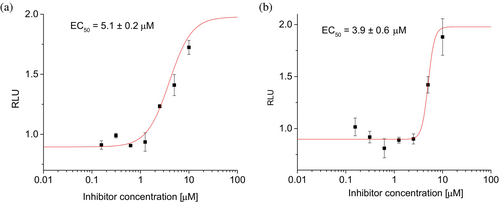
Due to the enhanced solubility of peptides from the last round of optimization, further experiments confirming their biological activity could be performed for most active miniproteins 25 and 32. The HTRF experiment performed using human PD-1 and PD-L1 proteins demonstrated the potential of studied molecules to inhibit this interaction with IC50 values of 18.5 and 29.2 μM, respectively (Figure S12 in Data S1). Subsequently, cell-based studies were performed for peptides 25 and 32 (Figure 9) using commercially available Promega PD-1/PD-L1 Blockade Bioassay. The bioluminescence resulting from the interaction of PD-1 and PD-L1 expressed on the two different cell types was decreased by adding an inhibitor. Both studied miniproteins showed significant activity in this assay with EC50 values of 5.1 and 3.9 μM, which confirmed their biological activity potential.
3 CONCLUSIONS
The use of miniproteins for constructing PPI inhibitors was found to be very effective; however, several factors have to be considered during the development process. Although miniproteins provide numerous points for modifications for building appropriate interfaces for interaction with the target molecules, these changes can affect studied molecules' conformational stability and solubility. Here, we presented a methodology that takes into account several miniprotein parameters. These include the control over binding affinity, conformational stability, and solubility. In the case of molecules interacting with PD-L1, the necessity of the construction of a relatively sizeable hydrophobic surface imposes the requirement of its compensation by the incorporation of additional charged residues to retain solubility. Interestingly, although introduced charged residues could not directly interact with the molecular target, the affinity to PD-L1 was also improved, which was attributed to either preorganization of neighboring residues that interact with PD-L1 or changing overall charge of miniproteins that affect nonspecific long-range Coulombic interaction. Taking into account all the above-mentioned parameters, we have developed highly active miniproteins in three rounds of optimization.
It is worth noting that several inhibitors of PD-l/PD-L1 interaction are known, and they can be grouped into two major classes, namely antibodies (Goldman et al., 2021) and small molecules (Koblish et al., 2022; Park et al., 2021; Sasikumar and Ramachandra, 2022). Both mentioned groups can show some drawbacks related to their synthesis and usage. The application of miniproteins provides an alternative way of developing PPI inhibitors with several beneficial features, including feasible synthesis, the possibility of incorporation of any functional group at any chosen position, and an expanded interaction surface. The molecule described here is characterized by its ability to inhibit the PD-1/PD-L1 interaction with an IC50 value similar to that of the published miniprotein inhibitor of the PD-1/PD-L1 interaction, MOPD-1, which is 3.3 μM (Yin et al., 2021). However, it should be noted that the tertiary structure of MOPD-1 is stabilized by covalent bonds, specifically by the presence of three disulfide bridges. In contrast, the structures of the inhibitors presented in this work are stabilized solely by a hydrophobic core.
In summary, the appropriate control over the proper construction of the interacting surface and distribution of charged residues over the whole surface of the miniprotein constitute major factors that lead successfully to obtaining highly active miniprotein-based inhibitors of PD-1/PD-L1 interaction.
4 MATERIALS AND METHODS
4.1 General
All commercially available solvents and reagents used in this work were purchased from Sigma-Aldrich, Merck, Iris Biotech, Lipopharm, Trimen, and Avantor and were not further purified.
4.2 Miniprotein design
The crystal structures of PD-L1 (PDB id 4ZQK) (Zak et al., 2015) and ENH miniprotein (PDB id 1ENH) (Clarke et al., 1994) were starting structures for all calculations. Rosetta v 3.10 software was used (Fleishman et al., 2011). Initially, ENH was manually truncated at C- and N-termini to produce miniprotein 1 (Discovery Studio 2022 Viewer, Biovia), which was docked to the PD-L1 using the RosettaDock protocol (Gray et al., 2003). RosettaDock was used with the following parameters: ex1, ex2aro, dock_pert 3 8, spin, randomize1, randomize2, use_ellipsoidal_randomization true, and nstruct 30000. The resulting complex was used as the input for the FastDesign algorithm. The FastDesign protocol was used with following parameters: task_operations=“limitchi2,resfile1” scorefxn=“sfxn_std” clear_designable_residues=“0” repeats=“4” ramp_down_constraints=“0”, where task operations and score were defined as follows <LimitAromaChi2 name=“limitchi2” include_trp=“1”>, <ReadResfile name=“resfile1” filename=“name.resfile”> and <ScoreFunction name=“sfxn_std” weights=“beta_nov15.wts”> <Reweight scoretype=“aa_composition” weight=“1.0”/> <Reweight scoretype=“p_aa_pp” weight=“0.8”/>. The RESFILE file with the range of possible mutations of ENH was used. The algorithm was run 5000 times, and filters required a number of unsatisfied hydrogen bonds lower than 5 were applied. The FastDesign script was run with following flags: -beta_nov15, -ex1, -ex2aro, -use_input_sc, -no_his_his_pairE, -nblist_autoupdate true, -chemical:exclude_patches LowerDNA UpperDNA Cterm_amidation SpecialRotamer VirtualBB ShoveBB VirtualDNAPhosphate VirtualNTerm CTermConnect sc_orbitals pro_hydroxylated_case1 pro_hydroxylated_case2 ser_phosphorylated thr_phosphorylated tyr_phosphorylated tyr_sulfated lys_dimethylated lys_monomethylated lys_trimethylated lys_acetylated glu_carboxylated cys_acetylated tyr_diiodinated N_acetylated C_methylamidated MethylatedProteinCterm, -relax::ramp_constraints true.
4.3 Peptide synthesis
Peptides were synthesized using the microwave-assisted SPPS method in Biotage® Initiator+Alastra or Liberty Blue CEM instruments. H-rink amide ChemMatrix® resin was used for the synthesis (loading: 0.59 mmol/g), which led to obtaining an amide group at the carboxyl end of the peptide on a scale of 0.06 mmol. In the first synthesis stage, the resin was swelled in DMF (20–60 min). The solvent used in peptide synthesis was DMF peptide synthesis grade.
DIC and Oxyma coupling reagents were used for the coupling reaction (0.5 M concentration in DMF, five-fold molar excess). The single coupling reaction was performed for Fmoc-α-amino acids (0.1 M concentration in DMF, five-fold molar excess) for 15 min at 75°C (Biotage® Initiator+Alastra) or 4 min at 90°C (Liberty Blue CEM). The reaction was carried out at a reduced temperature of 50°C for 10 min for histidine residue. A 20% solution of piperidine in DMF was used for the α-amino group deprotection reaction. After synthesis, the resin was rinsed with DCM and dried.
Subsequently, the resin with the attached peptide was transferred to a sintered polypropene syringe, where the peptide was cleaved from the resin, and the side chain protecting groups were removed. To detach the synthesized peptide from the resin, a cleavage mixture consisting of TFA:TIS:thioanisol:H2O (85:5:5:5) was used, in which the resin was shaken for 3 h. The peptide was then precipitated from the solution by adding ice-cold diethyl ether, and the resulting precipitate was centrifuged at 4°C and 8000 rpm. After HPLC purification, all peptides were over 95% pure.
4.4 HPLC
Peptides were purified by HPLC using the UltiMate 3000 LC System (Dionex) or Knauer Prep apparatus on a C18 preparative column (Thermo Scientific, Hypersil Gold 12 μm, 250 mm × 20 mm) in the MeCN/H2O solvent system with 0.05% addition TFA in both eluents. The purity of the peptides was determined using Shimadzu analytical UFLC HPLC on a C18 column (Reprosil Sapir 100 C18, 5 μm, 150 × 4.6 mm) in the same solvent system.
4.5 Mass spectrometry
The synthesized peptides were identified by mass spectrometry using an WATERS LCT Premier XE system with a time-of-flight detector and electrospray ionization.
4.6 CD
CD spectra were measured at a constant temperature of 25°C in the wavelength range of 195–250 nm, every 0.2 nm, scanning speed 50 nm/min, accumulating data from five scans. Measurements were performed for solution in 50 mM phosphate buffer pH 7.5, at a peptide concentration of 60 μM. The CD intensity was presented as molar residual ellipticity ([Θ]MRE) normalized according to the number of amino acid residues in the sequence.
4.7 PD-L1 binding studies
The analysis of the kinetics of the interaction with the molecular target, and thus the determination of the dissociation constant of the inhibitor's binding to PD-L1, were performed using BLI using Octet RED96 equipment (ForteBio). BLI is an optical technique that analyzes interference patterns generated by white light reflected from the biosensor tip surface, which contains the target protein (PD-L1). These interference patterns change according to the binding of the ligand (inhibitor) present in the solution within the biosensor well. Consequently, BLI-based measurements enable the analysis of interaction kinetics and the determination of the KD value.
The received data were processed using software integrated with the hardware using the global fitting procedure. The buffer used for measurements consisted of 10 mM HEPES (pH 6.9), 150 mM NaCl, 3 mM EDTA, 0.05% Tween20, and 0.5% BSA. In the first stage of the experiment, biotinylated human PD-L1 (Sino Biological #10084-H08H-B) at a concentration of 1 μg/mL (aliquots created by dilution of the material provided by the manufacturer) was bound to a streptavidin-coated biosensor (SAX, Sartorius #18-5117). The tested peptides dissolved at a concentration of 1 mM in H2O were diluted in the measurement buffer to the appropriate concentrations. The binding kinetics was measured by immersing the loaded biosensors in a peptide solution of appropriate concentration (association) and then dipping the biosensors in a buffer (dissociation). All experiments were done in duplicate.
4.8 PD-1/PD-L1 inhibition studies
Inhibition studies were conducted using the HTRF assay. In this assay, two antibodies (anti-Tag1 labeled with Europium, as the HTRF donor, and anti-Tag2 labeled with XL665, as the HTRF acceptor) along with two proteins of interest (Tag1-PD-L1 and Tag2-PD-1) are utilized. Upon binding of PD-L1 and PD1, the HTRF donor and acceptor are brought into close proximity. Excitation of the donor initiates fluorescence resonance energy transfer toward the acceptor, resulting in emission specifically at 665 nm. Therefore, the inhibition of the PD1/PD-L1 interaction will result in a decrease in the HTRF signal, which can be quantified and utilized to assess the level of inhibition.
The HTRF assay was performed using reduced-volume 96-well white plates (CisBio #66PL96025) and a PD-1/PD-L1 interaction kit (CisBio #64PD1PEG) according to the kit protocol at pH 6.9. HTRF measurements were performed in triplicate on a CLARIOstar® fluorimeter with an excitation filter at 337 nm by measuring the fluorescence intensity at wavelengths 620 and 665 nm. The background values were subtracted from the obtained data, the 665 nm/620 nm ratio was calculated, and the negative control values were subtracted, normalized to the positive control, and averaged. The measurement error was calculated using the sample standard deviation from three measurements. IC50 values were obtained from a set of measurements at different concentrations by fitting the results using the DoseResp function of OriginPro 2019 software.
4.9 Cell studies
The cell-based immune checkpoint blockade assay was performed using the commercially available PD-1/PD-L1 Blockade Bioassay (Promega, Madison, WI, USA) according to the protocol provided by the manufacturer.
This test involves the coculture of two cell lines: artificial cells presenting the CHO-K1 antigen with overexpression of PD-L1 and the TCR activator protein (CHO/TCRAct/PD-L1 cells) and Jurkat cells with overexpression of PD-1 and the luciferase gene controlled by NFAT (Jurkat Effector Cells, Jurkat-ECs). The binding of the TCR activator to the TCR results in the activation of Jurkat-EC cells, which results in the activation of the NFAT transcription factor and then the expression of luciferase, the effect of which is the luminescence measured in the experiment. PD-1/PD-L1 interaction reduces the activation level of Jurkat-EC cells. Blocking the PD-1/PD-L1 interaction allows for the restoration of activation of these cells.
CHO/TCRAct/PD-L1 cells were cultured in white 96-well plates (density: 10,000 cells/well) for the experiment. After 24 h of culture, inhibitors were added at appropriate concentrations. Due to discrepancies in the results, from that moment on, the experiment used an RPMI-1640 medium with a pH lowered by adding HCl to 6.9. Incubation with inhibitors lasted 24 h at 37°C in 5% CO2.
Preparation of inhibitor solutions consisted of preparing solutions with a concentration of 2 mM in water. Additionally, the correctness of the peptide concentration was checked by diluting the initial 2 mM solution to 100 μM in 8 M Gu*HCl, incubating for 30 min at room temperature, and then measuring the absorbance of the sample at a wavelength of 280 nm. Then, the concentration in the sample was calculated using the Lambert–Beer law, and further dilutions were corrected based on it.
After incubation with inhibitors, Jurkat T cells (density 20,000 cells/well) and inhibitors at the appropriate concentration were added. DMSO was used as a negative control, and durvalumab solution at a concentration of 2.5 mg/mL was a positive control.
Effector cell activation was evaluated by measuring bioluminescence after 6 h of incubation (37°C, 5% CO2) and 20 min of additional incubation with Bio-Glo reagent (Promega) at room temperature. Bioluminescence measurement was performed using a Tecan Spark 20M multimode microplate reader. The data were presented as fold induction of the luminescent signal relative to the control, that is, cells with DMSO. Data points represent mean values ± SD from three independent experiments. EC50 values were calculated by fitting a Hill curve to experimental data using OriginPro 2019 software with the lower and upper limits of these fits chosen on the basis of negative and positive control results. For 25 and 32 miniproteins, measurements were carried out at the following concentrations: 0.156, 0.312; 0.62; 1.25; 2.5; 5; 10 μM.
AUTHOR CONTRIBUTIONS
Agnieszka Ciesiołkiewicz: Conceptualization; investigation; formal analysis. Juan Lizandra Perez: Investigation; formal analysis. Łukasz Skalniak: Investigation; formal analysis. Paweł Noceń: Investigation; formal analysis. Łukasz Berlicki: Conceptualization; validation; supervision; project administration; writing – original draft; writing – review and editing; funding acquisition.
ACKNOWLEDGMENTS
The work was financially supported by the National Science Centre, Poland, Grant no. 2018/31/B/ST5/02631 (to Ł.B.). The software and hardware resources from the Wrocław Centre for Networking and Supercomputing were used in the present study.