Probing effects of site-specific aspartic acid isomerization on structure and stability of GB1 through chemical protein synthesis
Reviewing Editor: Hideo Akutsu
Abstract
Chemical modifications of long-lived proteins, such as isomerization and epimerization, have been evoked as prime triggers for protein-damage related diseases. Deamidation of Asn residues, which results in formation of a mixture of l- and d-Asp and isoAsp via an intermediate aspartyl succinimide, can result in the disruption of cellular proteostasis and toxic protein depositions. In contrast to extensive data on the biological prevalence and functional implications of aspartyl succinimide formation, much less is known about the impact of the resulting altered backbone composition on properties of individual proteins at a molecular level. Here, we report the total chemical synthesis, biophysical characterization, and NMR structural analysis of a series of variants of the B1 domain of protein G from Streptococcal bacteria (GB1) in which all possible Asp isomers as well as an aspartyl succinimide were individually incorporated at a defined position in a solvent-exposed loop. Subtle local structural effects were observed; however, these were accompanied by notable differences in thermodynamic folded stability. Surprisingly, the noncanonical backbone connectivity of d-isoAsp led to a variant that exhibited enhanced stability relative to the natural protein.
1 INTRODUCTION
The timescale of protein turnover, the carefully choreographed balance of synthesis and controlled degradation, varies over orders of magnitude within an organism and even within an individual cell (Toyama & Hetzer, 2013). Some proteins evade this turnover process entirely and are neither resynthesized nor degraded throughout the lifetime of an individual after being generated in utero (Lynnerup et al., 2008; Schey et al., 2020). Such long-lived proteins can become damaged over time through a variety of mechanisms, accumulating chemical changes as they age (Truscott et al., 2016). While some modifications stem from oxidation or reaction with cellular metabolites, the vast majority result from spontaneous transformations involving side chains (Truscott & Friedrich, 2016).
Asn and Asp are referred to as “hot spots” for spontaneous degradation due to their prevalence at protein damage sites, and Asn is generally the more reactive of the two at physiological pH (Geiger & Clarke, 1987). In one major pathway, the Asp/Asn side chain acts as a nucleophile and attacks the backbone amide carbonyl, resulting in cleavage of the protein chain and formation of a fragment with a C-terminal cyclic anhydride or imide (Figure 1, top). While the anhydride hydrolyzes rapidly, the C-terminal imide can persist and has been shown to target the cleavage product containing it for ubiquitination and proteasomal degradation (Ichikawa et al., 2022). In a related pathway (Figure 1, bottom), the polarity of the above reaction is reversed, and the backbone amide nitrogen at position i + 1 attacks the Asp/Asn side-chain carbonyl to form an intact protein chain with an aspartyl succinimide (Geiger & Clarke, 1987). Hydrolysis of the succinimide intermediate can occur from two sites, resulting in a mixture of α- and β-linked Asp residues, with the β-linked isoaspartic acid (isoAsp) product favored ~3:1 over α-linked Asp (Geiger & Clarke, 1987). Epimerization of the succinimide may also occur prior to hydrolysis, leading to a mixture of four possible isomeric products.
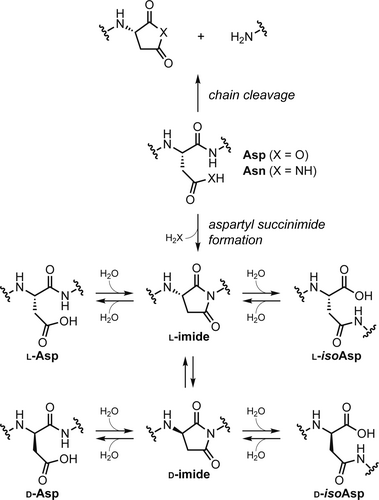
While protein chemical modifications associated with aspartyl succinimide formation can be innocuous in some cases, they have been shown to play important biological roles. Deamidation and isomerization have been observed in lens crystallins, where they accumulate in an age-dependent manner and have been implicated in age-related cataract formation (Fujii et al., 1994, 2011, 2016; Lampi et al., 2014; Lyon et al., 2019; Warmack, Shawa, et al., 2019). Degradation products associated with aspartyl succinimide have also been found to exist at elevated levels in tau and amyloid beta (Aβ) peptides isolated from the brains of Alzheimer's disease patients (Hasegawa et al., 1992; Lambeth et al., 2019; Roher et al., 1993; Watanabe et al., 1999). Beyond biological relevance in aging and disease, there is a growing evidence that both aspartyl succinimide and isoAsp may also exist as a defined backbone post-translation modification in some systems (Cámara-Artigas et al., 2010; Choi et al., 2022; Curnis et al., 2006; Kumar et al., 2016; Mallagaray et al., 2019; Wong et al., 2020).
In contrast to extensive data on the functional significance of spontaneous reactions at Asp/Asn, little is known about the impact of the resulting altered backbone composition on individual protein properties at a molecular level. The study of material isolated from biological sources or artificially aged under harsh conditions has led to information on sites prone to isomerization, but these proteins can only be isolated in small quantities and as complex mixtures. Mutagenesis can be used to examine effects of site-specific deamidation (i.e., substitution of Asn with Asp); however, this method cannot provide the corresponding d-Asp, l-isoAsp, or d-isoAsp isomeric products. The potential importance of this limitation is underscored by results obtained from biophysical studies of human crystallin proteins and variants, where Asn → Asp substitution has been observed to alter structure, stability, or aggregation propensity in some family members but not others (Forsythe et al., 2019; Guseman et al., 2021; Lampi et al., 2006; Pande et al., 2015; Takata et al., 2008).
Protein preparation via total chemical synthesis (Agouridas et al., 2019) or semisynthesis (Thompson & Muir, 2020) enables complete control over chemical composition throughout the chain and overcomes limitations inherent to reliance on natural biosynthetic machinery. This control includes the potential for site-specific incorporation of all possible isomeric backbone connectivities resulting from formation and subsequent hydrolysis of an aspartyl succinimide. Although widely used to generate short peptides for fundamental in vitro studies of succinimide formation and degradation as well as enzymatic repair of isomerized backbones, the application of chemical synthesis to interrogate isomeric backbone connectivity in the context of intact proteins is rare. In efforts involving intrinsically disordered sequences, site-specific incorporation of isomerized Asp residues has been shown to impact aggregation propensity in Aβ (Shimizu et al., 2002; Warmack, Boyer, et al., 2019) as well as islet amyloid polypeptide (Nguyen et al., 2017). In one example involving a defined tertiary fold, semisynthesis was used to produce variants of the enzyme ribonuclease A in which an Asp residue near the C-terminus of the chain was replaced by all possible isomeric analogs (Sakaue et al., 2017). Functional assays revealed a complete loss of catalytic activity from for all changes to backbone composition at the site in question; however, insolubility stymied attempted experiments aimed at understanding the molecular origins of this qualitative result (Sakaue et al., 2017).
To our knowledge, no prior study has reported a systematic examination of the consequences of site-specific Asp isomerization on protein structure or folding thermodynamics. In an effort to address this gap, we report here the total chemical synthesis, biophysical characterization, and NMR structural analysis of variants of the B1 domain of Streptococcal protein G (GB1) in which all possible Asp isomers as well as an aspartyl succinimide are individually incorporated at a position in the sequence prone to spontaneous degradation. Subtle effects on the fold of the protein variants resulting from backbone alterations were observed and accompanied by more significant impacts on folded stability. Particularly surprising is the observation that canonical backbone connectivity does not give rise to the most thermodynamically stable fold in this protein sequence.
2 RESULTS AND DISCUSSION
We selected the well-studied protein GB1 as a host sequence to quantify structural and thermodynamic effects of site-specific aspartic acid isomerization (Björck & Kronvall, 1984). GB1 adopts a compact tertiary fold (Figure 2a) in which a four-stranded β-sheet packs against an α-helix, and these structural elements are connected by two turns and two loops (Gronenborn et al., 1991). Prior work has reported a number of variants of GB1 with engineered backbone compositions, revealing the fold can tolerate diverse forms of non-canonical residue content (Odaert et al., 1999; Reinert et al., 2012, 2013; Walters et al., 2017). The propensity of a given Asp or Asn residue in a protein to spontaneously form an aspartyl succinimide depends on local sequence, conformational disposition, and solution conditions (Clarke, 1987; Stephenson & Clarke, 1989). In general, the reaction occurs more readily at sites where the C-terminal flanking residue is Gly and the Asx-Gly motif is found in a flexible, solvent-exposed region of the folded protein. In GB1, the loop connecting the α-helix to the C-terminal hairpin contains both Asn-Gly (N37-G38) and Asp-Gly (D40-G41) motifs that are potentially prone to aspartyl succinimide formation and subsequent isomerization (Figure 2a). Indeed, peptide bond cleavage involving cyclic imide formation has been previously observed at two positions in GB1 as evidenced by NMR and mass spectrometry (A.M. Gronenborn, personal observation). Therefore, we set out to synthesize and characterize a series of GB1 variants containing each of the four possible isomeric forms of Asp at position 40: l-Asp, d-Asp, l-isoAsp, and d-isoAsp (Figure 2b).
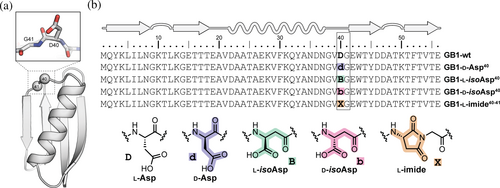
Wild-type GB1 and its Asp40 isomers were prepared by chemical synthesis, adapting Fmoc solid-phase methods previously developed for other backbone-modified variants of the domain (Reinert et al., 2012, 2013). Each protein was purified by preparative HPLC, and the identity and purity of isolated material assessed by analytical HPLC and ESI-MS. We additionally prepared a sample of wild-type GB1 by expression in E. coli for comparison to the synthetic material (Figure S1). The synthesis of the native protein (GB1-wt) and its epimer (GB1-d-Asp40) proceeded smoothly (Figures S2 and S3); however, the initial attempt to synthesize β-linked variant GB1-l-isoAsp40 under similar conditions resulted in the formation of a major product with mass corresponding to the intact protein with a loss of water. Purification of this dehydration product to homogeneity (Figure S6) and subsequent analysis by NMR and HPLC, detailed below, led us to assign it as the aspartyl succinimide at Asp40Gly41 with l-Asp derived stereochemical configuration (GB1-l-imide40-41). The succinimide was found to be quite stable in aqueous conditions at neutral pH, where 1H NMR analysis showed no evidence for a change in the sample composition after 24 h at room temperature (Figure S7). For comparison, aspartyl succinimide hydrolysis in a simple unstructured peptide takes place with a half-life of ~8 h under these conditions (Geiger & Clarke, 1987). This result suggests the local context of the GB1 tertiary fold provides a substantial degree of protection from succinimide reaction with water and motivated us to examine the folded structure and stability of this variant alongside the isomerization products that can form from it.
On-resin aspartyl succinimide formation is a well-documented side reaction in solid-phase peptide synthesis, occurring upon prolonged treatment of protected Asp residues with base (Behrendt et al., 2016). While typically a minor side reaction, base-mediated on-resin cyclization is much more rapid for β-linked ester-protected Asp residues compared to α-linked counterparts (Vadasz et al., 1996). Based on this, we hypothesized the high-yielding conversion to the succinimide observed above was a result of the presence of an isoAsp-Gly motif in the protected polypeptide chain on resin during 39 cycles of subsequent elongation (Figure S8). Interestingly, no evidence was observed in the crude material after cleavage for adducts resulting from the opening of the ring by 4-methylpiperidine during remaining Fmoc deprotections. To prevent the susceptible isoAsp moiety from reacting after it was introduced and provide access to the remaining GB1 variants, we utilized a 2,4-dimethoxybenzyl (Dmb) protecting group (Figure S8) to block the backbone amide nitrogen of Gly41 until acidic cleavage from resin (Cardona et al., 2008). This change to the synthetic strategy enabled isolation of variants GB1-l-isoAsp40 and GB1-d-isoAsp40 (Figures S4 and S5).
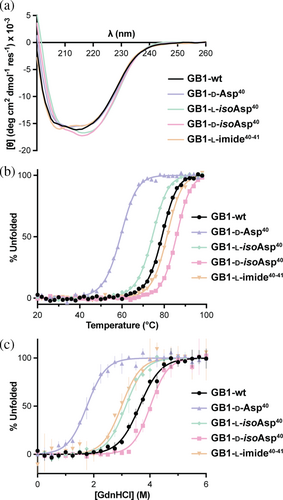
With the four authentic GB1 Asp40 isomers in hand alongside the aspartyl succinimide, we undertook a comparative investigation of the properties of these proteins. We first subjected each to analysis by circular dichroism (CD) spectroscopy. CD spectra of 40 μM protein in 20 mM sodium phosphate at pH 7 at 20°C are similar among all variants (Figure 3a), suggesting minimal impact on the overall fold of the domain. In contrast, noticeable changes in thermodynamic stability were observed, as evidenced in thermal denaturation experiments monitoring ellipticity at 220 nm (Figures 3b and S9). Fits to a two-state folding model yielded temperature midpoints (Tm) varying over a range of 26°C (Table 1). As a control, synthesized GB1 and E. coli-expressed GB1 exhibited identical Tm values within experimental error (Figure S10). Tm values for GB1-l-isoAsp40 and GB1-d-Asp40 were reduced by 5 and 20°C, respectively, while the Tm value for GB1-d-isoAsp40 was increased by 7°C relative to the wild-type protein. The cyclic imide variant exhibited an only slightly enhanced Tm value compared to wild-type GB1.
| Protein | Tm (°C)b | ΔTm (°C)c | Cm (M)d | m (kcal mol −1 M−1)e | ΔG°N-U (kcal mol−1)f | ΔΔG°N-U (kcal mol−1)g |
|---|---|---|---|---|---|---|
| GB1-wth | 78.9 ± 0.4 | – | 3.64 ± 0.07 | 1.6 ± 0.2 | 5.7 ± 0.7 | – |
| GB1-wti | 78.2 ± 0.5 | −0.7 ± 0.6 | n.d. | n.d. | n.d. | n.d. |
| GB1-d-Asp40 | 59.3 ± 0.3 | −19.6 ± 0.5 | 1.8 ± 0.1 | 1.7 ± 0.4 | 3.0 ± 0.8 | −2.7 ± 1.1 |
| GB1-l-isoAsp40 | 74.4 ± 0.2 | −4.5 ± 0.5 | 3.14 ± 0.05 | 1.7 ± 0.2 | 5.4 ± 0.6 | −0.3 ± 0.6 |
| GB1-d-isoAsp40 | 85.7 ± 0.7 | 6.8 ± 0.8 | 4.01 ± 0.07 | 1.9 ± 0.3 | 7.6 ± 1.1 | 1.9 ± 1.4 |
| GB1-l-imide40-41 | 81.3 ± 0.4 | 2.4 ± 0.6 | 3.0 ± 0.1 | 1.7 ± 0.4 | 5.2 ± 1.3 | −0.5 ± 1.4 |
- a Reported uncertainties are standard errors obtained from fits to experimental data or determined by error propagation (ΔTm, ΔΔG°N-U).
- b Temperature midpoint of thermal unfolding transition (Figure 3b).
- c Change in Tm relative to wild-type GB1 prepared by heterologous expression in E. coli.
- d Concentration midpoint of chemical unfolding transition in the presence of guanidinium chloride (Figure 3c).
- e Dependence of folding free energy on concentration of denaturant.
- f Free energy of unfolding at 25°C.
- g Change in free energy of unfolding relative to wild-type GB1 prepared by heterologous expression in E. coli.
- h Material obtained by heterologous expression in E. coli.
- i Material obtained by total chemical synthesis.
Thermodynamic stability was also assessed by guanidinium chloride (GdnHCl)-mediated chemical denaturation, monitoring intrinsic Trp fluorescence (Figures 3c and S11). The data were fit to a two-state folding model yielding an apparent unfolding free energy (ΔG°N-U) of 5.7 ± 0.7 kcal/mol for GB1-wt and ΔG°N-U ranging over ~5 kcal/mol around that value across the variant series (Table 1). GB1-l-imide40-41 and GB1-l-isoAsp40 showed folded stability similar to wild-type within uncertainty, while GB1-d-Asp40 was destabilized by 2.7 kcal/mol. The GB1-d-isoAsp40 variant, in which the backbone is both isomerized and epimerized, was unique among the series in showing a greater folded stability than the natural protein, with an increase in unfolding free energy of 1.9 kcal/mol.
Collectively, the above results revealed that alterations in backbone composition at Asp40 in GB1 led to measurable impacts on the folded stability of the protein. To probe whether these thermodynamic changes correlate with changes to folded structure, each protein was subjected to NMR analysis. As global isotopic labeling is impractical for synthetic samples, we relied exclusively on 2-dimensional homonuclear 1H experiments. We recorded solvent-suppressed 1H/1H TOCSY, 1H/1H magnitude COSY, and 1H/1H NOESY experiments, which enabled complete assignment of backbone and side chain resonances for each variant.
The absence of signal for the Gly41 amide proton in the putative succinimide and presence of this resonance for every other variant was consistent with the assigned covalent connectivity of the dehydration product. Given the possibility of epimerization during synthesis, we conducted additional experiments to establish the stereochemistry of the noncanonical residue. Previous kinetic studies involving aspartyl succinimide containing small peptides revealed that hydrolysis is ~20-fold faster than epimerization under physiological conditions (Geiger & Clarke, 1987). Thus, we assumed that the products obtained from the hydrolysis of the succinimide containing GB1 variant could be used to infer its stereochemistry. The l-configuration should yield a mixture of predominantly l-Asp and l-isoAsp hydrolysis products, while the d-configuration a mixture of d-Asp and d-isoAsp. The GB1 succinimide was incubated for 36 h at 37°C in 20 mM phosphate buffer, pH 7, and ESI-MS indicated that the protein was ~30% hydrolyzed (Figure S12). Isomers were resolved by HPLC (Figure S13), and GB1-wt and GB1-l-isoAsp40 were identified, with no evidence for GB1-d-Asp40 or GB1-d-isoAsp40 to be present (Figure S14). These data confirmed that the GB1 aspartyl succinimide had a stereochemical configuration matching the protected l-isoAsp monomer used in its synthesis.
For all variants, Hα chemical shift differences (CSD) compared to predicted random coil values and to the chemical shifts of GB1-wt (Figures 4 and S15) revealed that only very small differences are present for the loop resonances where the modified Asp40 residue resides, suggesting structural differences were restricted to this region of the protein. Structure calculations were carried out for all GB1 variants based on 855–939 NOE-derived distance restraints (19–21 total restraints per residue, Table S1). To assess the accuracy of structures determined using exclusively homonuclear measurements, the solution structure obtained for GB1-wt was compared to an ensemble consisting of four previously reported x-ray structures of the protein (Franks et al., 2006; Frericks Schmidt et al., 2007; Gallagher et al., 1994). The resulting overlay revealed that the NMR ensemble calculated here showed good agreement with the x-ray structures, albeit slightly inferior to a prior NMR structure of GB1 (Kuszewski et al., 1999) determined using isotopically labeled protein and a more extensive collection of experimental measurements (Figure S16).
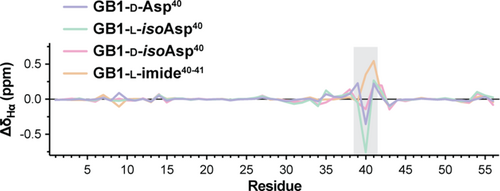
Comparison of the NMR ensembles among the variants showed the overall tertiary fold was conserved across the series (Figures 5a and S17). Pairwise backbone rmsd values for ensemble-to-ensemble overlays with GB1-wt ranged from 0.6 to 0.7 Å and were only slightly higher than corresponding pairwise backbone rmsd within each NMR ensemble (0.4–0.5 Å). Consistent with the Hα CSD analysis, subtle structural differences were observed in the loop where the backbone composition was altered (Figures 5b and S18).
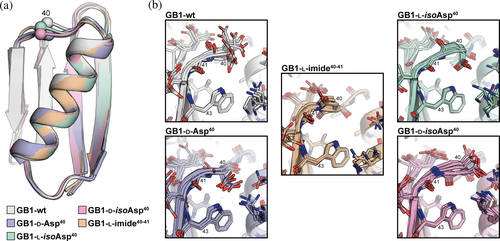
Evidence for an altered loop conformation in some variants can also be drawn from the cross-peaks involving the Trp43 NεH resonance observed in the NOESY spectra (Figure S19). Inversion of the Asp40 stereocenter in GB1-d-Asp40 is expected to position the Asp side chain methylene group close to the Trp43 indole. In contrast, extension of the backbone in the β-linked variants should yield new contacts with the Trp43 side chain. In GB1-d-isoAsp40, the backbone amide between residues 40 and 41 exhibits a flipped orientation relative to GB1-wt, and the carbonyl oxygen from residue 40 may form a hydrogen bond to the Trp indole NH. A similar conformation has been observed previously in an x-ray structure of a GB1 variant bearing an artificial β3-residue of similar connectivity to d-isoAsp at residue 40 (Reinert et al., 2013). In GB1-l-isoAsp40, the side chain carboxylate (formerly the backbone amide) may form a polar contact with the Trp43 indole NH. This is consistent with a significant downfield shift of the Trp indole NH resonance in this GB1 variant compared to the others (Figure S20). The NMR structure obtained for GB1-l-imide40-41 provides insight into the molecular origins of the observed stability of this variant to hydrolysis. One face of the succinimide ring is sterically blocked by the side chain of Glu56, and the other occluded by the side chain of Trp43 (Figure S21).
3 CONCLUSIONS
In summary, we report here a systematic examination of the impacts of site-specific dehydration and isomerization on the folded structure and thermodynamic stability of the protein GB1. Total chemical synthesis provided a series of samples of defined composition corresponding to the wild-type protein alongside variants that are formed from an aspartyl succinimide intermediate in a solvent-exposed loop. Biophysical characterization revealed that isomerization at Asp40 can result in significant changes in GB1 folded stability. While some forms of backbone alteration are detrimental to the overall stability, it is noteworthy that the canonical backbone is not the most thermodynamically stable fold among the variant series. The Asp→d-isoAsp substitution results in an increase in thermal stability by 7°C and a 1.9 kcal/mol larger free energy of unfolding. Analysis of folded structure by NMR showed that the overall fold of GB1 is minimally perturbed by the Asp cyclization and isomerization at position 40, although subtle differences are observed in backbone conformation and contacts involving the modified loop. These structural differences, combined with potential changes to backbone conformational preferences, may give rise to the observed thermodynamic effects of cyclization and isomerization.
The finding that a variant of GB1 that has undergone both epimerization and isomerization of the backbone compares more favorably to the canonical protein than a variant with one or the other modification alone is supported by results from prior work on biological repair pathways for isomerized Asp residues. In nature, enzymatic methylation of the l-isoAsp carboxylate is followed by spontaneous conversion to the succinimide, which opens to give a mixture of Asp and l-isoAsp; iteration of this process shifts the distribution at the site in question back toward Asp (McFadden & Clarke, 1987). These methyltransferase repair enzymes recognize both l-isoAsp and d-Asp residues but not l-Asp or d-isoAsp (Murray & Clarke, 1984). The structural basis for this substrate selectivity has been investigated through a combination of crystallography and modeling studies (Griffith et al., 2001), which revealed similarities between l-isoAsp/d-Asp and between l-Asp/d-isoAsp that make the former viable substrates for the enzyme but the latter not. In the case of the GB1 variants examined here, the overall conformation of the modified loop is constrained by the tertiary fold and consistent across the series; however, differences are observed in positioning of the side chain on the isomerized residue (Figure 5). The side chains of the Asp and d-isoAsp residues project toward solvent, while those of l-isoAsp and d-Asp residues project toward the hydrophobic core.
Although the scope of our present study was limited to a single site in a single protein, the structural and stability effects in the compact and simple GB1 fold underscore the vital role of backbone composition in protein structure and stability. The fact that one of the noncanonical backbone connectivities resulting from isomerization at Asp leads to a thermodynamic advantage in this protein lends support to the idea that these modifications may play roles beyond simple protein damage. Our results, alongside those reported previously for RNAse A (Sakaue et al., 2017), highlight the power of protein chemical synthesis to uniquely investigate molecular level consequences of backbone changes in the context of intact folded proteins. The present findings also open a number of questions for future work. How do the structure and stability of other proteins respond to site-specific changes to backbone composition and how do these influence biological properties, particularly for long-lived proteins and those implicated in disease? Are the effects of multiple modified residues additive or are there critical positions in a single sequence or fold? Continued efforts to bring the technologies of chemical protein synthesis to bear in this area will aid to address these questions and provide fundamental insights into common pathways of spontaneous protein modification.
4 MATERIALS AND METHODS
4.1 Materials
Fmoc-l-Asp(OtBu)-OH [β-tert-butyl ester], Fmoc-d-Asp(OtBu)-OH [β-tert-butyl ester], Fmoc-l-Asp-OtBu [α-tert-butyl ester], and Fmoc-d-Asp-OtBu [α-tert-butyl ester] were purchased from Novabiochem. HCTU [O-(1H-6-chlorobenzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate], PyAOP [(7-azabenzotriazol-1-yloxy)trispyrrolidinophosphonium hexafluorophosphate], HGlu-HMPB NovaPEG resin, and other protected amino acid derivatives were purchased from Novabiochem or ChemImpex. Solvents and all other chemicals were purchased from Fisher or Millipore-Sigma and used without further purification. Biologically produced wild-type GB1 was expressed in E. coli and purified following published protocols (Guseman & Pielak, 2017). HPLC experiments were performed using Hitachi instrumentation equipped with Phenomenex Jupiter C18 columns using gradients between 0.1% TFA in water (solvent A) and 0.1% TFA in acetonitrile (solvent B). Analytical-scale separations made use of a 4.6 × 250 mm column (5 μm particle size, 300 Å pore size) and a flow rate of 1 mL/min. Preparative-scale separations made use of a 21.2 × 250 mm column (10 μm particle size, 300 Å pore size) and a flow rate of 15 mL/min. ESI-MS experiments were performed on a Thermo Fisher Oribtrap Exploris 240 spectrometer.
4.2 Synthesis of wild-type GB1
Protein GB1-wt was synthesized manually at room temperature on HGlu(OtBu) HMPB NovaPEG resin (0.1 mmol). Prior to the start of the synthesis, the resin was swelled in DMF for 30 min. For a standard coupling reaction, Fmoc-protected amino acid (6 equiv) and HCTU (5.8 equiv) were dissolved in NMP (6 mL, 0.1 M monomer), and DIEA (10 equiv) was added. The solution was allowed to preactivate for 2 min, transferred to resin, and the reaction stirred at room temperature for 30 min. The monomer coupled at position 40 was Fmoc-l-Asp(OtBu)-OH. Residues Thr51-Lys50 and Thr25-Ala24 were introduced using pseudoproline dipeptides, and residue Glu15 was double coupled. Fmoc deprotection reactions were carried out by treatment of resin with 20% 4-methylpiperidine in DMF at room temperature for 10 min. The resin was rinsed three times with DMF between each step. Following the final Fmoc deprotection, the resin was rinsed three times each with DMF, DCM, and methanol, then dried under vacuum prior to cleavage.
4.3 Synthesis of GB1 d-Asp and succinimide variants
Proteins GB1-d-Asp40 and GB1-l-imide40-41 were synthesized at room temperature in automated fashion using a Biotage Alstra synthesizer on HGlu(OtBu) HMPB NovaPEG resin (0.1 mmol). Fmoc-amino acids were dissolved in DMF (0.3 M), and HCTU was dissolved in NMP (0.2 M) prior to the start of the synthesis. Standard coupling reactions consisted of Fmoc-protected amino acid (6 equiv), HCTU (5.8 equiv), and DIEA (10 equiv) in a 2:3 mixture of DMF and NMP (5 mL). Following preactivation for 1.5 min, the solution was added to resin and the reaction agitated for 45 min at room temperature. The monomer coupled at position 40 was Fmoc-d-Asp(OtBu)-OH in the synthesis of GB1-d-Asp40 and Fmoc-l-Asp-OtBu in the synthesis of GB1-l-imide40-41. Residues Thr51-Lys50 and Thr25-Ala24 were introduced using pseudoproline dipeptides. Fmoc deprotection reactions were carried out by treatment of resin with 20% 4-methylpiperidine in DMF for two 5-min cycles. The resin was rinsed three times with DMF between each step. Following the final Fmoc deprotection, the resin was rinsed three times each with DMF, DCM, and methanol, then dried under vacuum prior to cleavage.
4.4 Synthesis of GB1 isoAsp variants
Proteins GB1-l-isoAsp40 and GB1-d-isoAsp40 were synthesized manually using a combination of microwave assisted (CEM MARS) and room temperature methods on HGlu(OtBu) HMPB NovaPEG resin (0.075 mmol). Prior to the start of the synthesis, the resin was swelled in DMF for 30 min. For a standard coupling reaction, Fmoc-protected amino acid (6 equiv) and HCTU (5.8 equiv) were dissolved in NMP (6 mL, 0.1 M monomer), and DIEA (10 equiv) was added. The solution was allowed to preactivate for 2 min, then transferred to resin. For the segment of the protein corresponding to residues Ala48-Thr55, coupling reactions were carried out under microwave heating, with a 1.5 min ramp to 90°C and a 2 min hold at that temperature. For the remainder of the protein, coupling reactions were carried out at room temperature for 45 min. Fmoc deprotection reactions were carried out by treatment of resin with 20% 4-methylpiperidine in DMF. For the segment of the protein corresponding to residues Ala48-Thr55, Fmoc deprotections were carried out under microwave heating, with a 1.5 min ramp to 90°C and a 1 min hold at that temperature. For the remainder of the protein, Fmoc deprotections were carried out at room temperature for 10 min. The monomer coupled at position 41 was Fmoc-(Dmb)Gly-OH. The monomer coupled at position 40 was Fmoc-l-Asp-OtBu and Fmoc-d-Asp-OtBu in the synthesis of GB1-l-isoAsp40 and GB1-d-isoAsp40, respectively; these residues were double coupled using PyAOP (6 equiv) in place of HCTU. Residues Thr51-Lys50 and Thr25-Ala24 were introduced using pseudoproline dipeptides, and residue Glu15 was double coupled. The resin was rinsed three times with DMF between each step. Following the final Fmoc deprotection, the resin was rinsed three times each with DMF, DCM, and methanol, then dried under vacuum prior to cleavage.
4.5 Cleavage and purification of GB1 variants
All proteins were cleaved from the resin by treatment with a solution composed of 94% TFA, 2.5% ethane dithiol, 2.5% water, and 1% triisopropylsilane by volume. After 3 h, the solvent was separated from the resin by filtration and evaporated under a stream of nitrogen gas. Crude protein was precipitated by addition of cold diethyl ether, centrifuged to isolate the pellet, then decanted to remove the ether. The pellet was dissolved in a solution composed of 10% acetonitrile in water with 0.1% TFA (for GB1-wt, GB1-d-Asp40, and GB1-l-imide40-41) or in a solution composed of 6 M guanidinium chloride in water (for GB1-l-isoAsp40 and GB1-d-isoAsp40), then sonicated for 30 min. Synthetic proteins were purified by preparative reverse-phase HPLC chromatography. Identity and purity of isolated material was assessed by analytical HPLC and ESI-MS (Figures S1–S6). Following lyophilization, purified proteins were stored under vacuum as powders or dissolved in water and stored frozen.
4.6 CD spectroscopy
CD experiments were performed using an Olis DSM17 CD Spectrometer. Protein stock solutions were prepared in water, and the concentration of protein was determined by UV absorbance (Gill & von Hippel, 1989). An appropriate volume from the stock solution was aliquoted and lyophilized. The lyophilized powder was reconstituted in 300 μL of 20 mM sodium phosphate buffer pH 7.0 at a final protein concentration of 40 μM. CD scans were recorded at 20°C in the wavelength range 200–260 nm with 1 nm increments, 2 nm bandwidth, and 3 s integration time per data point. CD scan data were smoothed using GraphPad Prism. For thermal denaturation experiments, ellipticity was measured at 220 nm over a temperature range of 4–98°C in 2°C increment with a 2-min equilibration time between each step and a 0.05°C dead band. Temperature denaturation data were fit to a two-state unfolding model using GraphPad Prism to obtain melting temperatures (Tm).
4.7 Fluorescence spectroscopy
Fresh samples were prepared composed of 5 μM protein in 20 mM sodium phosphate pH 7.0 with 0–6 M guanidinium chloride (0.25 M increments). These samples were equilibrated overnight at 25°C and then dispensed in triplicate (20 μL per well) to a 384-well plate. Fluorescence intensity was recorded on a Tecan Spark multimode plate reader with an excitation wavelength of 295 nm (5 nm bandwidth) and emission wavelengths of 335 and 360 nm (5 nm bandwidth). The fluorescence intensity ratio at 360 versus 335 nm was plotted as a function of denaturant concentration and fit to a two-state folding model to obtain the free energy of unfolding (ΔGN-U), the concentration midpoint of the unfolding transition (Cm), and the dependence of folding free energy on denaturant concentration (m).
4.8 NMR sample preparation, data acquisition, and data processing
Lyophilized protein was dissolved in 20 mM sodium phosphate buffer pH 7.0 with 10% D2O and 0.1 mM DSS [3-(trimethylsilyl)-1-propanesulfonic acid sodium salt]. The pH of all samples was rechecked by pH meter, leading to observed values between 6.95 and 7.00 (uncorrected for presence of deuterium). Final protein concentrations were 0.12 mM for GB1-wt and GB1-d-Asp40, 0.18 mM for GB1-l-isoAsp40, 0.17 mM for GB1-d-isoAsp40, and 0.07 mM for GB1-l-imide40-41 based on integration of resolved signals in the 1D-1H spectrum for methyl groups from DSS and Val54. 1D-1H as well as 2D-1H/1H TOCSY (60 ms mixing time), NOESY (150 ms mixing time), and magnitude COSY spectra were acquired at 298 K on a 700 MHz Bruker Avance III NMR spectrometer equipped with a triple resonance inverse probe. Solvent suppression was achieved using excitation sculpted gradient pulse sequences, with parameters O1, P1, and SP1 optimized separately for each set of experiments. For each 2D experiment, 2048 data points were collected in the direct dimension, and 512 data points were collected in the indirect dimension. NMR data were processed using TopSpin (Bruker), phased, and calibrated to the DSS internal standard.
4.9 NMR data analysis and structure determination
NMR data analysis was performed using the program NMRFAM-SPARKY (Lee et al., 2015). Resonances of each protein were manually assigned, and peaks in the NOESY spectra were manually picked and integrated in SPARKY. Structure calculations were performed by simulating annealing using the program Ambiguous Restraints for Iterative Assignment (ARIA, version 2.3) (Rieping et al., 2007) in conjunction with Crystallography & NMR System (CNS, version 1.2) (Brunger, 2007). Topology and parameter definitions for noncanonical residues and links were generated based on analogous atom types already present in the topology and parameter files distributed with the program. ARIA run settings were modified from program defaults as described to improve accuracy and convergence (Mareuil et al., 2015). Backbone φ dihedral restraints were derived from measured 3JHα-HN coupling constants for resolved amide doublets in the 1D-1H spectrum (φ = −65° ± 25° for J ≤ 6.0 Hz and φ = −120° ± 40° for J ≥ 8.0 Hz). NOE distance restraints were generated automatically by the ARIA program, starting from a list of 1H resonances and a set of unassigned integrated NOESY peaks. The structure calculation proceeded in two rounds. The first ARIA run was initiated from a fully extended chain and included as input the resonance list, NOESY peak list, and dihedral restraints. The second ARIA run proceeded identically to the first, with the following three changes to program input and settings. The calculation was initiated from the run 1 ensemble. Backbone hydrogen bonds restraints were included based on the structure ensemble obtained from run 1 (1.9 ± 0.1 Å H − O distance and 2.9 ± 0.2 Å N − O distance for each such contact). Spin diffusion correction (Linge et al., 2004) was carried out using a calculated rotational correlation time from the lowest energy model in the run 1 ensemble by the program HYDROPRO (García de la Torre et al., 2000). The final set of 10 lowest energy structures resulting from the second run was taken as the final NMR ensemble for each variant (see Table S1 for structure calculation statistics) and used for subsequent analysis. Ensemble coordinates and additional data are deposited in the PDB (accession codes GB1-wt: 8UM7, GB1-d-Asp40: 8UM9, GB1-l-isoAsp40: 8UMS, GB1-d-isoAsp40: 8UMA, and GB1-l-imide40-41: 8UMB) and BMRB (accession codes GB1-wt: 31114, GB1-d-Asp40: 31115, GB1-l-isoAsp40: 31118, GB1-d-isoAsp40: 31116, and GB1-l-imide40-41: 31117). Pairwise rmsd values for ensemble to ensemble overlays were calculated using the program Ensemblator v3 (Brereton & Karplus, 2018). Reference random coil chemical shift values for wild-type GB1 used to generate plots in Figure 4a were calculated using the Poulsen IDP/IUP Random Coil Chemical Shift Server (https://spin.niddk.nih.gov/bax/nmrserver/Poulsen_rc_CS/) (Kjaergaard & Poulsen, 2011).
AUTHOR CONTRIBUTIONS
W. Seth Horne: Conceptualization; investigation; writing – review and editing; funding acquisition; formal analysis; writing – original draft. Shelby L. Heath: Conceptualization; investigation; writing – original draft; writing – review and editing; formal analysis. Alex J. Guseman: Conceptualization; investigation; writing – review and editing; formal analysis. Angela M. Gronenborn: Conceptualization; writing – review and editing; funding acquisition.
ACKNOWLEDGMENTS
Funding for this work was provided by the National Institutes of Health (R01GM107161 and R35GM149220 to W. Seth Horne, R01EY030057 to Angela M. Gronenborn). Alex J. Guseman is a Merck fellow of the Life Science Research Foundation and holds a Postdoctoral Enrichment Program Award from the Burroughs Wellcome Fund.