Structural evidence suggests that antiactivator ExsD from Pseudomonas aeruginosa is a DNA binding protein
Abstract
The opportunistic pathogen P. aeruginosa utilizes a type III secretion system (T3SS) to support acute infections in predisposed individuals. In this bacterium, expression of all T3SS-related genes is dependent on the AraC-type transcriptional activator ExsA. Before host contact, the T3SS is inactive and ExsA is repressed by the antiactivator protein ExsD. The repression, thought to occur through direct interactions between the two proteins, is relieved upon opening of the type III secretion (T3S) channel when secretion chaperone ExsC sequesters ExsD. We have solved the crystal structure of Δ20ExsD, a protease-resistant fragment of ExsD that lacks only the 20 amino terminal residues of the wild-type protein at 2.6 Å. Surprisingly the structure revealed similarities between ExsD and the DNA binding domain of transcriptional repressor KorB. A model of an ExsD-DNA complex constructed on the basis of this homology produced a realistic complex that is supported by the prevalence of conserved residues in the putative DNA binding site and the results of differential scanning fluorimetry studies. Our findings challenge the currently held model that ExsD solely acts through interactions with ExsA and raise new questions with respect to the underlying mechanism of ExsA regulation.
Introduction
The opportunistic pathogen Pseudomonas aeruginosa is the underlying cause of many serious and frequently fatal illnesses. It is the leading cause of nosocomial pneumonia and urinary tract infections, has been linked to severe corneal infections, and is the source of acute and chronic infections in predisposed individuals such as burn victims and cancer patients.1-5 The widespread emergence of antibiotic resistant strains of P. aeruginosa, particularly in hospital settings, has increased the pressure for the development of novel therapeutic options.6-8
Many gram-negative pathogens utilize a type III secretion system (T3SS), including Yesinia pestis, the Vibrio species, Salmonella species, and even plant pathogens such as Pseudomonas syringae. In P. aeruginosa, the T3SS has also emerged as a pivotal contributor to disease severity and persistence, particularly in acute infections. The T3S apparatus forms a syringe-like structure that, fueled by ATP hydrolysis, injects a number of cytotoxic proteins or effectors into host cells. While many structural components of the T3S machinery are broadly conserved, the translocated virulence factors may vary widely, as they have evolved to support the life style of the specific organism. In P. aeruginosa, at least four different cytotoxins (ExoS, ExoT, ExoU, and ExoY) enter the host cells via the T3SS. Inside the host these effectors trigger a range of events including a rearrangement of the actin cytoskeleton and the induction of apoptosis.9-13 Wild-type strains of P. aeruginosa produce either ExoS or ExoU but not both. This is remarkable, considering these two proteins are thought to be the most potent among the translocated virulence factors.14
Primary targeting of effector proteins to the secretion channel occurs via an amino terminal signal peptide. The debate is ongoing as to whether the targeting takes place at the level of the mRNA, after protein synthesis, or both.13, 15-17 In addition, some but not all effectors associate with cognate secretion chaperones. These chaperones may also assist in secretion targeting18 and maintaining the passenger proteins in a secretion-competent state by preventing their degradation and/or aggregation.19
In P. aeruginosa, transcription of all T3SS-related genes is directly dependent upon the AraC/XylS-type transcriptional activator ExsA. exsA− strains fail to produce functional T3SSs and are severely attenuated in acute infection models.20-22 AraC-type proteins feature characteristic DNA binding domains, containing two helix-turn-helix motifs. Many AraC-types possess a second domain that, upon ligand binding, mediates regulation of gene expression. Perhaps the most prominent member of this family is E. coli AraC, which uses a DNA looping mechanism for transcriptional repression.23 Bearing an amino-terminal regulatory domain and a carboxy-terminal DNA-binding domain the domain structure of ExsA mirrors that of AraC. Yet, unlike AraC, ExsA is not regulated through interactions with a small-molecule ligand such as arabinose, but through interactions with other macromolecules. As part of distinct signaling mechanisms the two proteins PtrA and ExsD directly bind to and repress ExsA. It is not known what aspect of ExsA function, DNA binding, dimerization, or recruitment of RNA polymerase, is affected by these proteins; and if PtrA and ExsD use the same mechanism to achieve their objectives. PtrA is produced in response to copper stress,24 while ExsD prevents the premature up-regulation of the expression of the T3SS before host cell contact. The ExsD-ExsA interactions are part of larger signaling cascade also involving the small secreted protein ExsE and its cognate T3SS chaperone ExsC. Together these four proteins ensure timely expression of the T3SS by coupling ExsA activation with the opening of the T3S channel. The T3SS is activated through direct contact between P. aeruginosa and host cells. Before T3SS activation, antiactivator protein ExsD is thought to sequester ExsA, while ExsC remains tightly bound to ExsE.25-29 Upon host cell contact, ExsE is translocated resulting in the release of ExsC. ExsC now sequesters ExsD forming a second tight protein–protein complex (Kd = 19 nM)29 and thus permitting up-regulation of ExsA-dependent gene expression.28 The role of ExsD as antiactivator of ExsA came to light when exsD− strains showed an accumulation of T3SS-associated toxins in the bacterial cytoplasm when the T3SS was inactive.25 Subsequently, direct ExsA-ExsD interactions were demonstrated in bacterial two-hybrid studies.25
At this point, our understanding of the molecular mechanism underlying the ExsACDE signaling cascade is very limited. We are particularly interested in characterizing the unusual interactions of ExsD with ExsC and ExsA. Unlike the commonly observed 2:1 chaperone to effector stoichiometry, ExsC and ExsD form a stable 2:2 complex; and represent a rare example of a T3SS chaperone associating with a protein not targeted for secretion.28, 29 The molecular basis for the ExsD-ExsA interactions is even more mysterious. The only evidence for direct interactions between ExsD and ExsA stems from the two-hybrid studies.25 Based on this finding, ExsD is thought to interfere with ExsA by either blocking its binding to DNA or by preventing the recruitment of RNA polymerase to the promoter site. Yet, evidence for either mechanism has remained elusive. To shed light into the molecular interactions that underlie this signaling cascade, we set out to characterize the crystal structures of the involved proteins and protein–protein complexes. Here we report the crystal structure of antiactivator protein ExsD. Unexpectedly, the amino-terminal domain of ExsD strongly resembles the DNA-binding domain of transcriptional regulator KorB, suggesting a more complex regulatory role for ExsD in the ExsACDE signaling cascade than originally presumed.
Abbreviations:
DSF, differential scanning fluorimetry; DTT, dithiothreitol; EMSA, electrophoretic mobility shift assay; IPTG, isopropyl-β-D-thiogalacto-pyranoside; MBP, maltose binding protein; PDB, Protein Data Bank; SAD, single anomalous dispersion; T3S, type III secretion; T3SS, type III secretion system; TCEP, tris(2-carboxyethyl) phosphine; TEV, tobacco etch virus.
Results
Crystal structure of the Δ20ExsD
Full-length ExsD proved recalcitrant to crystallization. Therefore, the full length protein was subjected to limited proteolysis with thermolysin. Remarkably, this experiment yielded a single highly protease-resistant product (Supp. Info. Fig. 1). Subsequent mass-spectrometric analysis revealed that the fragment encompassed all but the 20 amino-terminal residues of ExsD (Δ20ExsD). Δ20ExsD could be directly purified from the proteolysis reaction and readily crystallized. As the initial crystals gave only poor X-ray diffraction (>7 Å), we performed reductive methylation of the protein's lysine residues, a technique that is increasingly attempted to improve protein crystal quality.30 The modified sample crystallized under new conditions and gave dramatically improved X-ray diffraction. Seleno-methionine-substituted Δ20ExsD crystallized under the same condition and ultimately the structure was solved at a resolution of 2.6 Å using single anomalous dispersion (SAD) phasing. Data collection, structure solution, and refinement statistics are provided in Table I.
| Data collection statistics | |
| Δ20ExsD-Se-Met | |
| Molecules/a.u. | 3 |
| Wavelength (Å) | 0.9792 |
| Space group | P21212 |
| Unit cell parameters (Å) | a = 144.8; b = 69.35; c = 86.33 |
| Resolution (Å) | 30–2.6 |
| Total reflections | 299,234 |
| Unique reflections | 23,616 |
| Completeness (%)a | 92.5 (57.4) |
| Average I/σ | 18.3 (1.7) |
| Rmerge (%)b | 11 (39.4) |
| Phasing statistics (23–2.6 Å) | |
| Number of selenium atom sites | 9 |
| Overall FOM of phasing (RESOLVE) | 0.66 |
| R for FC vs. FP (%) | 27.0 |
| Refinement statistics | |
| Resolution range (Å) | 23–2.6 |
| R (%)c | 21.2 |
| Rfree (%)d | 27.1 |
| Root mean square deviation bond lengths (Å) | 0.006 |
| Root mean square deviation angles (°) | 1.0 |
| Temperature factor (Å2) | 49.2 |
| Number of protein atoms | 5754 |
| Number of solvent molecules | 136 |
- a The values in parentheses relate to the highest resolution shell from 2.69 to 2.6 Å.
- b Rmerge = Σ|I − 〈I〉|/ΣI, where I is the observed intensity, and 〈I〉 is the average intensity obtained from multiple observations of symmetry-related reflections after rejections.
- c R = Σ‖‖Fo| − |Fc‖‖/Σ|Fo|, where Fo and Fc are the observed and calculated structure factors, respectively.
- d Rfree defined in Ref.31.
Two views of the Δ20ExsD structure are presented in Figure 1. Reflecting the reported solution state of ExsD, there are three Δ20ExsD molecules in the asymmetric unit. A ribbon diagram of the trimer is presented in Figure 1(b) and a single Δ20ExsD molecule is drawn in Figure 1(a). In addition to the deleted first 20 amino acids, no electron density was observed for residues 21–36, suggesting that these are not ordered in free ExsD. Δ20ExsD may be divided into three distinct subdomains. A small helical amino terminal domain spans residues 37 to 109 and helices α-1 to α-5. The second domain, consisting of a small two-stranded beta sheet, β1 and β−2, and five alpha helices, α-6 and α-9 to α-12, encompasses amino acids 110–137 and 210–276. In the third domain, encompassing residues 139–208, helices α-7 and α-8 form an extensive coiled-coil domain that projects away from the remainder of the ExsD molecule. Coiled-coil domains are known to mediate protein oligomerization, yet the coiled-coil regions of ExsD contribute relatively little to trimer formation and assume slightly different conformations in all three molecules (Supp. Info. Fig. 2). The conformational flexibility in this part of the structure is also evident from the relatively weak electron density observed for residues 163–176 at the connected ends of α-7 and α-8 and a number of disordered side chains in this region. Taken together, these observations suggest that the biological role of the coiled-coil domains may go beyond aiding trimer formation and may facilitate other protein–protein contacts.
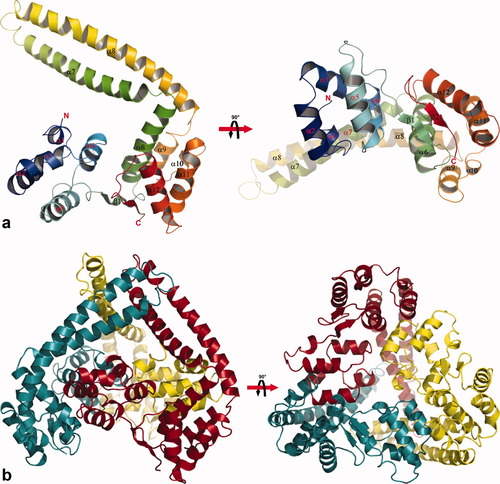
(a) Two orthogonal views of the rainbow-colored model of the Δ20ExsD crystal structure. Secondary structure elements are labeled and the termini are marked. (b) Reflecting the reported solution state of ExsD there are three molecules in the asymmetric unit. Protein–protein contact surfaces within the trimer amount to about 2000 Å2 for each molecule and involve all three sections of the protein. This figure was generated by PYMOL.32
ExsD resembles other bacterial transcriptional regulators
The refined model of Δ20ExsD was submitted to the DALI server,33 to identify related structures from the Protein Data Bank (PDB).34 Our initial search returned a number of structures with coiled-coil domains similar to that of Δ20ExsD. The top five hits gave Z-scores between 9.1 and 9.7 (a fit above Z = 2 is considered statistically significant) for the 69 overlapping amino acids forming the two extended helices. Most of the top-ranked coiled-coil regions belonged to larger enzymes with no functional relationship to ExsD. The second-ranked structure of the transcriptional activator AphA (PDB code: 1yg2) from Vibrio cholerae (Z = 9.4) stood out because of its functional similarities to ExsD. Dimeric AphA activates expression of a number of virulence-associated genes through interactions with transcription factor AphB and a specific DNA operator sequence.35 Even though ExsD was not thought to function through interactions with DNA the structural and functional parallels to AphA were striking. A second DALI search, this time without the coiled-coil domain, revealed a striking similarity between the amino terminal domain of Δ20ExsD and large parts of the DNA binding domain of transcriptional repressor protein KorB (see Fig. 2). The KorB structure can be divided into two subdomains, with residues 117–194 encompassing a small helical domain containing a classical helix-turn-helix DNA binding motif, while residues 194–252 form a second less conventional DNA binding domain. ExsD resembles the carboxyterminal domain of KorB, which mediates sequence specific KorB-DNA interactions.36 The root mean square deviation (RMSD) for the backbone atoms of the 52 overlapping residues is 2.6 Å resulting in a reported Z-score of 5.2.
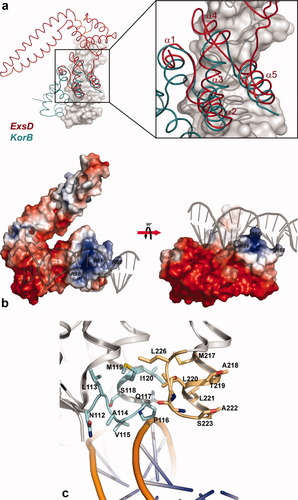
(a) Superposition of Δ20ExsD with the KorB-DNA complex. Two subdomains of transcriptional repressor KorB interact with the major grove of the operator sequence. The amino terminal domain of Δ20ExsD encompassing residues 37–108 and helices α1–α5 is structurally homologous to the carboxy-terminal domain of KorB (amino acids 194–252), which has been shown to confer specificity to the KorB-DNA interactions.36 DALI reported a Z-score of 5.2 and an RMSD of 2.6 Å for the backbone atoms of the 52 overlapping residues. (b) Electrostatic surface presentation of Δ20ExsD. The DNA molecule of the KorB-complex is also shown to mark the putative DNA binding site on Δ20ExsD. ExsD has a calculated isoelectric point of 5.15 and consequently displays large areas with negative surface potential. Remarkably, the putative DNA binding site of Δ20ExsD coincides with the only area of the protein with a significant concentration of positively charged residues. The arginine residues discussed in the text, such as R51, R58, and R86, are highlighted in the figure. (c) Close-up view of the second putative DNA binding site of ExsD. The cyan-colored section encompassing residues 112–120 appears poised for an interaction with the DNA backbone. Also highlighted are amino acids 217–226. This region would be positioned directly above the major groove of the DNA and might also be involved in binding. It is noteworthy that, although the surrounding areas are rich in acidic residues, no negatively charged residues are contained within either of the highlighted regions.
Based on the KorB-ExsD structural alignment a crude model of an ExsD-DNA complex was constructed by simply superimposing the putative DNA binding domain of ExsD with the corresponding residues of the KorB-DNA structure (PDB code: 1R71) (see Fig. 2). The resulting model appears to give a realistic approximation of the ExsD-DNA interface, as it produced no obvious clashes between protein and DNA. On the contrary, the DNA molecule passes in close proximity to the second globular domain of ExsD, which is not present in KorB, suggesting additional DNA contacts may be mediated by that part of the protein. Our model is also supported by the charge distribution of ExsD [Fig. 2(b)]. The putative DNA binding site constitutes the only extensive surface area of the protein with positive electrostatic potential, while most of the ExsD surface is covered by negatively charged residues, reflecting its calculated pI of 5.15. The ExsD-DNA model is additionally supported by the distribution of conserved residues in ExsD. There is a significant degree of divergence among known ExsD proteins with sequence identities ranging from 35 to 38% (Fig. 3). Yet, a number of residues predicted by the model to mediate contacts between the amino terminal domain of ExsD and DNA are either strictly conserved or, at the very least, retain their polar character. In particular, the ExsD-DNA model explains the concentration of conserved residues between amino acids Ile-47 and Arg-62. These residues form helix α-2, which, in our model, contributes a number of close contacts with the DNA. Ser-49, Arg-51, Gln-52, and Arg-58 appear to be ideally positioned to form additional hydrogen-bonding contacts. Gln-52 is poised for interactions within the major groove, while Arg-58 appears to mediate interactions with the DNA backbone. The side chains of Arg-51 together with Arg-86 also point directly into the major groove of the DNA double helix. While Arg-86 is strictly conserved, Arg-51 is replaced by a glutamine in most ExsD orthologs. Both glutamine and arginine residues feature prominently in DNA binding sites, because their side-chains may not only interact with the phosphodiester groups of the backbone but also convey binding specificity through interactions with DNA bases.37 Therefore, the difference in position 51 may well reflect a difference in the DNA recognition sequence among ExsD orthologs.

Sequence alignment of known ExsD orthologs. The secondary structure elements obtained from the Δ20ExsD structure are displayed above the sequence. Residues that constitute part of the ExsD-DNA interface in the modeled complex are marked by a triangle below the sequence.
Somewhat less compelling is the level of sequence conservation in the second putative DNA binding domain of ExsD and the primarily negative electrostatic surface potential in this region suggests weaker interactions. Noteworthy is a structural motif formed by conserved strand β-1, helix α-6, and the connecting loop [Fig. 2(c)] that appears to directly contact the DNA backbone. The interacting residues are poorly conserved. However, precise sequence conservation may not be necessary, because the contacts appear to be mediated by amide bonds rather than side chains. Such backbone–backbone contacts frequently provide additional affinity by mediating nonspecific protein-DNA interactions.37
A second stretch of residues, ranging from M217 to L226, appears to be positioned for interactions within the major groove of the DNA [Fig. 2(c)]. This region is also not well conserved. It is noteworthy that even though the surrounding regions are rich in acidic residues these are absent in this stretch of amino acids. Several ExsD orthologs do have negatively charged residues in positions equivalent to A218, S223, and G225. However, judging from their position near the bottom of the loop, at least the latter two residues may well be involved in hydrogen bonding interactions within the major groove, away from the negatively charged backbone.
While there are no obvious clashes within a single ExsD-DNA complex, the molecular arrangement of the trimer in the Δ20ExsD crystal structure strongly suggests that the trimer is not compatible with DNA binding. The predicted DNA binding sites are all oriented toward a central pore of the trimer. Therefore, a single DNA molecule completely occupies the pore, leaving no room for the binding of two additional DNA molecules [Fig. 4(a)]. We do not consider this to be a problem with respect to the validity of our model, since the ExsD trimer has previously been shown to dissociate when the 2:2 ExsC-ExsD complex is formed.29 Furthermore, the predicted dissociation of the trimer is also consistent with the results of the below described differential scanning fluorimetry (DSF) studies.
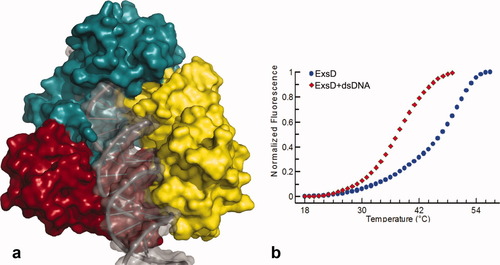
The ExsD trimer is incompatible with DNA binding. (a) Structure of the Δ20ExsD trimer with a single DNA molecule placed into the DNA binding site of molecule C (yellow). No space is available for the binding of two additional DNA molecules and the modeled-in DNA clashes with the two other ExsD molecules. (b) Results of DSF studies for (full length) ExsD-dsDNA interactions. For 10 μM ExsD alone a Tm of 49.7°C ± 0.3°C was observed, while 10 μM ExsD combined with 100 μM of 24-nucleotide double-stranded DNA produced a Tm of 37.7°C ± 0.2°C. The observed decrease in Tm is consistent with the predicted dissociation of the ExsD trimer upon DNA binding.
Evidence for ExsD-DNA interactions
Since we only recently discovered the putative DNA binding properties of ExsD, the actual ExsD binding site is currently unknown. Considering its proposed functional role we presumed the ExsD operator would be located in close proximity to the ExsA binding sites. However, initial electrophoretic mobility shift assay (EMSA) studies with the ExsA-dependent pD promoter templates failed to show ExsD binding in the absence and presence of ExsA (Supp. Info. Fig. 3). We therefore reasoned that either the actual operator of ExsD may not have been contained within the templates or that additional proteins other than ExsA might be required for tight binding.
DNA binding proteins generally possess nonspecific affinity for DNA with dissociation constants that are between 103 to 107 times higher than their dissociation constants for their specific operator sites.38 Presumably, this property permits these proteins to glide along the DNA in search of their actual recognition site. However, such weak interactions may well be outside the detection limits of EMSA and many other biochemical tools. We had previously used DSF to map ExsA-promoter interactions (unpublished results) with great success. DSF proved extremely sensitive to even single base changes in the operator sequence, suggesting that it might also be sensitive enough to detect nonspecific ExsD-DNA interactions. Usually, ligand binding results in increased thermal stability and a higher melting transition point (Tm) because of the additionally formed bonding interactions, where even a Tm increase of a single degree Celsius may be considered an indicator of binding.39, 40 However, in the case of ExsD the addition of DNA caused a 12°C decrease of the Tm [Fig. 4(b)]. The striking loss of thermal stability, at first glance perhaps unexpected, is consistent with the prediction that DNA binding necessitates dissociation of the ExsD trimer. Inside the trimer, extensive protein–protein contacts amount to a buried surface area of about 2000 Å2 for each ExsD molecule. In comparison, the ExsD-DNA interface covers only about 800 Å2 in the modeled complex, corresponding to a net reduction in contact area by about 1200 Å2. Therefore, our results are in line with previous studies, where reduced oligomerization and the accompanying loss of stabilizing intermolecular contacts area resulted in significant decrease of the melting temperature.41-43
Discussion
The central role of ExsA in the regulation of all T3SS-related genes in P. aeruginosa has been known for some time.21 McCaw et al.25 uncovered the importance of ExsD as antiactivator of ExsA, by demonstrating that an exsD− strain is deregulated with respect to T3SS gene expression. In the same study, results of a bacterial two-hybrid assay suggested a direct interaction between ExsA and ExsD. In the following years the existence of ExsC and ExsE were first postulated and subsequently proven.26, 44 The ExsACDE signaling cascade follows a unique mechanism to couple activation of the T3SS with up-regulation of T3SS-gene expression. Unlike in the more commonly encountered two-component and three-component signaling systems no covalent bonds are broken or formed and none of the participating proteins display catalytic activity. The entire cascade seems to be based on the formation and dissociation of three distinct protein–protein complexes: ExsE-ExsC, ExsC-ExsD, and ExsD-ExsA. The ExsE-ExsC and ExsD-ExsA complexes represent the inactive state of the T3SS as well as T3SS-related transcription.25-29 The host-cell-contact-triggered opening of the secretion channel is accompanied by the translocation of ExsE. The released ExsC sequesters ExsD and thus permits ExsA-mediated transcription activation. The entire signaling mechanism is thought to be based on the differential strength of the involved protein–protein complexes. This model is supported by the observed 19-fold higher affinity of ExsC for ExsE over ExsD.
The discovery that ExsD appears to function as a DNA binding protein has far reaching consequences for our understanding of the functional role of ExsD and the regulatory mechanism as a whole. ExsC is thought to alleviate ExsD-mediated repression of ExsA by disrupting ExsD-ExsA interactions. Previous studies have placed the ExsC binding site within the aminoterminal region of ExsD and thus in immediate proximity to the putative DNA binding domain of ExsD.28 Therefore, ExsC may well interfere with ExsD-DNA as well as ExsA-ExsD interactions. Alternatively, since ExsD-ExsA interactions are likely quite weak, it might be sufficient for ExsC to disrupt ExsD-DNA interactions alone.
Since ExsD is believed to directly interact with ExsA, we expected the ExsD binding site to be located near known ExsA consensus sequences. Yet, EMSA experiments with ExsA-dependent promoter constructs showed no measurable affinity of ExsD for the pD promoter (Supp. Info. Fig. 3). Furthermore, even in the presence of ExsA no additional gel shifts or “supershifts” were observed, suggesting neither ExsA nor the DNA sequences proximal to the promoter start sites suffice to promote tight ExsD binding. Although these results are in apparent contradiction with our current understanding of how ExsD regulates ExsA function, our findings may be reconciled with previously published data. Perhaps, a third as yet unknown protein is required for ExsD binding. Alternatively, ExsD, like many other repressor proteins, may not simply act as a roadblock of transcription but use a DNA looping mechanism to prevent ExsA-mediated transcription. In DNA looping, efficient transcription repression would not only be contingent on ExsD-ExsA interactions but also on the simultaneous binding of ExsD to a distant operator. Incidentally, KorB the structural homolog of ExsD not only utilizes a DNA looping mechanism to mediate transcription repression but also requires the presence of corepressors KorA or TrbA for full function from certain operator sequences.45 Conceivably, weak nonspecific ExsD-DNA interactions near the promoter are enhanced through ExsA binding and higher affinity interactions of ExsD with a distal specific operator sequence. The LysR-type transcriptional regulator CatR from Pseudomonas putida offers an excellent example for such asymmetric protein-DNA interactions. CatR is thought to facilitate repression of the catBCA operon by DNA looping through simultaneous interaction with an operator sequence just upstream of the −35 promoter element and a downstream operator at +162. In vitro studies showed that the barely detectable affinity of CatR for the downstream element was dramatically enhanced by the presence of the upstream operator.46 Another feature of the ExsD structure consistent with a DNA looping mechanism is the presence of the extensive coiled-coil region. Coiled-coil regions frequently mediate dimerization and could therefore facilitate the bridging of two distant DNA binding sites. Key to unraveling the molecular basis for the action of ExsD will be the identification of its DNA binding site, which will also provide additional corroboration of the protein's DNA binding properties. Future studies will also focus on the question whether or not additional proteins assist in the regulation of ExsA.
In summary, the crystal structure of the antiactivator protein ExsD has revealed striking similarities of ExsD with the transcriptional repressor protein KorB. A crude model of an ExsD-DNA complex was constructed. Electrostatic surface properties as well as a high degree of sequence conservation at the putative DNA binding site lend additional support to our model. While ExsD displayed no strong DNA binding affinity during EMSA studies, ExsD-DNA interactions were observed in DSF experiments. Also consistent with our model, the decrease in thermostability of ExsD suggests the dissociation of the ExsD trimer upon DNA binding. The conspicuous positioning of the DNA binding site in close proximity to the ExsC binding site suggests that ExsC-binding might impact ExsD-DNA interactions.
Materials and Methods
Expression and purification of ExsD
ExsD was overexpressed in E. coli from a vector constructed by Gateway recombinational cloning (Invitrogen, Carlsbad, CA). A tobacco etch virus (TEV) protease recognition site and the appropriate att recombination sites (attB1 and attB2) were added to the exsD gene during PCR and subsequently the amplicon was recombined into pDONR201 (Invitrogen). The nucleotide sequence of the ORF was verified, after which it was recombined into the destination vector pDEST-HisMBP47 to create the expression vector pFS1, which was designed to produce ExsD as a fusion to the C-terminus of an N-terminally His-tagged E. coli maltose-binding protein (MBP).
Single colonies of E. coli BL21(DE3) CodonPlus RIL cells (Stratagene, La Jolla, CA) containing pFS1 were used to inoculate 100 mL of Luria broth supplemented with glucose at 2 g/L, 100 μg/mL ampicillin, and 30 μg/mL chloramphenicol. The culture was grown with shaking (225 rpm) to saturation overnight at 37°C and then diluted 66-fold into 6 L of fresh medium. When the cells reached early log phase (OD600 = 0.5), the temperature was reduced to 30°C and isopropyl-β-D-thiogalacto-pyranoside (IPTG) was added to a final concentration of 1 mM. Four hours later, the cells were recovered by centrifugation at 5000g for 15 min. The cell paste was resuspended in 100 mL of 50 mM HEPES (pH 7.6), 150 mM NaCl, 25 mM imidazole, 2 mM DTT (buffer A) along with 150 μL of EDTA-free protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). The cells were lysed via sonication. The supernatant was filtered through a 0.45-μm polyethersulfone membrane and then applied to a 30 mL Ni-NTA Superflow affinity column (Qiagen, Valencia, CA) equilibrated with buffer A. The column was washed with 5 column volumes of buffer A and then eluted with a linear gradient from 25 to 250 mM imidazole. The His-MBP-ExsD was then digested with His-tagged TEV(S219V) protease48 overnight, using 1 mg of protease for every 100 mg of fusion protein. The sample was subsequently diluted sixfold in 50 mM HEPES (pH 7.6), 150 mM NaCl, 2 mM to reduce the imidazole concentration down to a final concentration of 25 mM. Next, it was once again applied to a 25 mL Ni-NTA Superflow affinity column (Qiagen,) equilibrated with buffer A. The flow-through fractions now contained the free ExsD while His-MBP and His-TEV(S219V) were retained on the column. The selenomethionine-substituted ExsD was produced using the saturation of the methionine biosynthetic pathway protocol49 and subsequently treated in the same manner as the original ExsD.
Limited proteolysis and purification of Δ20ExsD
A 1 mg/mL stock solution of thermolysin (Roche Molecular Biochemicals, Indianapolis, IN) in water was used for the limited proteolysis experiments. The ExsD stock solution consisted of the protein at 1 mg/mL in buffer A. The five individual reactions were composed of 25 μL of ExsD stock solution, 25 μL of 2× thermolysin buffer (20 mM Tris-HCl pH8.0, 4 mM CaCl2, 0.4M NaCl, and 10% Glycerol), and 0.5 μL of serial 1:4 dilutions of the thermolysin stock solution. The reactions were allowed to proceed for 1 h at 37°C, at which time they were stopped by the addition of 0.5 μL of 0.5M EDTA. The reaction products were initially analyzed by SDS-PAGE. The precise molecular weights of the fragments were obtained by electrospray mass spectrometry. The large-scale thermolysin digest of ExsD was performed by combining 5 mL of 5 mg/mL ExsD, 5 mL of 2× thermolysin buffer, and 0.1 mL of thermolysin at 0.25 mg/mL. The reaction proceeded for 1 h at 37°C and was stopped by the addition of 0.1 mL of 0.5M EDTA. The sample was concentrated to about 16 mg/mL using an Amicon YM10 membrane (Millipore, Billerica, MA) and applied to a 26/60 HiLoad Superdex 200 prep grade column (Amersham Biosciences, Piscataway, NJ) equilibrated with 25 mM HEPES (pH 7.4), 150 mM NaCl, 2 mM tris(2-carboxyethyl) phosphine (TCEP) (buffer B). The peak fractions containing the Δ20ExsD were pooled and concentrated to 15 mg/mL. Aliquots were flash-frozen with liquid nitrogen and stored at −80°C until use. The final product was judged to be >95% pure by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Crystallization of Δ20ExsD
Since full length ExsD and Δ20ExsD initially failed to crystallize, purified Δ20ExsD was subjected to reductive methylation following a previously published protocol30 and subsequently applied to a 26/60 HiLoad Superdex-200 prep grade column equilibrated with 25 mM Tris-HCl (pH 7.4), 150 mM NaCl, and 2 mM TCEP. After concentrating the purified and methylated Δ20ExsD to 15 mg/mL, high-throughput crystallization screening was conducted in the sitting drop format with commercially available crystallization matrices. Initial crystals were obtained from condition 79 of the Index crystallization screen (Hampton Research, Aliso Viejo, CA). The optimized condition containing 0.1M Bis-Tris (pH 6.8), 0.2M ammonium acetate, and 30% polyethylene glycol 3350 was combined at a 1:1 ratio with Δ20ExsD solution at 15 mg/mL in 25 mM Tris-HCl (pH 7.4), 0.15M NaCl, 2 mM TCEP.
X-ray data collection, structure solution, and refinement
Crystals of the Δ20ExsD were loop mounted without cryo-soaking and flash-frozen in liquid nitrogen. The dataset used for the structure solution was collected at beamline X29A (National Synchrotron Light Source, Brookhaven National Laboratory) using an ADSC Q315 CCD detector. We obtained a peak SAD data set for the selenomethionine-substituted sample with useful diffraction data extending to about 2.6 Å resolution. Data processing was carried out at the beamline with the HKL2000 program suite.50 Details of data collection and processing for all data sets are provided in Table I.
Location of heavy atom positions, initial phase calculations, phase improvement through density modification, and structure solution were all carried out with the PHENIX program suite.51 The initial 2.6 Å map and automatically generated backbone trace were of excellent quality, exhibiting clear protein/solvent boundaries and recognizable features of protein secondary structure. After density modification, nearly the entire backbone and most of the side-chains could be traced with the molecular modeling program O.52 The model was refined in PHENIX followed by manual adjustment against weighted difference Fourier maps. After several rounds of manual adjustment and refinement, 136 water molecules were added to the structure.
Model quality was assessed with PROCHECK.53 All nonglycine residues in both crystal structures resided either in the most favorable or in the allowed regions of the Ramachandran plot, and the overall geometry was better than average when compared to structures solved at the same resolution. Model refinement statistics are given in Table I. The atomic coordinates and structure factors for the Δ20ExsD structure have been deposited in the PDB34 with accession code (3FD9).
Differential scanning fluorimetry experiments with ExsD
The DSF experiments were carried out with an ABI 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA). Sypro Orange (Ex. 490 nm, Em. 530 nm) can be obtained as a 5000× solution in DMSO (Invitrogen). To minimize the exposure of our ExsD to DMSO, we produced a 50× Sypro Orange solution by diluting the dye into protein buffer (20 mM NaCl, 50 mM Hepes, 2 mM TCEP, pH7.6). Each reaction had a final volume of 30 μL containing 5 μM of ExsD and 5× Sypro Orange. The complementary DNA oligomers were purchased from Operon Biotechnologies (Huntsville, AL). Several different templates were used in the DSF experiments. Since no significant differences were observed for the binding of the various templates, only the curve obtained for the binding of a 46-mer dsDNA strand is shown in Figure 4. All oligomers were prepared in 500 μM stock solutions and their final concentration in the experiment was kept at 50 μM, so that each reaction contained 5 μM full-length ExsD protein, 50 μM dsDNA, 20 mM NaCl, 60 μM TCEP, and 50 mM Hepes pH 7.6. Starting at 10°C the temperature was incrementally increased to 85°C at a rate of 1°C per minute. Data analysis was carried out with XLfit (IDBS, Bridgewater, NJ). The sequences of the used double-stranded DNA strands were 5′-CGA-ATG-CCG-GGC-TAA-AAA-TAA-CTG-3′ and its reverse complement.
Acknowledgements
Funding for data collected at beamline × 29 NSLS is provided by DOE/DER and NIH/NCRR.




