Doses for X-ray and electron diffraction: New features in RADDOSE-3D including intensity decay models
Joshua L. Dickerson and Patrick T. N. McCubbin contributed equally to this study.
Review Editor: Nir Ben-Tal
Abstract
New features in the dose estimation program RADDOSE-3D are summarised. They include the facility to enter a diffraction intensity decay model which modifies the “Diffraction Weighted Dose” output from a “Fluence Weighted Dose” to a “Diffraction-Decay Weighted Dose”, a description of RADDOSE-ED for use in electron diffraction experiments, where dose is historically quoted in electrons/Å2 rather than in gray (Gy), and finally the development of a RADDOSE-3D GUI, enabling easy access to all the options available in the program.
1 INTRODUCTION
Structural biology has until recently relied on X-ray crystallography to provide much of the three-dimensional information on proteins and other macromolecules that inform biological function. Recently, single-particle cryogenic electron microscopy and electron diffraction techniques have advanced to the point where results from them are also giving new and exciting contributions to our knowledge. However, in all of these experimental methods, the samples suffer from radiation damage (RD) inflicted by the incident X-rays/electrons, and this RD remains one of the major bottlenecks to accurate structure determination. RD in macromolecular crystallography (MX) has been characterised over the last 60 years (for a recent review see Garman & Weik, 2023) and manifests in both reciprocal space and in real space. In reciprocal space, there is fading of the diffracted signal, starting with the highest resolution reflections and gradually extending inwards to lower resolution as irradiation continues. Finding an appropriate model for this intensity decay has proved challenging and this issue is addressed in more detail below. Diffraction fading ultimately affects the biological detail that can be gleaned from the structure, so it has become a mainstream concern for MX. Concomitant with the reflection intensity decrease, for cryo-cooled crystals at a synchrotron, the unit cell volume is seen to expand, the scaling B-factors increase linearly with exposure, the internal agreement quality indicators for the dataset become worse (e.g., higher R values), and the mosaicity often increases. In real space, atomic B-factors become larger, and specific structural damage to particular moieties is observed in a reproducible order; for example, reduction of metal ions and disulphide bond scission (also observed at room temperature) occur before decarboxylation of aspartate and glutamate residues.
In MX, the primary metric against which the rates of damage have been monitored is the absorbed dose, defined as the absorbed energy (J) per mass (kg) in units of gray (Gy = J/kg). The dose in an experiment cannot be measured, it can only be estimated from the properties of the beam (incident beam flux density, beam profile, and energy) and the sample (atomic composition, dimensions of the crystal) so that the absorption coefficients can be calculated. To enable experimenters to more easily estimate dose, we have written and freely distributed an open-source software program called RADDOSE-3D (Bury et al., 2018; Zeldin, Gerstel, & Garman, 2013) which allows time- and space-resolved modelling of dose. Due largely to the initial object-orientated modular architecture of the code, we have been able to continually develop and improve it for the last 11 years. In RADDOSE-3D, an experiment is represented by three objects, the “Crystal”, “Beam”, and “Wedge” blocks. By defining these objects in the program input, RADDOSE-3D can “simulate” the experiment and estimate the absorbed dose within the sample (Figure 1). A full description of progress from the first release in 2013 until 2018 was given by Bury et al. (2018). Other papers since then have detailed various extensions to the code and RADDOSE-3D can now be used to estimate the absorbed dose for a wide range of structural biology modalities. Specifically, modifications to the code have been implemented which allow dose estimations for small angle X-ray scattering (SAXS) investigations (Brooks-Bartlett, 2016) and for small molecule crystallography experiments (Christensen et al., 2019), with a subsequent improvement to include the energy carried away from the sample by fluorescent photons (Fernando et al., 2021). A more sophisticated treatment of photoelectron escape (which reduces the absorbed energy and thus lowers the dose) has been implemented to cater for the increased use of microbeams and microcrystals, and now includes an option for Monte Carlo simulations to provide more accurate calculations (Dickerson & Garman, 2021). We have also released RADDOSE-XFEL (Dickerson et al., 2020) which can provide estimates of the dose absorbed during very short X-ray pulse (fs) experiments at X-ray free electron lasers (XFELs) by tracking the time taken for the various energy loss processes.
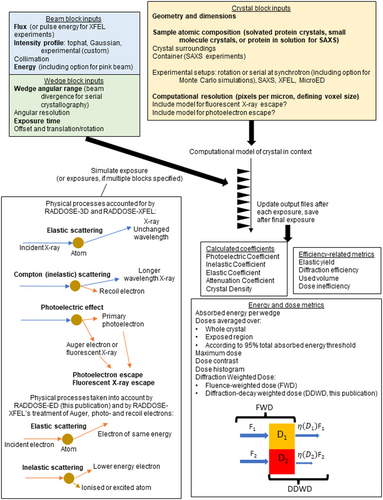
In this article, we will summarise the unpublished capabilities recently implemented in RADDOSE-3D. We divide the descriptions of new work into three main sections below: (1) the option to specify an intensity decay model, (2) a description of RADDOSE-ED for use in microcrystal electron diffraction (MicroED) studies where traditionally the effects of RD have been monitored against fluence (electrons/area, e−/Å2) rather than against dose in gray, and (3) an introduction to a new graphical user interface (GUI) that gives researchers all the current capabilities in a user-friendly form.
However, to ensure clarity between the different fields involved in the descriptions below, here we provide unambiguous definitions of certain key terms. Fluence is defined as photons (or electrons) per unit area (ph/mm2 or e−/Å2), respectively, flux is photons (or electrons) per second, and flux density is photons (or electrons) per unit area per second. The information coefficient (or “diffraction efficiency” for modalities involving diffraction), is defined as the signal intensity per MGy of absorbed dose. It should be noted that cryoEM papers in the literature frequently discuss quantities in terms of “per unit damage,” which often refers to global damage measured by an increase in overall B-factor. In MX papers, (where is the total intensity of a dataset or section of data and is the intensity of the same sweep of data extrapolated to zero dose) is used as a unit of global damage and Bnet is a new unit of specific damage (Shelley & Garman, 2022).
2 INTENSITY DECAY MODELS IN RADDOSE-3D
Intensity decay models (IDMs) describe the decrease in the intensity of diffracted X-rays as the absorbed dose increases. Table 1 shows commonly used or recently proposed IDMs. The associated parameters are either purely empirical or have some physical justification (for further discussion of the general form of IDMs, see Section 2.2.1). In the first part of this section, we will demonstrate the implementation of a previously published IDM (that of Leal et al. (2013)) into RADDOSE-3D and show how dose estimates by this implementation can explain the progression of specific and global RD in diffraction data. In the second part, we place this IDM in a broader context, show how we estimated its parameter values through fitting to diffraction data, and analyse its physical basis in order to motivate further work on how the parameter values for each crystal might be predicted from physical principles. Finally, we discuss the significance of this work for crystallographic data processing and the analysis of electron density maps.
| Model (citation) | Form of relative intensity decay with dose | Fitted parameters | Resolution dependence of dose-dependent decay |
|---|---|---|---|
| None | This is the IDM used in the original implementation of DWD as a fluence-weighted dose (Zeldin, Brockhauser, et al., 2013). | N/A | |
| Linear (Owen et al., 2006) | (for ) (for ) |
is the half-dose, experimentally measured at 43 MGy in Owen et al. (2006) for intensities in the resolution range 50–2.4 Å | varies with resolution shell, see discussion in Owen et al. (2006), but no explicit relationship incorporated into model. |
| Standard dose–response (Owen et al., 2014) | is the lower asymptote (i.e., the final diffracting power). is the decay curve midpoint, is the Hill slope. |
The values of the midpoint and Hill co-efficient may depend on the resolution. | |
| Four-state kinetic (Sygusch & Allaire, 1988) | is the relative intensity for a small region of the crystal as used in Equation (3). is the contribution of the undamaged fraction of the crystal that decreases linearly with dose. is the contribution of a fraction only slightly perturbed by damage (e.g., by site-specific damage or only a few ionisation events per unit cell) such that the scattering power is still similar to the native state. is the contribution of a fraction of the crystal that has been significantly disordered but is still capable of contributing to diffraction. The fractions evolve according to a sequential kinetic scheme with rate constants (with respect to dose) that are fitted empirically. The amorphous fraction does not contribute to diffraction and thus does not appear in the equation. |
The resolution-dependence of intensity decay is captured in the term. | |
| Exponential decay (Holton, 2009; Holton & Frankel, 2010) | is the Howell criterion (units MGy Å−1), derived from meta-data for a range of experimental measurements, of 10 MGyÅ for cryo-temperature experiments in Howells et al. (2009) based on data in resolution range 100–1 Å. is the Wilson B-factor at zero dose. |
||
| Gaussian blurring of electron density (Atakisi et al., 2019) | would be expected if intensity decay is due to Gaussian blurring of electron density at random locations in the crystal. However, empirical fitting suggests values of less than 2 [18]. is a proportionality constant. This model is equivalent to a model with linearly increasing B-factor (with initial value ) but no dose-dependent scale factor. |
||
| Adapted scaling model (general form) (Beilsten-Edmands et al., 2020; Borek et al., 2013; Evans & Murshudov, 2013; Kabsch, 2010; Leal et al., 2013) | is the overall B-factor that increases linearly with dose. How this behaviour is implemented can vary, for example, Leal et al. (2013) suggest as described below, and Borek et al. (2007) suggest using a relative B-factor with where is a constant estimated to be 1 Å2 MGy−1 (Borek et al., 2007; Kmetko et al., 2006; Krojer & von Delft, 2011) is an empirical scale factor, sometimes denoted instead by . Assuming no other variables are affecting the intensities (such as changes to illuminated volume), this is usually taken to be unity, as in Borek et al. (2007). This is equivalent to assuming that the effects of global damage are captured entirely by the linear B-factor increase. However, Leal et al. (2013) suggest a dose-dependent form of the scale factor . This can improve the fit especially for room temperature diffraction data. |
||
| Adapted scaling model (as parameterized in RADDOSE-3D, after Leal et al. (2013)) | is the overall B-factor at zero dose (units Å2), quantifies rate of increase in overall B-factor with dose (units Å2 MGy−1). (units MGy−1) determines the behaviour of the Gaussian scale factor. is small (0.04 MGy) at cryogenic temperatures (Leal et al., 2011; Leal et al., 2013). |
- Note: The linear and dose–response models are given in terms of the relative intensity for the nth image, , as a function of the cumulative dose in the nth image, . For the remaining models where the resolution-dependence of intensity decay is more precisely defined, the IDM is given as the function in the appropriate form as to be substituted into Equation (3) below to calculate the relative diffraction efficiency for a region of a crystal. is a function of the dose, , and the magnitude of the scattering wavevector, where is the spacing between Bragg planes. It should be noted that in practice the relative intensity is taken instead to be where is the first measured intensity at some small initial dose, so care should be taken to account for the fact that this is not the true intensity at zero dose. Similarly, care should be taken if data are normalised independently for each individual resolution bin since information on the resolution-dependence of intensity at zero dose will be lost during this normalisation procedure.
2.1 Implementing an IDM in RADDOSE-3D
In this part, we first describe how, through the incorporation of the IDM proposed by Leal et al. (2013), the diffraction-weighted dose (DWD) metric of RADDOSE-3D has been modified from a fluence-weighted dose (FWD) to a diffraction-decay weighted dose (DDWD). We then show how the DDWD estimated by RADDOSE-3D can explain the extent of RD in electron density maps, using the dataset collected by de la Mora et al. (2020) as an example. Finally, we discuss how DDWD compares to other dose metrics that are output by RADDOSE-3D.
2.1.1 Diffraction-weighted dose in RADDOSE-3D
This is an advantageous metric compared to the total average dose across the whole crystal (weighting all voxels equally), the maximum dose (weighting the voxel with the highest dose as one, and all other voxels as zero) or the average dose in the exposed region (defining an exposed region by an incident intensity threshold and weighting all voxels in this region equally). This is because voxels that are irradiated by more intense regions of the beam contribute proportionally more to the FWD, and voxels outside the beam have negligible incident intensity and thus make negligible contribution to the FWD. However, as pointed out in the original publication (Zeldin, Brockhauser, et al., 2013) and by other studies thereafter (Brooks-Bartlett, 2016; de la Mora et al., 2020; Warkentin et al., 2017), weighting by incident fluence alone does not account for the decay in relative intensity due to RD as the dose increases. For a true DWD, an appropriate intensity decay model (IDM) that can be applied for each volume element of the crystal must be incorporated into the definition of DWD.
2.1.2 Diffraction-decay weighted dose in RADDOSE-3D
The representative and values used to evaluate the integrals are those from the BEST diffraction data collected on 72 different proteins (scaled together, and with varied folds, molecular weights, space groups, and data resolutions at both cryo and room temperature) (Popov & Bourenkov, 2003). The integral is taken over all resolution shells in the BEST data (12.0–0.9 Å resolution window) and thus the DDWD output is what would be expected of a typical protein with relative intensities evaluated over this resolution window. Using representative values, rather than requiring the user to input their own [] values, allows these to be coded directly into RADDOSE-3D, thereby reducing its execution time.
The modular nature of the RADDOSE-3D code means new models that may be proposed by the crystallography community can easily be included. If no IDM is specified, the program defaults to outputting the FWD, by setting .
2.1.3 Example use of RADDOSE-3D with incorporated IDM
To validate the implementation of this DDWD in RADDOSE-3D, we reanalysed the high dose rate, room temperature dataset from de la Mora et al. (2020). RADDOSE-3D was first used to calculate the FWD with respect to exposure time (input parameters are shown in the middle column of Supplementary Table S2). The Python script used to generate the modelled beam profile is available on the RADDOSE-3D GitHub repository and can straightforwardly be adapted to generate any modelled beam profile for input into RADDOSE-3D. Appropriate parameter values for the Leal et al. (2013) IDM were estimated as described in Section 2.2.2. To give the estimated DDWD with respect to the exposure time, the implementation of the Leal et al. (2013) model in RADDOSE-3D was then run (inputs are shown in the right column of Supplementary Table S2, which are the same inputs as for the FWD calculation, except for the specification to use the Leal et al. (2013) model with the appropriate parameter values).
The results in Figure 2 show that whilst the FWD increases linearly with exposure time, DDWD increases to a maximum before gradually decreasing, because at high total doses the less damaged regions that have absorbed lower doses contribute more to the diffraction pattern. Furthermore, this behaviour correlates with the degree of damage observed in a disulphide bond: the absolute value of the integrated difference electron density for this bond is shown in Figure 2, calculated as described in de la Mora et al. (2020), where a greater value indicates a more damaged bond. DDWD gives information on the extent to which the absorbed dose manifests in the electron density map and thus correlates with the damage to this disulphide bond.
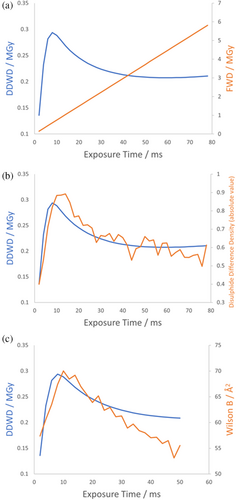
Because the integrals in Equation (3) are evaluated over the BEST data for the resolution range 12–0.9 Å, our implementation will be accurate when the resolution range of the data being analysed matches this resolution range. However, Figure 2 shows that even if the resolution ranges do not match exactly, the resultant error is systematic such that the calculated DDWD is still useful semi-quantitatively for understanding the dose effects that manifest in the electron density map. What is critical for this analysis is that the IDM fits the relative intensity decay curve well across the full range of doses analysed in the experiment. In the original analysis in the supplementary material of de la Mora et al. (2020), an exponential decay IDM is used and the calculated DDWD significantly increases again at the highest doses, implying that the disulphide bond damage should increase again, but this behaviour is not observed in the electron density.
2.1.4 Interpreting dose metrics output by RADDOSE-3D
Accurate dose estimation is important for designing a data collection strategy. For rotation crystallography, it is necessary to ensure a full crystal rotation of data are collected before RD has significantly affected the signal. Since IDMs are all smoothly decreasing functions, often well-approximated by the simple linear model at low/medium doses for cryo-temperature experiments, the original implementation of DWD as the FWD (Zeldin, Brockhauser, et al., 2013) output by RADDOSE-3D remains a useful dose metric for designing a data collection strategy; RADDOSE-3D is implemented at multiple beamlines (such as I04 at DLS, BL12-1 and BL12-2 at SSRL (Garman & Weik, 2023)). Furthermore, dose estimates are essential inputs for dedicated programs that optimise data collection strategy. For example, the program BEST (Bourenkov & Popov, 2010) (within the EDNA framework (Incardona et al., 2009)) implements the Leal et al. (2013) IDM alongside a model for radiation-induced non-isomorphism, taking dose rate estimates from RADDOSE version 1 (Murray et al., 2004), to design an optimal data collection strategy based on a few initial diffraction images. Similarly, the program KUMA within the ZOO framework (Hirata et al., 2019) implements RADDOSE version 2 (Paithankar & Garman, 2010) to suggest exposure conditions with an absorbed dose of 10MGy (Hirata et al., 2019). For optimisation programs that implement an IDM internally, such as BEST, if it is necessary to input a single dose metric then the FWD (Zeldin, Brockhauser, et al., 2013) is the output from RADDOSE-3D that is generally applicable for more complex exposure schemes (e.g., helical) as discussed in Bury et al. (2018). However, the behaviour of DDWD shows the advantage, particularly for room temperature data collection, of explicitly accounting for the spatial distribution of dose within the crystal and applying the model of RD to each small region of the crystal (see Section 2.2.2 for a related discussion on using the FWD to fit IDMs). Among the outputs of RADDOSE-3D is a file containing the dose for each voxel of the crystal (Bury et al., 2018).
A second important application for dose estimation is to understand the extent of RD in electron density maps. It is essential to avoid the misinterpretation of radiation-damage-induced changes as biologically significant. DDWD is more informative than FWD about the extent of RD in the final electron density map, as illustrated by the analysis in Section 2.1.3.
When evaluating different dose metrics, it is important to account for how robust the dose metric is to inaccuracies in the model for the beam intensity profile, particularly for the low-intensity edges of the beam. For example, the average dose in the exposed region is sensitive to such inaccuracies, whereas FWD and DDWD are relatively insensitive to them. Another strategy is to make a conservative approximation of the beam as purely Gaussian (i.e., neglecting any low-intensity tails), as discussed in de la Mora et al. (2020).
2.2 Understanding the IDM implemented in RADDOSE-3D
In this part we first place the IDM proposed by Leal et al. (2013) and implemented in RADDOSE-3D in the context of some general properties of all IDMs (Section 2.2.1), then show how this IDM was fitted to diffraction data to obtain parameter values for the DDWD calculation in Section 2.1.3 (Section 2.2.2), and finally explore the physical basis for the terms of this IDM (Sections 2.2.3 and 2.2.4) to motivate further work towards an IDM with predictable parameter values.
2.2.1 Introduction to the general form of IDMs
IDMs all contain an implicit assumption of uniform irradiation of the crystal or region of the crystal to which the IDM applies. To achieve uniform irradiation in practice in experimental studies, it is possible to use a beam with an exceptionally flat “top hat” intensity profile, such as is implemented at the EMBL beamline, P14, at PETRA III, Hamburg (Garman & Weik, 2017). Alternatively, a more common approach is to apply an appropriate correction for non-uniform illumination during analysis, for example, the three-beam model in the supplementary material of de la Mora et al. (2020) which combines a model for the beam and the exponential “H-model” IDM (see next paragraph) (Holton & Frankel, 2010) into a single model. In the definition of DDWD, the IDM is applied to many small regions of the crystal, each of which is small enough to be treated as uniformly irradiated. The comparative advantage of using RADDOSE-3D for the analysis of IDMs is that it directly calculates the impact of non-uniform illumination on the distribution of dose in the crystal (Bury et al., 2018).
Figure 3 shows typical diffraction intensity data from the room-temperature high dose rate dataset described in de la Mora et al. (2020). The data are plotted as the average intensity for a series of small resolution shells, for each of a series of sequential small exposures (for this dataset, an exposure time of 2 ms per exposure). The figure demonstrates that intensity decay depends on both dose and resolution. The definition of the relative diffraction efficiency , Equation (3), encodes this dependence on dose and resolution. To account for the fact that spherically averaged squared structure-factor magnitudes are a complicated function of resolution, Equation (3) uses the empirical approximation given by the BEST data (Popov & Bourenkov, 2003). However, it is important to stress that these data do not include the effect on intensity of the atomic B-factors at zero dose, so this effect must be encoded into . The simplest way to do this is through a term where is an average isotropic B-factor at zero dose (using an average B-factor assumes the distribution of atomic B-factors is not too broad or skewed). includes further terms that describe the dose-dependence of intensity decay. For example, a “scale” term describes any resolution-independent contribution to intensity decay (such as within the model of Leal et al. (2013). A final term describes dose-dependent intensity decay where the decay varies depending on the resolution. The two main hypotheses for this term are the “B-model” , as suggested in the Leal et al. model (Leal et al., 2013) and other scaling models, and the “H-model” (Holton & Frankel, 2010) (see Table 1 for further citations, parameter definitions, and units).
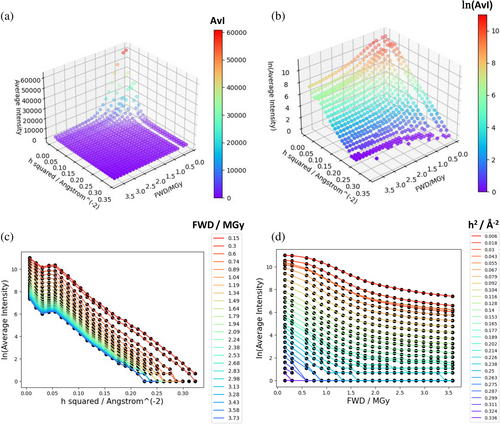
The evaluation of IDMs requires meta-analyses of many datasets to increase the statistical power of the hypothesis testing. This approach has been implemented multiple times (Atakisi et al., 2019; Holton & Frankel, 2010; Howells et al., 2009; Leal et al., 2013). The resolution-dependence of IDMs is especially significant because the loss of diffraction efficiency in the higher resolution shells has implications for the ability of the structure to inform biological hypotheses (Owen et al., 2006). An advantage of the B-model is that it directly encodes the robust linear relationship observed between scaling B-factor and dose (Borek et al., 2010; Bourenkov & Popov, 2010; Kmetko et al., 2006; Leal et al., 2013). A linear increase of B-factor as dose increases is expected under a central limit theorem if radiation-induced atomic displacements are randomly distributed, small but numerous, and accumulate in proportion to the dose (Borek et al., 2013). It has also been suggested that a different resolution-dependence, and thus form of IDM, might apply at medium to high resolution (<10 Å) compared to low resolutions (>10 Å) (Atakisi et al., 2019). Central to crystallographic data analysis is the equivalence of modelling unit cell constituents as a collection of point scattering sources (i.e., atoms) versus scattering from a continuous electron density. In the context of IDMs, it has been shown that increasing the scaling B-factor is an equivalent model to that of the Gaussian blurring of electron density at random locations in the unit cell (Atakisi et al., 2019).
Before we consider how the parameter values of the Leal et al. (2013) IDM were fitted for use in our DDWD calculation, it is worth emphasising that the parameter values of IDMs are temperature-dependent. For example, the parameter of this IDM is approximately zero only for cryogenic datasets (Leal et al., 2013) whereas for room temperature datasets the scale factor term becomes strongly dose-dependent. Temperature will affect not only the energy required to break bonds (and hence the rate of bond breakage per unit dose), but also the mobility of ions and radicals and their subsequent radiation chemistry, and thus the distribution of the absorbed dose both within unit cells and through the crystal (Weik & Colletier, 2010). Finally, none of the models in Table 1 explicitly account for the possibility of dose-rate effects (see (Garman & Weik, 2023) for further discussion).
2.2.2 Fitting the adapted scaling model
To estimate parameter values for use in the DDWD calculation in Section 2.1.3, the Leal et al. (2013) model was fitted to the room-temperature, high dose rate data from de la Mora et al. (2020). Merged (but not scaled) reflection files for each sequential 2 ms exposure dataset were reanalysed: note that no B-factor or scale factor correction had been applied to the intensities before this analysis. We stress that the appropriate intensities to use for fitting the model are the result of reflection integration (in this case by CrystFEL (White et al., 2012)) and thus have no contribution from background, and the intensity values tend to zero at high doses as shown in Figure 3.
Wilson B-factors and scale factors were calculated for each 2 ms exposure dataset by AIMLESS (Evans & Murshudov, 2013). A maximum resolution limit of 1.71 Å was always specified, because for the data at the longest exposure times higher resolutions than this showed some noise in their Wilson plots, and it was important to ensure the same region of reciprocal space was followed over all exposure times. The Wilson B-factor and the scale factor (the reciprocal of the Wilson scale factor, ) were plotted against the FWD estimated by RADDOSE-3D. Fitting of the B-factor term of the Leal et al. (2013) model was performed by least-squares regression over the region where the B-factor plot is still linear (FWD 0.8 MGy), as shown in Figure 4. The scale factor term, , was fitted by least-squares regression over the whole data range as indicated in Figure 4. The estimated parameter values agree well (within a factor of two) with previously reported values for chicken egg white lysozyme (HEWL) (Leal et al., 2013). The cryo-temperature dataset from the same study was also analysed for the same range of FWD values and similarly gave parameter values broadly consistent with previous studies (Leal et al., 2013).
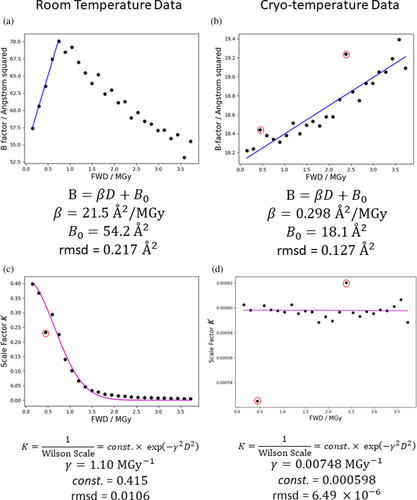
2.2.3 Interpretations of the adapted scaling model
For the purposes of DDWD calculation, the underlying meaning of the terms in the Leal et al. (2013) model is not important, as it is only used to provide an accurate prediction of the diffracted flux exiting each region of the crystal. However, to motivate further work towards an IDM that has predictable parameters, the physical basis of the Leal et al. (2013) model will now be discussed.
However, it is also necessary to account for the conversion of PBragg to the fraction of atoms that no longer contribute to Bragg diffraction due to RD (PNone). This may be related to the progression of defects in the crystal lattice on large scales that effectively reduce the number of unit cells exposed to the beam, as proposed by Leal et al. (2013) and discussed further in Section 2.2.4.
The parameter could in principle be predicted from the rate constants of the various physical and chemical processes that bring about the “Bragg to None” conversion.
Another hypothesis for the origin of a dose-dependent term besides the B-factor term is that RD-induced changes to unit cell size and mosaicity occur at a greater rate than the increase in overall B-factor with dose (proposed by Warkentin et al. (2017) in the context of lag phases). Depending on the crystal orientation relative to the beam, for a subset of diffraction images, this may cause a few reflections to broaden or migrate into the measured region of reciprocal space and thus their measured intensity will increase. However, the total diffracted intensity should be calculated over a large region of reciprocal space sampling thousands of reflections and so the impact of a few reflections on the intensity statistics should be small.
2.2.4 Physical limits for B-factors and implications for macroscopic crystal stability
The Leal et al. model predicts that the average B-factor increases indefinitely as dose increases, which is physically impossible within the constraints of a crystal lattice. According to this model, with parameters fitted as in Figure 4, within the dose range of the room temperature dataset the average B-factor calculated according to increases to as high as 129 Å2 at 3.5 MGy. We might compare this to the B-factor of a bulk solvent model where no solvent mask is specified: usually 125–200 Å2 (Weichenberger et al., 2015) (although this is not formally a B-factor of the solvent atoms it does quantify, for the whole unit cell, the contribution of the solvent in reciprocal space). Eventually, the contribution to diffraction of an atom with high B-factor becomes negligible relative to the sensitivity of the detector. However, probably before it reaches these large values, the average B-factor will be so high that it is physically unreasonable for describing atoms constrained in an ordered crystalline lattice. This is because the integrity of the crystal lattice at the mesoscopic/macroscopic scale emerges from the structural integrity of the macromolecules within the lattice and the contacts between them. It is therefore sensitive to microscopic atomic displacements that result from ionisation events and subsequent radiochemistry; large atomic displacements should disrupt the integrity of the crystal. IDMs should have a term to account for this, and this may be the origin of the scale factor term in the Leal et al. (2013) model.
As described in Section 2.2.1, a linear increase of B-factor as dose increases is expected only if certain conditions are met: that radiation-induced atomic displacements are randomly distributed, small but numerous, and accumulate in proportion to the dose (Borek et al., 2013). Furthermore, the definition of the B-factor assumes a crystal with intact unit cells. Most microscopic phenomena such as bond breakage are likely to satisfy these conditions. By definition, microscopic phenomena involve small perturbations, and since the perturbations are small and localised they are more likely to be randomly distributed from the perspective of a whole crystal. Conversely, mesoscopic/macroscopic structural breakdown within the crystal may involve larger displacements (on the scale of whole unit cells) which may be concerted (i.e., not totally random). Thus, crystal lattice breakdown may be a RD process that is not well modelled by a linear average B-factor increase. Again this is consistent with the scale factor term in the model of Leal et al. being due to the contribution of crystal structural breakdown, as suggested when the model was originally proposed (Leal et al., 2013). Because breakdown of the macroscopic crystal lattice causes atomic displacements, we might expect it to contribute to changes in the apparent average B-factor. However, crystal breakdown should instead reduce the number of intact unit cells because the apparent B-factor loses its physical meaning if we consider scattering from regions of the sample that are no longer crystalline.
More comprehensively than an average B-factor, we might consider the dose-dependent shift to the full distribution of atomic B-factors, . Global damage is then this whole distribution shifting to higher values, whereas specific damage is represented by specific atoms that have B-factors that shift by relatively more than other atoms as dose increases (Gerstel et al., 2015). To formulate an IDM in the form we need to consider the distribution that would be calculated for a small region of the crystal if we had knowledge of the true atomic positions and motions of all atoms in each unit cell within this region (if we fit the IDM to the FWD, the relevant is for the whole crystal and has the contribution of unit cells weighted by their incident fluence). Importantly, as dose increases these distributions will become significantly different to the diffraction-decay weighted atomic B-factor distribution that would be calculated from processing a diffraction pattern all the way to a refined structure, to which only measurable diffraction contributes.
is defined in terms of individual atoms (and assuming a crystal with intact unit cells). By the same logic as applied in the preceding discussion of the average B-factor, shifts in are most easily rationalised in terms of microscopic phenomena, for example bond breakage is associated with increased B-factors of the atoms involved in the bond. We would like to model how RD at this microscopic level might propagate to mesoscopic/macroscopic defects in the crystal. For the model of Leal et al., the parameters and are correlated (as shown in Figure 5), which is consistent with the scale factor and B-factor terms of this model both ultimately arising from the same or related phenomena at the microscopic level.
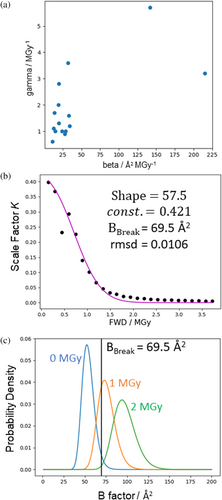
Figure 5 shows the simplest possible model for how a dose-dependent shift to could result not only in an increasing average B-factor but also a term like the scale-factor . For the purposes of illustration, is taken to be an inverse gamma function with a mean that increases according to the B-model (i.e., , see Supplementary materials, section 1.2.3 for details). To produce a term with behaviour similar to the scale factor term , we make two further assumptions. First, the extent of mesoscopic/macroscopic lattice defects is proportional to the fraction of atoms with B-factors above a certain threshold, , because we assume the crystal lattice is robust to displacements of individual atoms only up to a certain limit. Second, the decay in diffraction intensity which cannot be explained by the linear average B-factor increase is directly proportional to the extent of these defects, so the defects proportionally reduce the effective number of exposed unit cells. Figure 5 shows the fitting of this model to the variation of with dose and shows how varies with dose according to the fitted model. Inspection of Figure 5c suggests the exact distribution used to model should not have a huge impact on the predictions of this model so long as it is approximately bell-shaped and the whole distribution shifts past to higher values as dose increases.
The best fit for approximately matches the value at which the observed linear relationship between Wilson B-factor and dose breaks down. This is expected because in this model the combination of the linear relationship () and the parameter determines the doses at which the scale factor term goes through a steep decrease, which has a large effect on the relative intensity and thus the measured value of diffraction-weighted quantities (see end of this section for further discussion). This value of represents a mean square atomic displacement of . If the many assumptions underlying this simple model hold, we can interpret this as a maximum average square atomic displacement that can be tolerated by the intact crystal lattice for this particular sample.
In this formulation, we have considered the B-factor distribution of all atoms. However, it is necessary to give greater weight to the subset of atoms that are relatively more important for the structural integrity of the lattice (e.g., atoms at crystal contact sites). This has no effect on the model predictions if the atomic B-factor distribution of these atoms mirrors the B-factor distribution of all atoms. However, there will evidently be sample-dependent exceptions to this. A particularly striking example is crystals of oligomeric dodecin, where the decarboxylation of Glu57 probably causes a destabilisation of oligomers (and hence crystalline order) leading to global RD much faster than expected (Bieger et al., 2003; Murray et al., 2005). Further limitations of this treatment include the fact that by considering only atoms with B-factors it neglects any effect of radiation-induced changes to bulk solvent on the crystal lattice (e.g., an internal pressure due to gas generation) unless these have a comparable effect on the atomic B-factor distribution. Furthermore, it does not directly model phenomena where causation occurs at the macroscopic level of the crystal lattice (e.g., mechanical forces propagated by the lattice, which might drive cooperativity and spreading of defects) and reduces mesoscopic/macroscopic lattice stability to just a single parameter, . Evidently, our understanding would also be improved by a more accurate model for and how changes with dose, and a way to quantify the contribution of individual atoms to mesoscopic/macroscopic lattice stability. We hope that future models can improve on the simplistic model.
The linear increase in the apparent Wilson B-factor with dose breaks down at ≈0.8 MGy (see Figure 2). This is likely because the calculated Wilson B-factor is itself “diffraction decay-weighted” given it is calculated from the measurable diffraction. The behaviour of the Wilson B-factor approximately matches the behaviour of DDWD (see Figure 2c), which is calculated assuming the model of Leal et al. (2013) holds over the full dose range analysed. The scale factor term, , has a large effect on the diffracted intensity and the linearity of the Wilson B-factor plot breaks down as rapidly decreases between 0.5 and 1.0 MGy. If we accept that the decreasing scale factor term represents a structural breakdown of the crystal lattice, beyond FWD ≈0.8 MGy it is unclear whether defining B-factors is even meaningful for the most damaged regions of the “crystal” which receive a local dose well in excess of 1 MGy and have probably essentially become amorphous. The fact that calculating Wilson B-factor from the whole crystal is at all possible is due to diffraction from the weakly illuminated regions of the crystal that have only received a relatively low dose.
2.3 IDMs and DDWD: Outlook
In this work we have shown how, by incorporating the IDM by Leal et al. (2013) into RADDOSE-3D, the code can be used to calculate the DDWD. This DDWD can be used to understand the extent of RD in an electron density map, exemplified by its correlation with the difference density of a disulphide bond (de la Mora et al., 2020). The parameters of the IDM, as estimated by fitting to global intensity statistics (Wilson B-factor and scale factor), could be used in the DDWD calculation (which applies the IDM to each region of the crystal to determine the diffracted flux scattered from that region).
However, it would be even more advantageous to be able to predict the parameter values of the IDM in advance of the experiment, without the need to fit them to each specific diffraction dataset. This work has examined a range of possible frameworks for describing the physical and chemical changes to the crystal as dose increases, which give rise to the form and parameter values of the IDM. These include conversion between crystal fractions according to kinetic rate equations, and dose-dependent increases to atomic B-factors (the average B-factor or more exactly a shift to the entire distribution of atomic B-factors). There is sample-dependent influence on these processes, as evidenced by the large table of parameter values in Leal et al. (2013), and also suggested by the range of exponent values in Atakisi et al. (2019), which will need to be understood before a generally predictive IDM can be formulated. Sample sensitivity to global damage will vary due to a range of factors relating to the unit cell contents and the structural integrity of the crystal lattice. First, analogous to how different proteins have different rates of specific damage with respect to dose due to their exact structure (e.g., disulphide bond breakage depending on the angle made with a carbonyl oxygen (Bhattacharyya et al., 2020), and staple-group disulphides having greater susceptibility (Gerstel et al., 2015)), different proteins may have slightly different susceptibility to breakage of bonds between their light elements, even if the large number of such bonds in a given protein molecule should average out most variation. Second, the complex chemistry known to occur during RD (including the formation and propagation of radicals (Owen et al., 2012) and of hydrogen gas (Meents et al., 2010)) suggests that slightly different amino acid compositions and environments within the crystal may have some influence on radiation sensitivity. Thirdly, differences in crystal packing may impose different constraints on unit cell expansion and increased mosaicity which may contribute to the intensity decay, in addition to more direct effects such as those observed for the highly radiation-sensitive crystals of oligomeric dodecin discussed in Section 2.2.4 (Bieger et al., 2003; Murray et al., 2005). These sample-dependent influences could be dissected experimentally by using an independent technique to monitor the damage state of the crystal as data collection progresses (e.g., spectroscopy (Fernando et al., 2021) or X-ray topography (Suzuki et al., 2022)). Also useful would be a systematic comparison of intensity decay curves for a broader range of proteins than the standard model proteins (HEWL, insulin, thaumatin). In particular, it may be desirable to study different crystal forms of the same protein (due to alternative functional conformations or induced, for example, by changing the crystal temperature (Weik et al., 2001)), or compare sequence variants that have different melting temperatures or are functional at different temperatures in vivo. We speculate that strategies optimised for extracting predictive power despite a large parameter space will be optimal for solving this problem, as would a more detailed understanding of the dynamics of radiolysis and subsequent chemistry.
Improved predictive power of IDMs would have implications for crystallographic data analysis. Scaling programs, such as AIMLESS (Evans, 2006; Evans & Murshudov, 2013), XDS (Kabsch, 2010), HKL3000 (Minor et al., 2022), and DIALS (Beilsten-Edmands et al., 2020), must account for intensity decay due to RD as part of placing reflections on a consistent scale through application of scale, decay and absorption terms, leveraging multiplicity within the dataset. The decay factor is usually an overall B-factor correction analogous to that described in the IDMs in Table 1. The scale factor also contributes to correction for the effects of global RD, where these effects cannot solely be taken into account by the B-factor correction (i.e., the scale factor is dose-dependent as in the IDM by Leal et al. (2013)) but in the context of scaling it is also important as a more general correction, for example, for changes to the illumination volume. Because scaling must only be consistent within a dataset, these programs use an experimental coordinate such as frame number, rotation angle, or exposure time as a proxy for dose, rather than the absolute dose estimated through consideration of the physics of X-rays interacting with the crystal, for example, by RADDOSE-3D. Some scaling programs provide an option for zero-dose extrapolation for individual reflections using linear, polynomial, or exponential functions (Borek et al., 2007; Diederichs et al., 2003; Kabsch, 2010). However, these attempts are complicated by the fact that individual reflections do not all follow the average trend described by IDMs (e.g., some reflection intensities actually increase, as noted by Blake and Phillips in 1962 [Blake & Phillips, 1962]), and by the need to handle negative values of experimentally measured intensities and weak intensities. Attempts to improve zero dose extrapolation have included using the Wilson intensity distribution as a Bayesian prior or encoding IDMs into the process function of a Hidden Markov model describing the evolution of the crystal with increasing dose (Brooks-Bartlett, 2016). Additional corrections may also be applied to account for anisotropy induced by RD.
It has generally been assumed that the intensity decay due to global damage is largely separable from the effects on the intensities due to specific damage processes. Specific damage requires that damage events occur at the same atomic position in all unit cells of the crystal. Hence, it is usually detectable in real space only for radiation-sensitive sites that damage at a greater rate (with respect to dose) than the majority of positions within the unit cell. Recent progress in quantifying specific damage includes using singular value decomposition to model it as a component distinct from global damage in reciprocal space (Borek et al., 2013), its separation into individual components in real space through independent component analysis (Borek et al., 2018), and analysis of atomic B-factors in real space through the BDamage metric (Gerstel et al., 2015), and the related metric Bnet that can be calculated for a whole structure and enable comparison between structures (Shelley & Garman, 2022). Conversely, inherent in the derivation of an isotropic global scaling B-factor (i.e., that encoded in the “B-model”) through a central limit theorem is the fact that damage events occur randomly according to a uniform distribution across the atomic positions in the unit cell. Conceptually, the observed distribution of BDamage values (Gerstel et al., 2015) could result from either a homogeneous distribution of absorbed dose within an average unit cell but different activation energies for damage at different positions, or an inhomogeneous distribution of absorbed dose within an average unit cell, or probably a combination of the two possibilities. The input energy required for atomic displacements and bond breakage is a function of temperature, as is the distribution of absorbed dose due to molecular motion, secondary damage, and mobile ions/radicals. The DDWD estimated by RADDOSE-3D is proportional to the dose per average unit cell represented by an electron density map, and so would be the appropriate dose metric for comparison to atomic B-factor distributions of refined structures. Therefore, we expect the implementation of DDWD in RADDOSE-3D will be a further useful tool for scientists wanting to characterise the extent of RD in their structures.
3 RADDOSE-ED
Although X-ray crystallography has enabled us to determine the structures of a wide variety of molecules from small molecules to comparatively large macromolecular complexes (Shi, 2014), the technique is reliant on the successful production of large, well-diffracting crystals. To successfully obtain a structure from a single protein crystal, Holton & Frankel (2010) predicted that a spherical crystal must be at least 1.2 μm in diameter, or potentially 0.34 μm if there is significant photoelectron escape. This theoretical limit has not yet been reached, with the smallest crystals successfully used for structure determination having scattering powers larger than a 1.2 μm sized crystal. This size limit can be somewhat reduced by using multiple crystals, as in serial synchrotron crystallography (SSX) (Gati et al., 2014; Stellato et al., 2014), and reduced even further if RD can be outrun, as is the case for SFX at XFELs (Chapman et al., 2014; Nass, 2019). Nonetheless, although possible using SFX (Colletier et al., 2016), sub-micron-sized crystals still present major difficulties for successful structure determination using X-rays.
Electrons are theoretically much more suited than X-rays for imaging thin samples. As calculated by Henderson (1995) by comparing scattering cross sections, electrons offer approximately three orders of magnitude more signal per unit radiation dose for very thin specimens. As a result of this, there is a long history of structure determination using electron crystallography. Traditionally, this has involved using 2D crystals that consist of just a single monolayer of molecules. The first protein structure to be determined by 2D crystallography was of purple membrane (Henderson & Unwin, 1975) using a combination of both diffraction patterns and images. More recently, the methodology has been extended further to 3D crystals, in a technique often called MicroED (Shi et al., 2013). This has proved useful in determining structures from crystals that do not grow large enough for successful structure determination using X-rays (Clabbers et al., 2022), and structures from single crystals more than 2 orders of magnitude smaller than that achieved using MX have been solved (Rodriguez et al., 2015).
On the other hand, the much higher scattering cross sections of electrons compared to X-rays make it exceedingly difficult to determine structures from samples more than just a few hundred nanometres thick using beam energies commonly available in modern transmission electron microscopes. Experimental studies suggest that solving structures from crystals thicker than 2 inelastic scattering mean free path lengths (MFP lengths, ≈600 nm for 300 keV electrons) is extremely difficult (Martynowycz et al., 2021). As a result, crystals too large for MicroED are often thinned using focused ion beam (FIB) milling (Duyvesteyn et al., 2018; Martynowycz et al., 2019).
The fundamental cause of the limitation in crystal size for both electrons and X-rays is RD since it limits the amount of signal we can achieve before the molecules are destroyed. RD studies in X-ray crystallography have benefited from using dose as a metric against which to monitor its manifestations, enabling us to compare its progression between datasets from a variety of samples and under different data collection conditions. This has accelerated our understanding of RD to biological macromolecules from ionising radiation as well as allowing the optimisation of data collection strategies to improve the chance of successful structure determination. In electron crystallography, “dose” has typically been reported in terms of fluence (e−/Å2), but this fails to account for other factors that determine the dose such as primary beam energy and sample composition. Since this makes inter-comparisons and thus finding optimisation strategies challenging, some efforts have been made to convert e−/Å2 to gray (Baker & Rubinstein, 2010; Egerton, 2021), but this change has so far not been widely adopted.
RADDOSE-3D (Bury et al., 2018; Zeldin, Gerstel, & Garman, 2013) has been used by many as a simple tool to estimate dose for X-ray crystallography experiments, and more recently SAXS (Brooks-Bartlett et al., 2017) and SFX experiments (Dickerson et al., 2020). However, it has so far been limited only to calculations for incident X-rays, not having been written for other projectiles. We have now extended RADDOSE-3D to calculate doses for electron irradiation; specifically electron crystallography experiments in the subprogram RADDOSE-ED. We demonstrate that RADDOSE-ED can be used to convert fluence to dose for electron crystallography experiments and that for a given amount of absorbed dose, the extent of RD is comparable to that in X-ray crystallography. Moreover, by calculating an information coefficient (Peet et al., 2019), defined as the signal intensity per MGy of absorbed dose, we demonstrate how RADDOSE-ED can be used to optimise the beam energy for a given specimen thickness.
3.1 Methods
RADDOSE-ED calculates electron stopping powers to estimate the energy absorbed by the sample, and hence estimate the dose. It also calculates elastic and inelastic scattering cross sections, allowing estimation of the information coefficient. RADDOSE-ED can be run using a standard RADDOSE-3D input file, but with an additional flag “Subprogram EMED” in the crystal block. The electron fluence is also given in e−/Å2 instead of specifying an X-ray flux in photons/s.
3.1.1 Calculation of dose for incident electrons
To calculate the dose in Gy, we must calculate the mass of the exposed volume, as well as the energy absorbed by the sample. The mass is calculated as the sum of the masses of all atoms in the exposed area. The absorbed energy is calculated using the electronic stopping power for electrons. The total electronic stopping power is the sum of two stopping power components, collision stopping power, , and radiative stopping power, . The collision stopping power is the average energy loss per unit path length as a result of Coulomb collisions with bound atomic electrons, resulting in ionisations and excitations (Brice, 1985). The radiative stopping power is the average energy loss per unit path length due to the emission of Bremsstrahlung in the electric field of the atomic nucleus and atomic electrons.
3.1.2 Scattering cross sections
To calculate both inelastic and elastic scattering cross sections for the entire sample, the cross sections are summed for each atom present in the exposed volume and then converted to a MFP. Poisson statistics are then used to determine the number of elastic and inelastic scattering events, which is appropriate since scattering events are independent.
3.2 Results
3.2.1 Parameters important for dose calculation
We have investigated which parameters are important for accurately estimating the dose in electron crystallography experiments. Doses were estimated in RADDOSE-ED for a 200 nm cubic crystal of pure low-density amorphous ice, and the incident beam energy was varied between 10 and 2000 keV in 10 keV steps (Figure 6a). Doses initially steeply drop with increasing beam energy due to a decrease in collision stopping power, before beginning to rise very gradually above 1160 keV as a result of the increasing radiative stopping power.
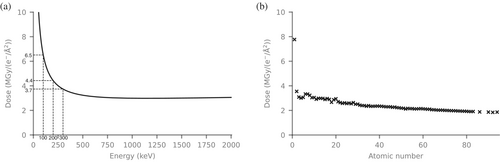
As well as varying the beam energy, the effect of atomic composition on dose was tested. We used a 200 nm crystal containing 500 of the same atom, ranging in atomic number from 1 to 83, and we also made estimations for radon, thorium, uranium, and plutonium samples. The doses generally decrease with increasing atomic number (Figure 6b), which agrees well with those calculated by Egerton (2021).
3.2.2 Comparison to MX
To determine if RD progresses similarly at cryo-temperatures in MicroED as it does in X-ray crystallography at 100 K, the absorbed dose of a MicroED dataset for proteinase K (Hattne et al., 2018) was calculated with RADDOSE-ED (Table 2). The reduction in the intensity of the diffraction pattern is shown in Table 2 and compares well with values from X-ray crystallography (e.g., for lysozyme at a temperature of 100 K), a of 12–14 MGy at 1.6–2.5 Å resolution (Teng & Moffat, 2000) and of 12.5–12.9 MGy at 1.8–35 Å resolution (de la Mora et al., 2011) has been observed. In terms of specific damage in MicroED, the disulphide bonds break before decarboxylation first appears (Hattne et al., 2018), as also observed in X-ray crystallography (de la Mora et al., 2011).
| Damaging effect | Exposure (e−/Å2) | Dose (MGy) |
|---|---|---|
| (1.7–21 Å) | 2.6 | 12 |
| S–S bond breakage | 0.9 | 4.0 |
| Decarboxylation first appears | 2.0 | 8.8 |
| All carboxylate groups removed | 5.0 | 22 |
- Note: The doses are calculated for the point at which the intensity drops to 50% of that in the first frame (), as well as for where the disulphide bond breakage and decarboxylation of aspartate and glutamate side chains were observed at cryo-temperature (Hattne et al., 2018).
3.2.3 Information coefficient
As well as estimating the absorbed dose, RADDOSE-ED also estimates the diffracted intensity per unit dose (“the diffraction efficiency” [Dickerson & Garman, 2019]), which is similar to the information coefficient defined by Peet et al. (2019) but applied to MicroED instead of to single particle cryoEM (SPA). The number of incident electrons that contribute useful signal is assumed to be those that elastically scatter once, and only once, in the sample and do not inelastically scatter. This is because inelastically scattered electrons will broaden the diffraction spots, and multiple elastic scattering (dynamical scattering) breaks the relationship between the Bragg intensities and the single-scattering values used for structure determination (Glaeser & Downing, 1993; Subramanian et al., 2015).
This number is then divided by the absorbed dose to give the information coefficient. For any given crystal, RADDOSE-ED will also estimate and output the beam energy that maximises the information coefficient.
We used RADDOSE-ED to estimate the information coefficient for crystals of pure low-density amorphous ice, with thicknesses varying from 10 to 1000 nm in 10 nm steps, and beam energies of 100, 200, 300, 500, 1000, and 2000 keV (Figure 7). RADDOSE-ED was also used to estimate the beam energy that maximises the information coefficient for a given crystal thickness (inset of Figure 7).
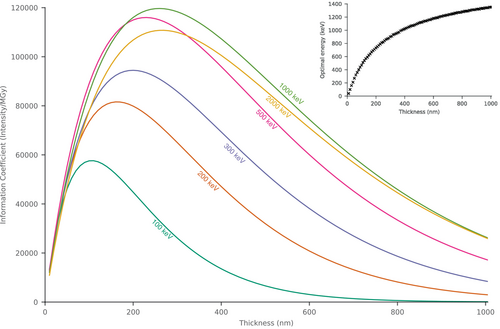
For very thin specimens, such as 2D crystals, lower incident electron energies are more favourable, with an optimal energy of 100 keV. As the specimen gets thicker, the information coefficient increases for all incident energies and peaks between 110 nm (100 keV) and 270 nm (2000 keV). The peak information coefficient increases as the energy increases, with the overall highest being with an 820 keV beam and a 250 nm thick crystal. At energies above this, the peak information coefficient begins to reduce, and there is little or no extra improvement for crystals <1 μm thick. This behaviour is caused by several factors. For thin specimens, lower energies are more beneficial since the ratio of elastic scattering to inelastic scattering is higher (Peet et al., 2019), meaning that there is more signal per unit dose. As specimen thickness increases, the information coefficient increases since the number of scatterers, and hence the diffracted intensity, increases. For thicker specimens, higher incident energies, and thus lower scattering cross sections, are required to minimise losses in signal from inelastic and dynamical scattering. However, since the ratio between elastic and inelastic scattering continues to reduce with increasing energy, there is eventually no improvement for samples thicker than ≈250 nm, leading to an optimum energy of 820 keV.
3.3 Discussion: RADDOSE-ED
We have extended RADDOSE-3D to include a new subprogram, RADDOSE-ED, to convert fluences in e−/Å2 into doses in MGy for electron diffraction experiments. Since account is now taken of primary beam energy and specimen composition, the biggest error in dose is likely to be the fluence measurement itself. Methods to measure this as accurately as possible are discussed by Krause et al. (2021). Unless beam currents are very low, one must be careful when using the counts from a direct electron detector, because of coincidence loss (Li et al., 2013). Measuring the beam current as indicated by the built-in screen ampere meter can also often be inaccurate. Installing a Faraday cup, or using the drift tube of a spectrometer as a Faraday cup, is often the most accurate method (Krause et al., 2021), and can also be used to properly calibrate the screen ampere meter.
We compared the symptoms of RD (specifically the rates of intensity decay, disulphide bond breakage, and decarboxylation) at cryo-temperatures in MicroED and X-ray crystallography, and determined that both global and specific damage events happen at similar doses. Considering that the majority of damage in X-ray crystallography is from photoelectrons and subsequent electrons produced (O'Neill et al., 2002), this is not surprising.
The information coefficient output by RADDOSE-ED allows beam energy and crystal thickness to be optimised. For thin crystals, such as 2D crystals, lower energies are predicted to be more optimal. As specimen thickness increases, signal losses due to multiple elastic scattering and inelastic scattering mean that higher energies, where the cross sections are lower, become more favourable. In fact, much higher energies than we are currently using should be used to both maximise the information coefficient and extend the upper limit of crystal thickness that can be productively investigated by MicroED, which has been experimentally measured to be two inelastic MFPs (Martynowycz et al., 2021). However, as the accelerating voltage increases, the size and cost of the microscope also rise, making energies above 300 keV a potentially costly endeavour. Although our results suggest 820 keV is optimum, the improvement above 500 keV becomes relatively modest, making 500 keV perhaps an ideal compromise between cost and maximising the information coefficient.
It is important to note that the information coefficient is not the only metric that will determine the quality of electron diffraction data. The information coefficient only considers signal and not noise; it assumes that electrons that have inelastically scattered, or elastically scattered more than once, are removed. Although this is true for inelastically scattered electrons if an energy filter is used, electrons that elastically scatter more than once (also termed dynamical scattering) are only removed if they scatter beyond the objective aperture. Dynamical scattering is particularly problematic since it breaks the kinematic approximation, meaning that kinematically forbidden reflections will now appear. As a result, diffraction from thick specimens is likely to be worse than predicted by a simple information coefficient, and higher energies thus may be more beneficial than suggested by the results shown in Figure 7. On the other hand, the harmful effects of dynamical scattering can potentially be reduced both experimentally (Clabbers & Abrahams, 2018; Subramanian et al., 2015) and computationally (Clabbers et al., 2019; Klar et al., 2023; Palatinus et al., 2015; Spence & Donatelli, 2021).
For crystals still too thick to be amenable to MicroED, specimens can be thinned by FIB milling to reduce them to the optimum thickness for a given primary beam energy (Duyvesteyn et al., 2018; Martynowycz et al., 2019). However, this will leave a layer of damage, estimated to be 30–60 nm thick for FIB milling ion energies of 30 keV (Berger et al., 2023; Lucas & Grigorieff, 2023; Parkhurst et al., 2023; Tuijtel et al., 2023; Yang et al., 2023). As a result, any FIB milled specimen will have less signal than expected for its thickness and the information coefficients will thus be reduced.
Lastly, although written for electron diffraction, RADDOSE-ED can in principle be used to convert fluences in SPA or cryogenic electron tomography (cryoET) into doses in MGy. The doses are likely to be a slight overestimate for very thin specimens used for SPA since RADDOSE-ED does not consider the escape of secondary electrons from the sample. The information coefficient described here does not apply to SPA or cryoET, since in those regimes the experimenter is mostly interested in a specific molecule of a particular size. As specimen thickness increases beyond this size, the signal will only decrease as a result of extra scattering (Dickerson et al., 2022; Russo et al., 2022).
4 RADDOSE-3D GUI
RADDOSE-3D has been interfaced at several synchrotron beamlines worldwide and is becoming embedded in assisting in data optimisation strategies. For instance, it has been integrated into Blu-Ice, used to control data collections at all the MX beamlines at the Stanford Synchrotron Radiation Laboratory, and into GDA at beamline I04 at Diamond Light Source (Masmaliyeva & Murshudov, 2019; McPhillips et al., 2002). To aid experimenters in planning their experiments, we have written an open-access RADDOSE-3D GUI in C++ which is suitable for running on both Windows and Linux-based operating systems (Figure 8). The GUI allows the user to select which RADDOSE utility they wish to run on a drop-down menu tab at the top of the right hand of the screen which lists: “Standard RADDOSE-3D”, “XFEL”, “Monte-Carlo”, and “RADDOSE-ED.” Once selected, the appropriate data entry boxes are displayed with 3 different tabs for the three blocks of “Crystal”, “Beam”, and “Wedge”. For instance, if a standard RADDOSE-3D run for MX, SAXS, or SMX is required, the following information about the sample can be entered in the “Crystal” tab: its shape (cuboid, polyhedron, cylindrical spherical), its XYZ dimensions (in μm), and the desired Pixels per Micron (default 0.1: this affects the voxelation and hence the resolution of the calculation). The next drop-down menu allows the user to specify the absorption coefficient calculation (ACC) appropriate to their experimental modality (MX, SAXS, or SMX): the ACC in-built in RADDOSE-3D, EXP (uses a PDB file), SEQUENCE, SAXS, SAXSSEQ, SMALLMOL, or CIF as input. Once an option is selected, the data entry boxes change to match the application (e.g., for SAXS, the protein concentration and an “advanced input” tab at the bottom allow entry of the sample container characteristics: type, thickness, density, composition). For an MX run, the crystal unit cell, the number of monomers, the number of residues per monomer, and the heavy elements per monomer are entered as well as the solvent atom composition (mM) and the solvent fraction. Input can then be manually edited using a tab at the bottom of the screen. The “Beam” and “Wedge” blocks can be similarly completed before running the program by pressing “Run”. The dose estimation results appear on the screen. Note that a full description of all the options is available in the User Guide (https://github.com/GarmanGroup/RADDOSE-3D/blob/master/doc/user-guide.pdf).
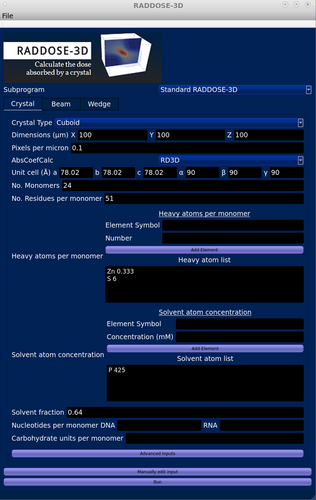
The GUI can be downloaded from https://github.com/GarmanGroup/RADDOSE-3D. To run it, Java must already be working, and if R (https://www.r-project.org/) is installed, a 3D representation of the dose distribution for the sample can be produced from the RADDOSE-3D output.
5 CONCLUSIONS
In this article, we have presented three related new developments that augment the toolbox available to structural biologists faced with the challenge of RD to their samples. First, we have now incorporated an option to include an intensity decay model into the calculation of the previous “fluence weighted dose” to give a “diffraction-decay weighted dose” and discussed the interpretation and context of this intensity decay model. Second, we have extended the capabilities of the open source RADDOSE-3D code which originally gave dose estimates solely for incident X-rays but which now gives the option, in its subprogram RADDOSE-ED, of calculating the dose absorbed for incident electrons as used in MicroED experiments. Since the use of gray should allow a more realistic comparison of RD between different instruments and samples, the dose given by RADDOSE-ED is in gray instead of , which are historically used in electron microscopy. Thirdly, we have written a RADDOSE-3D GUI to provide a simple platform that includes options for estimating the absorbed dose for a wide range of structural biology experiments (MX, SMX, SAXS, XFEL, ED). We hope that these developments will augment the experimentalists' capabilities and contribute to a better understanding of RD effects, as well as enable further optimisation of data collection strategies.
AUTHOR CONTRIBUTIONS
Joshua L. Dickerson: Conceptualization; formal analysis; investigation; methodology; project administration; software; supervision; validation; visualization; writing – original draft; writing – review and editing. Patrick T. N. McCubbin: Conceptualization; formal analysis; funding acquisition; investigation; methodology; software; validation; visualization; writing – original draft; writing – review and editing. Jonathan C. Brooks-Bartlett: Conceptualization; formal analysis; funding acquisition; investigation; methodology; software; validation; writing – original draft; writing – review and editing. Elspeth F. Garman: Conceptualization; funding acquisition; project administration; supervision; resources; writing – original draft; writing – review and editing.
ACKNOWLEDGEMENTS
We thank Eugenio De La Mora, Nicolas Coquelle, and Martin Rosenthal for kindly sharing past data with us. We are grateful to Ian Carmichael and Martin Weik for constructive comments on the manuscript. The ideas on IDMs presented here have been developed over a number of years and we would particularly like to thank Gleb Bourenkov and Thomas Schneider for their collaboration in 2015 when we collected IDM test data on beamline P14, PETRA III, for which they provided a beautiful “top-hat” X-ray beam. We have used these data to test a range of IDMs which have informed the current work but are not included in this manuscript.
FUNDING INFORMATION
We acknowledge the UK Engineering and Physical Sciences Research Council (EPSRC) for a studentship in the Systems Biology programme of the University of Oxford Doctoral Training Centre (JCBB) and for support from the EPSRC Undergraduate Vacation Placement Programme (PTNM). We are also grateful to the Collaborative Computing Project Number 4 (CCP4), UK for short-term postdoctoral funding (JCBB).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
RADDOSE-3D, along with the GUI and User Guide, is available at https://github.com/GarmanGroup/RADDOSE-3D.